Abstract
Neutrophils are an essential protective component of the innate immune system. However, in severe bacterial infections, neutrophils are known to mis-localise from the primary site of infection to other organs, where excessive release of cytokines, chemokines, and neutrophil extracellular traps (NETs) can induce organ damage and death. In this study, we use an animal model of bacterial infection originating in the peritoneum to show that hydrogen peroxide (H2O2, a potent neutrophil chemoattractant) is initially released in high concentrations both in the peritoneum and in multiple ‘off-target’ organs (lungs, liver and kidneys). The initial high H2O2 release inhibits neutrophil chemotaxis, but after 24 h concentrations of H2O2 reduce and can promote neutrophil migration to organs, where they release pro-inflammatory cytokines and chemokines along with NETs. The antimalarial compound artesunate potently inhibits neutrophil migration to off-target organs. It also abolishes cytokine, chemokine, and NET production, suggesting that artesunate may be a valuable novel therapy for preventing off-target organ inflammation associated with severe bacterial infections. Finally, the potency of H2O2 as a chemoattractant is shown by in vitro experiments in which, faced with competing gradients of H2O2 and other chemoattractants, neutrophils preferentially migrate towards H2O2.
1. Introduction
Neutrophils are early responders to pathogen invasion. In minor bacterial infections, neutrophils exhibit rapid chemotaxis to the site of pathogen infection [1], followed by effector actions, such as pro-inflammatory cytokine and chemokine release, that activate and attract further neutrophils and other immune cells [2], and microbiocidal actions, such as phagocytosis [3] and neutrophil extracellular trap (NET) production [4,5], that neutralise the invading organism.
However, in more severe infections, higher pathogen loads can lead to dysregulation in neutrophil function. During severe bacterial infections, neutrophils can become impaired in their initial chemotactic ability [6]. The reason for this phenomenon of ‘neutrophil paralysis’ remains debated [7], with some studies suggesting that it is due to the high pathogen load inducing an overwhelming number of chemoattractant molecules, thereby preventing the generation of detectable concentration gradients [8,9,10]. In contrast, others have suggested that it is due to upregulation of receptors on the neutrophils themselves, such as G-protein-coupled receptors (GPCRs) and proliferator-activated receptor γ (PPAR-γ), resulting in neutrophil desensitisation to chemoattractants [11].
Instead of displaying their typical chemotactic abilities to move to a site of infection, in severe infections, neutrophils can also mis-localise and lodge in vital organs other than the initial target of infection [12,13,14]. The reason for this is not understood, but autopsy examinations have revealed localisation of neutrophils varying from sequestration and aggregation in renal blood vessels to large-scale tissue infiltration of multiple organs in the body, including the lungs, liver and kidneys [15,16,17,18,19]. Once in ‘off-target’ organs, neutrophils, through the release of cytokines, chemokines, and NETs, as well as the production of reactive oxygen species (ROS) and enzymatic degranulation, can cause host organ damage, organ failure, and death [20,21,22,23,24]. The neutrophil-mediated release of pro-inflammatory molecules, including cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-1β, chemokines, such as C-X-C motif chemokine ligand (CXCL)1 and IL-8, and the induction of chemotactic proteins such as Complement component C5a (C5a), can cause positive feedback in which the neutrophil-induced hyper-inflammatory milieu within the organ causes the recruitment of further neutrophils, prolonging the damaging inflammation [25,26,27].
The mechanism which governs the mis-localisation of neutrophils to organs other than the site of the initial infection is currently unknown. One suggestion is that during severe bacterial infections, hydrogen peroxide (H2O2) may accumulate in organ parenchymal cells as a toxic by-product of the increased cellular metabolism caused by the patient’s hypermetabolic state [28,29,30]. Neutrophils can migrate strongly towards a source of H2O2, with leading-edge intracellular calcium ‘pulses’ central to guiding their migration, but higher H2O2 concentrations saturate calcium release, thus inhibiting chemotaxis [31].
In the present paper, we show that H2O2 is initially present at high concentrations in multiple off-target organs during a model of severe bacterial infection in mice. This elevated H2O2 paralyses neutrophils, but after 24 h the H2O2 concentration reduces, and mediates an influx of neutrophils to the organs, thus causing off-target release of cytokines, chemokines and NETs. We also show that artesunate, a widely used derivative of the antimalarial artemisinin, prevents organ infiltration by neutrophils and consequent high levels of cytokines, chemokines and NETs, suggesting that artesunate may be a valuable novel therapy in preventing the multiple organ inflammation associated with life-threatening bacterial infections.
2. Materials and Methods
2.1. Animals and Ethics
Cold Spring Harbour 57 Black 6 mouse strain (C57BL/6) wild-type (WT) mice (6–8 weeks old, 20 g) were purchased from Charles River Inc. (Kent, UK). All animal work was conducted under UK Home Office personal and project licences, approved by the Animal Welfare Ethical Review Board (AWERB) of King’s College London (London, UK) and carried out in accordance with the Animals (Scientific Procedures) Act 1986 and in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
2.2. Human Blood and Ethics
Human blood samples were collected by a trained phlebotomist and used under approved University of Westminster (London, UK) ethics, number ETH2324-0913.
2.3. Chemicals and Reagents
ibidi µ-slide chemotaxis assays were purchased from Thistle Scientific Ltd. (Glasgow, UK). Hydrogen Peroxide (H2O2, 31642), N-formylmethionine-Leucyl-Phenylalanine (fMLP, F3506), Lipopolysaccharide (LPS, LPS25), Interleukin-8 (IL-8, CXCL8 SRP3098), Complement Component C5a (C5a, C5788), CXCL2 (SRP4251), Tumour Necrosis Factor-alpha (TNF-α, T6674), Interleukin-1beta (IL-1β, SRP3083) and Thioglycolate (70157) were purchased from Sigma-Aldrich (Gillingham, UK). RAL-DIFF-QUIK kit (a modified version of the May-Grünwald-Giemsa stain) (RAL555) was purchased from RAL Diagnostics (Martillac, France). Hydrogen Peroxide Assay Kit (H2O2 assay kit, ab102500) was purchased from Abcam (Cambridge, UK). Artesunate (A3731), Dulbecco’s Modified Eagle’s Medium (DMEM, D0822), Foetal Bovine Serum (FBS, F7524), Phosphate-Buffered Saline (PBS, P4474) and Liberase Thermolysin Low (TL) (05401020001) were purchased from Merck Life Sciences (Feltham, UK). DuoSet Enzyme-Linked Immunosorbent Assay (ELISA) kits for CXCL1 (DY453), CXCL2 (DY452), IL-6 (DY406) and IL-1β (DY401) were purchased from R&D Systems (Bio-Techne, Oxford, UK). Cell-free (Cf) Deoxyribonucleic acid (DNA) kit (Quant-IT™ PicoGreen®, P7589) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Magnetic-Activated Cell Sorting (MACS)xpress Separator (130-098-308), MACSmix™ Tube Rotator (130-090-753) and MACSxpress® Whole Blood Neutrophil Isolation Kit, human (130-104-434) were purchased from Miltenyi Biotec (Woking, UK).
2.4. In Vivo Experiments
Mouse LPS Models
WT mice were intraperitoneally (i.p.) injected either with a low concentration of lipopolysaccharide (LPS, a major component of the Gram-negative bacterial cell wall) to induce a local inflammation (30 ng, c. 0.15 mg/kg) or with a higher concentration to induce more severe inflammation (either 30 µg, c. 15 mg/kg or 300 µg, c. 150 mg/kg), as used in previous studies [32,33,34]. After 5–24 h, mice were euthanised and immune cells were extracted from the peritoneum, lungs, liver or kidneys as follows. For peritoneal lavage, the peritoneal-covering skin was removed, and 5 mL of PBS was injected into the peritoneal cavity, which was massaged gently for 60 s to dislodge cells. The peritoneal fluid was gently extracted by syringe, as described previously [31,35]. For lung lavage, mice were placed in a supine position, limbs were secured, and the skin around the neck was removed. Salivary glands were separated to reveal the sternal hyoid muscle, and forceps were used to incise the muscle around the trachea. A cotton suture was then threaded under the tracheal tissue, and a needle was used to puncture the middle of the trachea between two cartilage rings. A pre-made plastic catheter was inserted ~0.5 cm into the tracheal lumen and stabilised with the suture. A syringe, loaded with 1 mL of PBS, was then attached to the catheter, and PBS was slowly injected. The thorax was massaged gently for 60 s before bronchoalveolar lavage fluid (BALF) was aspirated. This was repeated 3 times to maximise the BALF recovery, as described previously [35]. For cell extraction from the liver and kidneys, organs were excised and finely minced in a solution of 0.2125 mg/mL Liberase TL in DMEM at 37 °C for 20 min. DMEM + 10% FBS was then added, and the solutions were placed on ice to stop the digestion. The digested tissue was then passed through a 70 µm cell strainer and centrifuged at 427× g for 7 min to collect cells, following an established protocol [36]. In all cases, cells were then spun down onto glass slides using a cytocentrifuge and stained using RAL-DIFF-QUIK stain to quantify cells present (described in detail below). In experiments where the effect of artesunate was to be tested, mice were subcutaneously (s.c.) injected with either artesunate (28 mg/kg or 6 mg/kg) or PBS alone 30 min prior to being injected i.p. with LPS or control (PBS). Further artesunate/control s.c. injections were administered at 2, 4, 6, 8, 10, 12, 14, 20 and 22 h, before mice were euthanised at 24 h and cells extracted and identified as described above. Supernatant fluid samples from all peritonea, lung, liver and kidney samples were saved and analysed for cytokines/chemokines by ELISA (described below), and levels of H2O2 and cf-DNA were quantified as also described below.
2.5. In Vitro Experiments
2.5.1. Isolation of Mouse Peritoneal Neutrophils and Macrophages
Mice were i.p. injected with 3% thioglycolate solution (10 μL/g) and, after 4 h (for neutrophils) or 4 d (for macrophages), were euthanised by cervical dislocation. The peritoneal-covering skin was removed, and 5 mL of PBS was injected into the peritoneal cavity, which was massaged gently for 60 s to dislodge cells. The peritoneal fluid was gently extracted using a syringe and centrifuged for 10 min at 200 relative centrifugal force (RCF). The supernatant was discarded, and cells were resuspended in DMEM + 10% FBS. These methods generated cell suspensions containing > 95% of either neutrophils or macrophages, identified through RAL-DIFF-QUIK staining as previously described [31,35] (see also below).
2.5.2. Isolation of Human Neutrophils
Human neutrophils were obtained from whole blood using the MACSxpress® Whole Blood Neutrophil Isolation Kit as per the manufacturer’s instructions (Supplementary Figure S1). Neutrophil suspensions were centrifuged for 10 min at 200 RCF. The supernatant was discarded, and cells were resuspended in DMEM + 10% FBS. Cells were identified through RAL-DIFF-QUIK staining as previously described [31,35] (see also below).
2.5.3. ibidi µ-Slide Chemotaxis Assays
ibidi µ-slide chemotaxis assay chambers were purchased pre-coated with collagen IV along the central migration strip. Neutrophils or macrophages, isolated as above from the peritonea of WT mice or from human blood, were re-suspended within 30 min of collection in DMEM + 10% FBS at a concentration of 5 × 105 cells per ml. In some models, cells were pre-incubated with an inflammatory pool of LPS (50 ng/mL), C5a (10 nM), CXCL2/IL-8 (10 nM), fMLP (1 µM), TNF-α (150 pg/mL) and IL-1β (150 pg/mL). The cell suspension (6 µL) was seeded along the central migration strip of an ibidi µ-slide chamber as per the manufacturer’s instructions, and slides were then incubated for 1 h at 37 °C in humidified 95% air/5% carbon dioxide (CO2), to allow neutrophil/macrophage adherence to the central migration strip. DMEM (no added FBS) with and without added chemoattractant was then added to the wells on opposite sides of the central migration strip. For some models, competing gradients of chemoattractants were set up on opposite sides of the assays. For experiments in which the effects of artesunate were to be tested, equal concentrations were added to both sides of the assays to create a uniform concentration across the entire assay. Slides were incubated at 37 °C in 95% air/5% CO2 for 20 min to allow the generation of gradient(s) of chemoattractant across the 1 mm wide × 70 μm deep central cell migration strip. Live-cell time-lapse microscopy was then conducted using a 10× lens and dark-field illumination on a Nikon Eclipse Ti-E inverted microscope equipped with the Nikon Perfect Focus System (PFS) (Surbiton, UK). The microscope was housed in a temperature-controlled Perspex box (Solent Scientific (Portsmouth, UK)) at 37 °C, with slides housed in a stage-mounted block in a humidified 95% air/5% CO2 environment. A maximum of 12 individual chambers (4 individual slides, 3 chambers per slide) could be imaged per experiment by using a motorised stage. Stage movement, lens focus and image acquisition were controlled by the Nikon Imaging Software (NIS)-Elements Advanced Research (AR) programme (version 5.01). Experiments were conducted over 2 h, with images of each assay compartment taken every 2 min. The ‘Fiji is just ImageJ’ (FIJI) (version 1.54p) TrackMate plug-in was employed to track individual neutrophils/macrophages. A chemotaxis and migration plug-in, provided by ibidi, was used to calculate forward migration index (FMI) data from the neutrophil/macrophage tracks.
2.6. Identification of Isolated Cells in Both In Vitro and In Vivo Experiments
Cell suspension, isolated from peritonea/lungs/liver/kidneys of WT mice or from human blood as above, was spun down onto glass slides using a cytocentrifuge at 400 revolutions per minute (RPM) for 5 min and left to air-dry overnight. RAL-DIFF-QUIK staining was then used to identify cells as follows: Slides were suspended in RAL-DIFF-QUIK fixative solution (methanol based solution to stabilise cellular components) for 1 min, in RAL-DIFF-QUIK solution I (Xanthene solution; a buffered solution of Eosin Y) for 1 min and in RAL-DIFF-QUIK solution II (a buffered solution of thiazine dyes, consisting of methylene blue and Azure A) for 1 min. Nuclei were meta-chromatically stained red/purple, and cytoplasm pink/yellow.
2.7. Quantification of Cytokines, Chemokines, NETs and H2O2
Supernatant fluid, extracted from organs as indicated above, was collected, and IL-6, IL-1β, CXCL1 and CXCL2 concentrations in the fluid were measured by ELISA using commercial kits, as previously described [37], with absorbance readings taken at 450 nm on FlexStation 3 Microplate Reader (Molecular Devices, San Jose, CA, USA). The results are expressed as pg/mL of each cytokine/chemokine (assay minimum detection limits for all cytokines/chemokines: 1.56 pg/mL). As a control, concentrations of these cytokines/chemokines were measured in mice injected with vehicle (PBS). In the same samples, the amount of cf-DNA was quantified using the Quant-iT™ PicoGreen® kit, according to the manufacturer’s instructions, which serves as an accurate measure of NET release [20,35]. The fluorescence intensity (excitation at 488 nm and emission at 525 nm wavelength), a measure of the amount of dye bound to DNA, was quantified by a fluorescence reader (FlexStation 3 Microplate Reader) as previously described [20]. The results are expressed as ng/mL of cf-DNA (assay minimum detection limit: 0.002 ng/mL). H2O2 concentrations in samples were measured using the H2O2 assay kit, as per the manufacturer’s instructions. Samples were incubated with the OxiRed probe, developed with horseradish peroxidase (HRP), and absorbance read at 570 nm on a FlexStation 3 Microplate Reader, as described previously [38,39]. The results are expressed as µM of H2O2 (assay minimum detection limit: 0.04 µM).
2.8. Statistical Analysis
Data is presented as bars ± standard error of the mean (SEM). Statistical analysis of data was conducted using GraphPad Prism (version 10.5). For two-group comparisons, an unpaired t-test was used; for three-group comparisons, one-way Analysis of Variance (ANOVA) was used; and for ≥three-group comparisons where the effect of artesunate was tested, two-way ANOVA was used. For all ANOVA tests, the Tukey–Kramer post hoc test was also employed. Before any of these statistical tests were carried out, normality and homogeneity of variance were assessed using GraphPad Prism as standard. The assumptions were met, and no significant deviation from normality was observed. The N number, number of independent experiments, and p-values are stated in the legend of each Figure and Supplementary Figure.
3. Results
3.1. Suppressed Neutrophil Chemotaxis but Increased H2O2 in LPS Infection Model
Injection of low doses of antigens such as bacterial LPS or Coronavirus Disease 2019 (COVID-19) viral spike protein causes potent neutrophil chemoattraction to the site of injection, and these invading neutrophils then release cytokines, chemokines and NETs [35]. However, at the higher levels of pathogens typical of more severe infections, neutrophil chemotaxis towards the site of infection can be impeded [7] and neutrophils can instead show chemotaxis towards off-target organs such as lung, liver and kidney [13,14,16], where they release cytokines, chemokines and NETs that cause off-target inflammation [12,17,23].
To explore the cause of this phenomenon, we injected mice with LPS i.p. in order to model bacterial infections of varying severity. Extracellular LPS activates Toll-Like Receptor 4 (TLR4) to cause a complex inflammatory response, including activation of the Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway leading to release of pro-inflammatory cytokines/chemokines [40] and activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enzymes that synthesise superoxide radicals, highly reactive molecules that are rapidly converted to H2O2 [41]. Intracellular LPS can also activate cysteine-dependent aspartate-specific protease (caspase)-4, leading to nucleotide-binding domain, leucine-rich-repeat family pyrin domain-containing 3 protein (NLRP3) inflammasome activation, resulting in pro-inflammatory actions such as cytokine production [42,43]. A by-product of this production is the further activation of NOX enzymes, leading to further H2O2 production [44]. H2O2 itself can then also activate NLRP3, leading to a positive feedback loop of H2O2 production in response to LPS [45].
We used a low dose of LPS (30 ng, c. 0.15 mg/kg, i.p.) to induce a localised peritoneal inflammation, and in parallel experiments, we injected a 100× higher LPS concentration (3 µg, c. 15 mg/kg, i.p.) to model a more severe bacterial infection [32,33,34] (Figure 1). The lower LPS concentration caused neutrophil invasion into the peritoneum 5 h after injection (Figure 1A), similar to results reported previously [35], and also caused the release of around 40 µM H2O2 in the peritoneum (Figure 1B). However, at a higher dose of 3 μg LPS, no neutrophil chemotaxis to the injection site was seen (Figure 1A), a phenomenon sometimes referred to as neutrophil paralysis. The peritoneal H2O2 concentration, at around 200 µM, was significantly higher than with the 30 ng LPS dose (Figure 1B). The 3 μg dose of peritoneal LPS also induced no increase in neutrophil entry into the lung (Figure 1C) and elevated the concentration of H2O2 in the lung to around 130 μM (Figure 1D), suggesting that neutrophil migration was paralysed. (Lung neutrophils were not assessed following 30 ng i.p. LPS, as this dose induces only a localised peritoneal infection, not expected to have a system-wide effect and thus induce lung inflammation.)
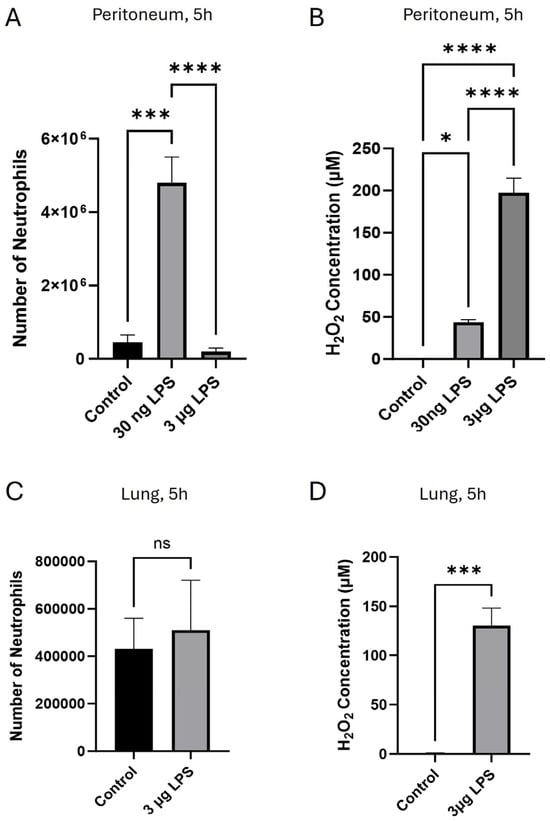
Figure 1.
H2O2 released in response to i.p. injection of LPS promotes neutrophil chemotaxis at low concentrations but inhibits chemotaxis at high concentrations. (A) Invasion of neutrophils into the peritoneum was high 5 h after i.p. injection of 30 ng LPS but was suppressed with 3 μg LPS; (B) H2O2 i.p. concentration was 43.7 ± 3.04 μM 5 h following i.p. injection of 30 ng LPS but 197.5 ± 17.12 μM following 3 μg LPS. (C) Invasion of neutrophils into the lung was suppressed 5 h following i.p. injection of 3 μg LPS; (D) lung H2O2 concentration following i.p. injection of 3 μg LPS was 129.5 ± 17.73 μM. Bars show mean ± SEM, N = 6 mice per bar, with 6 independent experiments per result. Results with no visible bars were below the assay detection limits (see Section 2) and, for statistical purposes, were assumed to be equal to the detection limit. * p < 0.05; *** p < 0.001, **** p < 0.0001, ns = no significant difference (for (A,B): one-way ANOVA and Tukey–Kramer post hoc test, for (C,D): unpaired t-test).
In previous work, we had found that in an in vivo model similar to that used in Figure 1, i.p. injections of H2O2 between 10 nM and 10 μM caused strong neutrophil chemotaxis into the peritoneum, while at 100 μM H2O2 chemotaxis was suppressed (see Figure 1A of ref. [31]). Using in vitro models with both mouse and human neutrophils, chemotaxis towards H2O2 was also found to be strong in the range 1 nM–10 μM, but chemotaxis was suppressed at 100 μM (see Figure 1B–E of ref. [31]). These results show that low concentrations of H2O2 are chemoattractive to neutrophils, while at higher concentrations of H2O2, chemoattraction is suppressed. In calcium-imaging experiments, we found that the mechanism underlying chemotaxis up a gradient of H2O2 of maximal concentration < 10 μM is preferential activation of calcium-permeable Transient Receptor Potential Cation Channel 2 (TRPM2) ion channels at the neutrophil leading edge, causing leading-edge calcium pulses which drive the forward movement of the neutrophil (see Figure 4 of ref. [31]). However, at higher concentrations of extracellular H2O2 (≥100 μM), we found that overactivation of TRPM2 ion channels caused calcium to be elevated throughout the whole cell, so that the critical intracellular calcium gradient is lost and chemotaxis is suppressed (see Figure 4A of ref. [31]). Inhibition of neutrophil chemotaxis at an H2O2 concentration > 100 μM therefore provides a plausible explanation for the inhibition of neutrophil chemotaxis that is observed at higher levels of LPS in Figure 1. In subsequent experiments, we explored this hypothesis as an explanation for neutrophil paralysis and for invasion of neutrophils into off-target organs.
3.2. Lower H2O2 and Increased Neutrophil Chemotaxis After 24 h
In contrast to the complete failure of neutrophils to invade either the peritoneum or the lung 5 h after i.p. administration of 3 μg LPS, we found that neutrophil migration to the lungs increased significantly after 24 h, to a greater than 10-fold higher level (Figure 2A). Similar levels of neutrophil invasion into the lungs were also observed 24 h after a 10× higher i.p. dose of 30 μg LPS (Figure 2A). As outlined above, a likely explanation for the delayed movement of neutrophils into the lung is that the concentration of H2O2 in lung had decreased from an inhibitory level of c. 130 μM at 5 h (Figure 1D) to a level of c. 40 μM at 24 h (Figure 2B), a concentration of H2O2 that had previously been shown to induce chemotaxis of isolated neutrophils [31]. These experiments therefore support the idea that a concentration of H2O2 ≤ c. 40 μM is chemoattractive for entry of neutrophils into the lung (Figure 2A,B), while a concentration of H2O2 above ~100 μM prevents neutrophil entry (Figure 1B,D), as was found in earlier studies with both in vitro and in vivo studies [31].
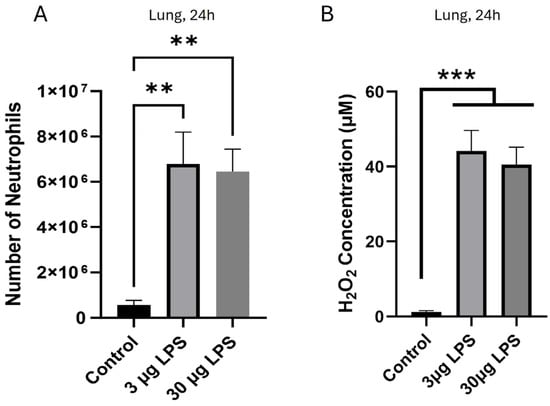
Figure 2.
H2O2 released in response to i.p. injection of high LPS (≥3 μg) is reduced at 24 h, allowing neutrophil entry to the lung. (A) Strong invasion of neutrophils into the lung was similar 24 h after injection of 3 μg and 30 μg LPS; (B) H2O2 concentrations in the lung were similar (c. 40 µM) 24 h after injection of 3 μg and 30 μg LPS. Bars show the mean ± SEM, N = 6 mice per bar, with 6 independent experiments per result. ** p < 0.01, *** p < 0.001 (one-way ANOVA and Tukey–Kramer post hoc test).
Together, these results show that high amounts of LPS injected into the peritoneum initially cause the release of elevated levels of H2O2, sufficient to inhibit chemotaxis of neutrophils and therefore to prevent neutrophil entry. High peritoneal LPS also induces the release of high H2O2 concentrations in the lungs, where it also initially prevents neutrophil entry. After 24 h, however, a decrease in H2O2 concentrations allows potent recruitment of neutrophils to the off-target lungs.
3.3. H2O2, Neutrophils, Cytokines/Chemokines, NETs and Artesunate in Lung, Liver and Kidney
The lessening of H2O2 concentrations to c. 40 μM in the lung 24 h after i.p. LPS injection (3 μg), and the accompanying influx of neutrophils, was confirmed in Figure 3A,B. Moreover, the neutrophil influx caused levels of pro-inflammatory cytokines, chemokines and NETs to be significantly elevated in the lungs (Figure 3C–G), with the potential to induce damaging inflammation. These findings therefore provide a basis for off-target inflammation in organs other than the initial target of infection, as is often seen in life-threatening bacterial infection.
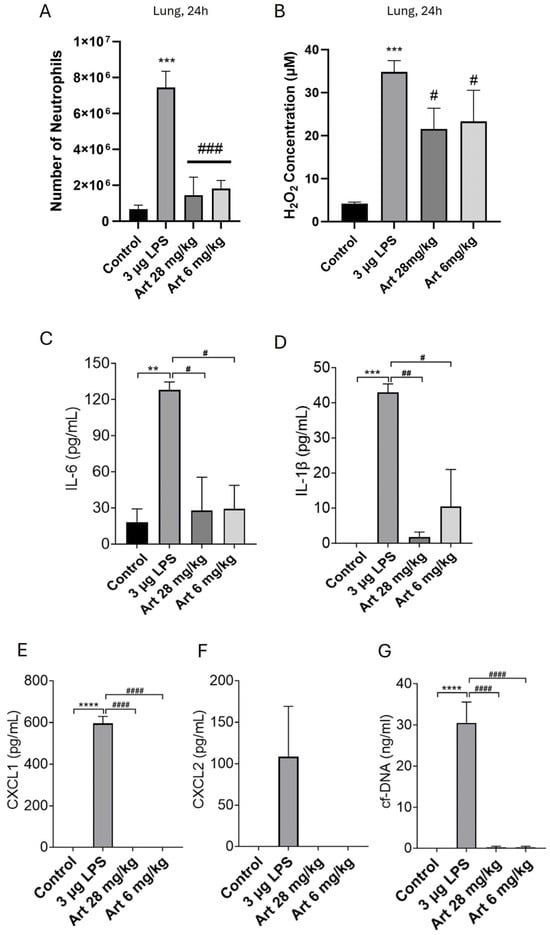
Figure 3.
Injection of LPS i.p. increases lung neutrophils, H2O2, pro-inflammatory cytokines and chemokines, and NETs. Artesunate inhibits the increase in lung neutrophils, pro-inflammatory cytokines and chemokines and NETs but has a lesser effect on H2O2. Mice were injected i.p. with 3 µg LPS to induce a severe bacterial-like infection and s.c. with 28 mg/kg or 6 mg/kg artesunate repeated at c. 2 h intervals (see Section 2). After 24 h, mice were euthanised and BALF extracted from lungs. (A) Strong neutrophil migration into the lung following 3 µg i.p. LPS was suppressed by both artesunate doses; (B) H2O2 concentrations of c. 35 µM observed in the lung following 3 µg i.p. LPS and c. 20 µM with both artesunate doses; (C–F) release of pro-inflammatory cytokines/chemokines IL-6, IL-1β, CXCL1 and CXCL2 were quantified using ELISA, with strong release in the lung following 3 µg i.p. LPS, suppressed by both artesunate doses; (G) cf-DNA in lung fluid was quantified as a measure of NET release, showing strong release caused by 3 µg i.p. LPS, suppressed by both artesunate doses. Bars show mean ± SEM, N = 4 mice per bar, with 4 independent experiments per result. Results with no visible bars were below the assay detection limits (see Section 2) and, for statistical purposes, were assumed to be equal to the detection limit. * for comparison between control and LPS to show the effect of LPS injection vs. sham, # for comparison between LPS and artesunate groups, to show the effect of artesunate on LPS-treated groups. ** p < 0.01, *** p < 0.001, **** p < 0.0001, # p < 0.05, ## p < 0.01, ### p < 0.001, #### p < 0.0001 (two-way ANOVA and Tukey–Kramer post hoc test).
In previous work, we showed that artesunate, an antimalarial, can significantly reduce neutrophil invasion and the levels of cytokines, chemokines and NETs released from neutrophils following an inflammatory stimulus directly applied to the peritoneum or lungs [35]. In Figure 3, we extend this work to off-target organs by showing that artesunate at a concentration of 6 mg/kg, close to clinically used doses for the treatment of malaria, together with a higher dose of 28 mg/kg, both cause a significant inhibition of neutrophil recruitment to lungs (Figure 3A) following injection of a high dose of LPS into the peritoneum to model a severe bacterial infection. Artesunate, however, had a much lesser effect on H2O2 levels in the lungs (Figure 3B), and the small effect is likely to be a by-product of inhibiting neutrophil chemotaxis and thus reducing neutrophil-mediated contribution to H2O2 levels through ROS release. Artesunate also caused near-complete inhibition of cytokine, chemokine and NET release in the lungs (Figure 3C–G). These results are similar to those obtained with the direct injection of inflammatory stimuli into the peritoneum or lung [35], and they suggest that artesunate may have therapeutic value in off-target organs, as well as in those directly targeted by an infection.
In other off-target organs, we found similar results. 24 h after i.p. injection of a high dose (3 μg) of LPS, concentrations of H2O2 were significantly elevated in both liver and kidney, at c. 25–35 μM but remained below an inhibitory concentration (Figure 4B and Figure 5B). Neutrophil migration correspondingly increased by ~5× (Figure 4A and Figure 5A). Pro-inflammatory cytokines, chemokines and NETs were significantly elevated in the liver (Figure 4C–G) and in the kidney (Figure 5C–G), with the potential to trigger off-target organ inflammation. The inhibitory effect of artesunate in liver and kidney on neutrophil migration (Figure 4A and Figure 5A), concentrations of H2O2 (Figure 4B and Figure 5B), cytokines/chemokines (Figure 4C–F and Figure 5C–F) and NETs (Figure 4G and Figure 5G) all mirrored those in the lung (Figure 3). Together, these results show that in a model of severe bacterial infection, elevated H2O2 mediates neutrophil chemotaxis to multiple off-target organs, together with cytokine, chemokine and NET release within these organs. Artesunate is a potent inhibitor of all of these pro-inflammatory manifestations.
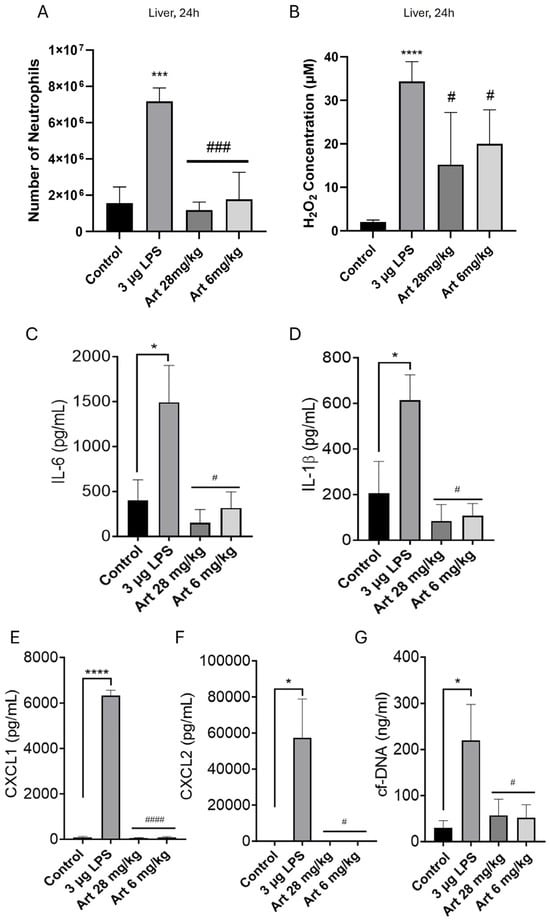
Figure 4.
Injection of LPS i.p. increases liver neutrophils, H2O2, pro-inflammatory cytokines, chemokines, and NETs. Artesunate inhibits the increase in liver neutrophils, pro-inflammatory cytokines, chemokines and NETs, but has a lesser effect on H2O2. Similar experiment to Figure 3 (where Figure 3A–G correspond here to (A–G)), except that lavage fluid was obtained from the liver (see Section 2). Bars represent mean ± SEM. N = 4 mice per bar, with 4 independent experiments per result. Results with no visible bars were below the assay detection limits (see Section 2) and, for statistical purposes, were assumed to be equal to the detection limit. * for comparison between control and LPS to show the effect of LPS injection vs. sham, # for comparison between LPS and artesunate groups, to show the effect of artesunate on LPS-treated groups. * p < 0.05, *** p < 0.001, **** p < 0.0001, # p < 0.05, ### p < 0.001, #### p < 0.0001 (two-way ANOVA and Tukey–Kramer post hoc test).
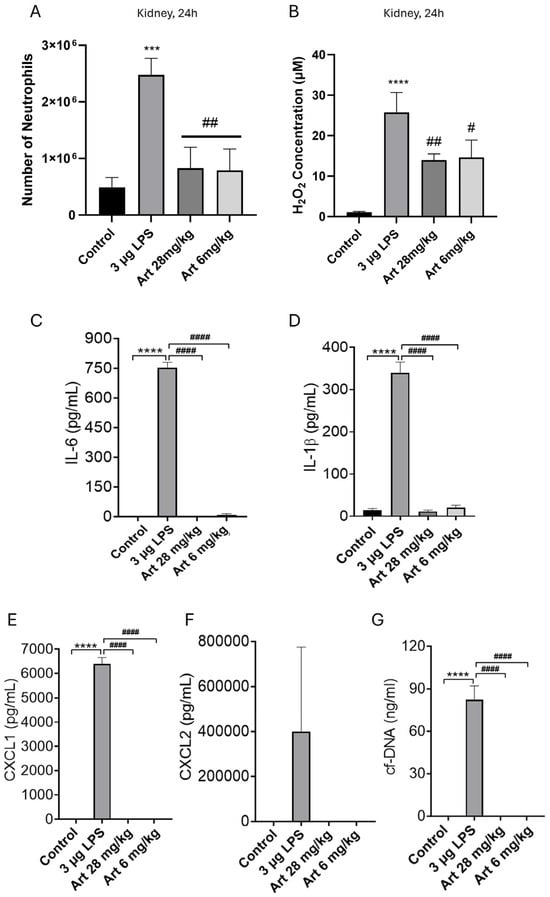
Figure 5.
Injection of LPS i.p. increases kidney neutrophils, H2O2, pro-inflammatory cytokines, chemokines, and NETs. Artesunate inhibits the increase in kidney neutrophils, pro-inflammatory cytokines, chemokines and NETs, but has a lesser effect on H2O2. Similar experiment to Figure 3 (where Figure 3A–G correspond here to (A–G)), except that lavage fluid was obtained from the kidney (see Section 2). Bars represent mean ± SEM. N = 4 mice per bar, with 4 independent experiments per result. Results with no visible bars were below the assay detection limits (see Section 2) and, for statistical purposes, were assumed to be equal to the detection limit. * for comparison between control and LPS to show the effect of LPS injection vs. sham, # for comparison between LPS and artesunate groups, to show the effect of artesunate on LPS-treated groups. *** p < 0.001, **** p < 0.0001, # p < 0.05, ## p < 0.01, #### p < 0.0001 (two-way ANOVA and Tukey–Kramer post hoc test).
3.4. Neutrophils Preferentially Migrate Towards H2O2 over Other Chemoattractants and Migration Is Inhibited by Artesunate
We previously showed that H2O2 potently induces neutrophil chemotaxis at concentrations of 10 µM or below [31]. In Figure 6A, we used ibidi µ-slide chemotaxis assays to show that neutrophils display comparable chemotaxis towards low levels of H2O2 and to other chemoattractants, including LPS, CXCL2, C5a, and fMLP, which each act through different intracellular signalling pathways. However, when exposing neutrophils to competing gradients (H2O2 vs. LPS, H2O2 vs. CXCL2, H2O2 vs. C5a and H2O2 vs. fMLP), neutrophils preferentially migrated towards H2O2 in all cases (Figure 6B), showing that signalling pathways activated in response to relatively low concentrations of H2O2 are more potent than those of other pro-inflammatory stimuli. Directly comparable results were observed with macrophages (Supplementary Figures S2 and S3).
We next aimed to model in vitro the inflammatory milieu seen in severe bacterial infections (visual methods shown in Supplementary Figure S4). For this, we pooled together pro-inflammatory compounds likely present in this hyper-inflammatory environment, namely LPS, CXCL2, C5a, fMLP, TNF-α and IL-1β [2,25,26,27]. When exposed to this inflammatory milieu, neutrophils did not undergo chemotaxis despite the abundance of chemoattractants present (Figure 6C), as shown in other reports [46,47]. However, when introducing a competing gradient of H2O2 on the other side of the assay to the inflammatory milieu, neutrophils exhibited strong chemotaxis towards H2O2 (Figure 6C). Thus, in a hyperinflammatory environment where neutrophil chemotaxis is impeded, chemotaxis can be restored by exposure to H2O2 at low concentrations.
To more faithfully replicate the inflammatory environment in such infections, we repeated the experiments in Figure 6C, but first incubated neutrophils in the inflammatory milieu (Figure 6D). Results mirrored those seen in Figure 6C, with chemotaxis observed only in repose to a gradient of H2O2. Directly comparable results were observed with macrophages (Supplementary Figure S5A,B), and similar results were also obtained with human neutrophils (Supplementary Figure S6A,B).
We have previously shown that artesunate acts as a potent inhibitor of neutrophil and macrophage chemotaxis to all chemoattractants, by inhibiting the subcellular calcium pump Sarco/Endoplasmic Reticulum Calcium adenosine triphosphate (ATP)ase 3 (SERCA3) and thus inhibiting the intracellular calcium pulses which steer neutrophil migration [35]. In Figure 6A and Supplementary Figure S2, we again show that neutrophil and macrophage chemotaxis towards all chemoattractants is inhibited by artesunate. The preferential neutrophil and macrophage chemotaxis observed towards H2O2 vs. other chemoattractants was also abolished by artesunate (Figure 6B, Supplementary Figure S3), as was chemotaxis restored by H2O2 (Figure 6E, Supplementary Figure S5C). Similar results were also obtained with human neutrophils (Supplementary Figure S6C).
Overall, these results show that a low concentration of H2O2 is a powerful neutrophil and macrophage chemoattractant and can restore impeded chemotaxis in the face of a hyper-inflammatory environment. Moreover, artesunate is a potent inhibitor of this chemotaxis in both single- and multi-chemoattractant assays.
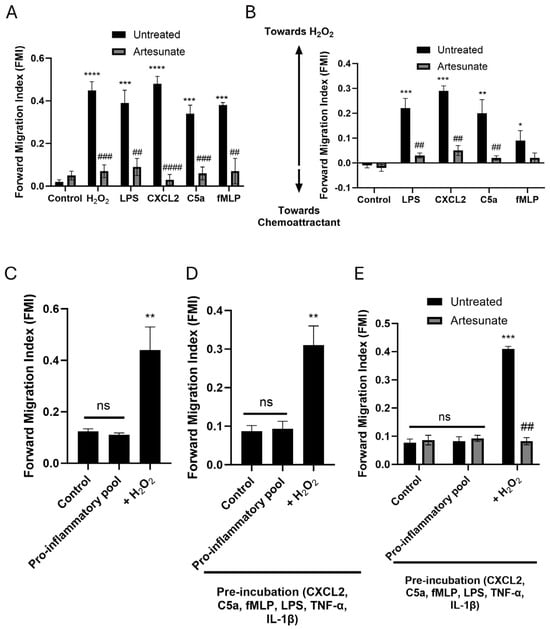
Figure 6.
Neutrophils preferentially migrate towards H2O2 over other chemoattractants in vitro, and migration is inhibited by artesunate. (A) Neutrophils migrated towards all individual chemoattractants (H2O2, 10 nM; LPS, 50 ng/mL; C5a, 10 nM; CXCL2, 10 nM; fMLP, 1 µM). Migration towards all chemoattractants was inhibited by artesunate (10 µM). (B) Neutrophils preferentially migrated towards H2O2 over other chemoattractants (same concentrations as above) when tested with competing gradients. Chemotaxis was inhibited by artesunate (10 µM). (C) A pro-inflammatory pool (LPS, 50 ng/mL; C5a, 10 nM; CXCL2, 10 nM; fMLP, 1 µM; TNF-α, 150 pg/mL; IL-1β, 150 pg/mL) did not cause neutrophil chemotaxis, but superimposing a gradient of 10 nM H2O2 restored chemotaxis. (D) A repeat of C but including pre-incubation of neutrophils with the inflammatory pool to more faithfully replicate the inflammatory environment. (E) Repeat of D but also showing that artesunate (10 µM) can inhibit the restored chemotaxis towards a gradient of H2O2. All experiments conducted in ibidi-µ slide chemotaxis assays. For the specific set-up of slides in each experiment, see Section 2 and Supplementary Figure S4. The forward migration index (FMI, vertical axis), which represents the mean ratio of distance travelled in the direction of the chemoattractant gradient to total distance travelled, is a measure of chemotaxis. Bars represent mean ± SEM. Cells extracted from N = 4 mice per bar, with ~200 cells analysed for each bar in 4 independent experiments per result. * for comparison between controls and chemoattractants; # for comparison between chemotaxis without and with artesunate. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ## p < 0.01, ### p < 0.001, #### p < 0.0001, ns = no significant difference (for (A,B,E): two-way ANOVA and Tukey–Kramer post hoc test, for (C,D): one-way ANOVA and Tukey–Kramer post hoc test).
4. Discussion
4.1. Role of H2O2 in Neutrophil Chemotaxis and Paralysis
The work described here shows that in a mouse model of bacterial infection, H2O2 plays important roles both in directing neutrophil chemotaxis and in triggering release of pro-inflammatory cytokines, chemokines, and NETs. At early times, 5 h after i.p. injection of a low dose of the bacterial antigen LPS into the peritoneum, a large influx of neutrophils was seen at the site of injection (the ‘target’ organ), as we have previously shown in low-dose LPS-lung infusion models [35]. Injection of a higher dose of LPS i.p., however, to model a more severe bacterial infection, blocked neutrophil entry into the target location, analogous to the neutrophil paralysis observed clinically [6,7] (Figure 1). Previous in vitro experiments had shown that H2O2 is a potent activator of neutrophil chemotaxis at lower concentrations (≤40 μM) but completely inhibits chemotaxis at higher concentrations (≥100 μM) [31]. We propose that the block of chemotaxis in vivo at high LPS concentrations is due to the high concentration of H2O2 (≥100 μM) liberated in the peritoneum, the site of LPS injection. With high i.p. LPS injections, we also noted high (≥100 μM) concentrations of H2O2 released in off-target organs, such as the lung, where neutrophil chemotaxis was also prevented (Figure 1). At longer times (24 h post-injection of LPS), we found a resumption of neutrophil entry and the release of both cytokines/chemokines and NETs in the lung, liver, and kidney, correlated with a reduction in H2O2 concentration to chemotactic levels (Figure 3, Figure 4 and Figure 5).
Several previous studies have shown H2O2 to be a potent neutrophil chemoattractant [48,49,50,51], and other studies have demonstrated increased H2O2 in bodily organs during inflammatory conditions [28,29,30,52]. Here we extend these findings by showing that low amounts of LPS cause the release of modest levels of H2O2 (≤40 μM) that are potently chemoattractive and promote the entry of neutrophils into target organs. High levels of LPS, on the other hand, caused the release of a higher concentration of H2O2 (≥100 μM) that completely blocks neutrophil chemotaxis. Similar results were observed both at the site of LPS injection and in off-target organs, likely because the hyper-inflammatory state in response to higher LPS injection induces system-wide hypermetabolism [28], which increases mitochondrial oxidative phosphorylation in the host cells of organs with high energy demand, thus producing H2O2 as a byproduct of increased ATP production, as has been shown in previous studies [53,54,55]. Hypermetabolism can also induce overwhelming demands on protein folding, inducing the unfolded protein response (UPR) and endoplasmic reticulum (ER) stress in organs [56]. The UPR transcription factor, activating transcription factor 6 (ATF6), has recently been shown to target the cytochrome b-245 alpha chain (p22phox) protein, a key subunit of NOXs, thus increasing ROS production and thereby contributing to H2O2 formation [57]. We propose that these increased levels of H2O2 are the cause of the phenomenon of neutrophil paralysis. In agreement, at longer times (24 h) after injection of an elevated level of LPS, we found that a fall in the concentration of H2O2 to permissive levels allowed neutrophil invasion and release of pro-inflammatory cytokines, chemokines and NETs to resume.
4.2. Neutrophils Migrate Preferentially Towards H2O2
Previous work in our lab has shown that two independent pathways determine the direction of chemotaxis in innate immune cells: H2O2 (≤40 µM) activates calcium influx through TRPM2 ion channels, leading to calcium release from subcellular stores, while chemokines coupled to GPCRs discharge calcium from subcellular stores via a distinct mechanism (see Supplementary Figure S8 of ref. [31]). Here we found that when neutrophils are faced with gradients of chemoattractants that conflict with a gradient of a chemotactic level of H2O2, neutrophils preferentially migrate towards a source of H2O2 rather than to other chemoattracts, either alone or in combination (Figure 6, Supplementary Figure S6). We found comparable results with macrophages (Supplementary Figures S3 and S5) that are also well-known to induce organ damage during life-threatening bacterial infections [58,59,60]. The ability of lower concentrations of H2O2 to ‘overrule’ other chemoattractants demonstrates the primacy of H2O2 as a chemoattractant.
4.3. Inhibition of Neutrophil Function by the Antimalarial Artesunate
Artesunate is a derivative of artemisinin, widely used as a frontline anti-malarial therapy [61,62]. We have recently found that artesunate is a potent inhibitor of neutrophil and macrophage chemotaxis, as well as cytokine, chemokine, and NET release [35]. Artesunate is also protective of neutrophil-mediated lung inflammation in pre-clinical COVID-19 infection models [35]. As COVID-19 can induce a hyperinflammatory state similar to that seen in severe bacterial infections [63,64,65], we sought to explore whether artesunate may also be protective in pre-clinical LPS models. Here, we show that artesunate inhibits chemotaxis of neutrophils to off-target organs (lungs, liver and kidneys) in a mouse LPS-infection model and can potently suppress the levels of cytokines and chemokines in these organs (Figure 3, Figure 4 and Figure 5), as we had previously observed in COVID-19 lung models [35]. The inhibition of chemotaxis by artesunate is attributed to the irreversible alkylation of SERCA3 [35], but the mechanism behind the prevention of cytokine/chemokine release remains unknown and may involve the suppression of the translocation of NF-κB, as suggested in previous studies [66,67,68]. Finally, we also observed that artesunate significantly reduces NET release from neutrophils in infection models (Figure 3, Figure 4 and Figure 5), which is therapeutically relevant because NET release during severe bacterial infections has been shown to be a major contributor to patient morbidity and mortality [69,70].
Previous studies have shown that compounds from the artemisinin family can be protective in models of widespread inflammation by inhibiting cytokine release, reducing organ damage and significantly enhancing survival [67,71,72,73]. We build on these previous studies to show that artesunate can induce its anti-inflammatory effects at therapeutically relevant concentrations and, moreover, that artesunate exhibits effects specifically through inhibition of neutrophil chemotaxis, cytokine, chemokine and NET release in LPS models. This work suggests that artesunate may be a useful novel therapeutic in the fight against neutrophil-mediated multiple-organ inflammation seen during life-threatening bacterial infection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/immuno5040047/s1. Figure S1: Human neutrophils isolated from whole blood; Figure S2: Artesunate is a potent inhibitor of macrophage chemotaxis towards all tested chemoattractants; Figure S3: Macrophages preferentially migrate towards H2O2 vs. other chemoattractants and migration is inhibited by artesunate; Figure S4: Diagrams of experimental procedure used in Figure 6C–E; Figure S5: A pool of pro-inflammatory molecules does not cause chemotaxis of macrophages, but chemotaxis is restored by a distinct H2O2 stimulus and inhibited by artesunate; Figure S6: A pool of pro-inflammatory molecules does not cause chemotaxis of human neutrophils, but chemotaxis is restored by a distinct H2O2 stimulus and is inhibited by artesunate.
Author Contributions
Conceptualization, H.O.J.M. and P.A.M.; methodology, H.O.J.M., L.G.-P. and P.A.M.; formal analysis, H.O.J.M., L.G.-P., G.C., F.D. and A.M.; investigation, H.O.J.M., L.G.-P., G.C., F.D. and A.M.; data curation, H.O.J.M., L.G.-P., G.C., F.D. and A.M.; writing—original draft preparation, H.O.J.M. and P.A.M.; writing—review and editing, H.O.J.M., L.G.-P., G.C., F.D., A.M. and P.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Wellcome Trust, grant number 205006/Z/16/Z, the Quintin Hogg Trust (QHT), grant number IP15, and the University of Westminster School Funding.
Institutional Review Board Statement
All animal work was conducted under UK Home Office personal and project licences, approved by the AWERB of King’s College London and carried out in accordance with the Animals (Scientific Procedures) Act 1986 and in compliance with the ARRIVE guidelines. Human blood samples were used under approved University of Westminster ethics, number ETH2324-0913.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NET | Neutrophil Extracellular Trap |
| H2O2 | Hydrogen Peroxide |
| PPAR-γ | Proliferator-Activated Receptor γ |
| ROS | Reactive Oxygen Species |
| TNF | Tumour Necrosis Factor |
| IL | Interleukin |
| WT | Wild-Type |
| AWERB | Animal Welfare Ethical Review Board |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| fMLP | N-formylmethionine-Leucyl-Phenylalanine |
| LPS | Lipopolysaccharide |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Foetal Bovine Serum |
| PBS | Phosphate-Buffered Saline |
| Cf | Cell-free |
| DNA | Deoxyribonucleic Acid |
| i.p. | Intraperitoneal |
| BALF | Bronchoalveolar Lavage Fluid |
| s.c. | Subcutaneous |
| CO2 | Carbon Dioxide |
| RCF | Relative Centrifugal Force |
| PFS | Perfect Focus System |
| RPM | Revolutions per Minute |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| TLR4 | Toll-like Receptor 4 |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NOX | NADPH Oxidase |
| ANOVA | Analysis of Variance |
| SEM | Standard Error of the Mean |
| TRPM2 | Transient Receptor Potential Cation Channel 2 |
| C5a | Complement Component 5a |
| SERCA3 | Sarco/Endoplasmic Reticulum Calcium ATPase 3 |
| GPCR | G protein-Coupled Receptor |
| COVID19 | Coronavirus Disease 2019 |
| NLRP3 | Nucleotide-Binding Domain, Leucine-Rich-Repeat Family Pyrin Domain Containing 3 Protein |
| ATP | Adenosine Triphosphate |
| UPR | Unfolded Protein Response |
| ER | Endoplasmic Reticulum |
| ATF6 | Activating Transcription Factor 6 |
| CXCL | C-X-C motif Chemokine Ligand |
| C5a | Complement Component C5a |
| C557BL/6 | Cold Spring Harbour 57 Black 6 Mouse Strain |
| UK | United Kingdom |
| TL | Thermolysin Low |
| MACS | Magnetic-Activated Cell Sorting |
| NIS | Nikon Imaging Software |
| AR | Advanced Research |
| FIJI | Figi Is Just ImageJ |
| USA | United States of America |
| HRP | Horseradish Peroxidase |
| Caspase | Cysteine-dependent Aspartate-specific Protease |
| FMI | Forward Migration Index |
| P22phox | Cytochrome b-245 Alpha Chain |
References
- De Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 115765. [Google Scholar] [CrossRef]
- Nordenfelt, P.; Tapper, H. Phagosome dynamics during phagocytosis by neutrophils. J. Leukoc. Biol. 2011, 90, 271–284. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simonm, H. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Gierlikowska, B.; Stachura, A.; Gierlikowski, W.; Demkow, U. Phagocytosis, degranulation and extracellular traps release by neutrophils—The current knowledge, pharmacological modulation and future prospects. Front. Pharmacol. 2021, 12, 666732. [Google Scholar] [CrossRef] [PubMed]
- Leliefeld, P.H.C.; Wessels, C.M.; Leenen, L.P.H.; Koenderman, L.; Pillay, J. The role of neutrophils in immune dysfunction during severe inflammation. Crit. Care 2016, 20, 73. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; Spiller, F.; Cunha, F.Q. Neutrophil paralysis in sepsis. Shock 2010, 34, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tharp, W.G.; Yadav, R.; Irimia, D.; Upadhyaya, A.; Samadani, A.; Hurtado, O.; Liu, S.-Y.; Munisamy, S.; Brainard, D.M.; Mahon, M.J.; et al. Neutrophil chemorepulsion in defined interleukin-8 gradients in vitro and in vivo. J. Leukoc. Biol. 2006, 79, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; De Freitas, A.; Russo, M.; Cunha, F.Q. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit. Care Med. 2006, 34, 461–470. [Google Scholar] [CrossRef]
- Hartung, T.; Sauer, A.; Hermann, C.; Brockhaus, F.; Wendel, A. Overactivation of the immune system by translocated bacteria and bacterial products. Scand. J. Gastroenterol. Suppl. 1997, 32, 98–99. [Google Scholar] [CrossRef]
- Reddy, R.C.; Narala, V.R.; Keshamouni, V.G.; Milam, J.E.; Newstead, M.W.; Standiford, T.J. Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-γ. Blood 2008, 112, 4250–4258. [Google Scholar] [CrossRef]
- Brown, K.; Brain, S.; Pearson, J.; Edgeworth, J.; Lewis, S.; Treacher, D. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- Balamayooran, G.; Batra, S.; Fessler, M.B.; Happel, K.I.; Jeyaseelan, S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am. J. Respir. Cell Mol. Biol. 2010, 43, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Navarini, A.A.; Lang, K.S.; Verschoor, A.; Recher, M.; Zinkernagel, A.S.; Nizet, V.; Odermatt, B.; Hengartner, H.; Zinkernagel, R.M. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc. Natl. Acad. Sci. USA 2009, 106, 7107–7112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-F.; Wang, Y.-P.; Xie, J.; Zhao, Z.-Z.; Gupta, S.; Guo, Y.; Jia, S.-H.; Parodo, J.; Marshall, J.C.; Deng, X.-M. Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood 2021, 138, 806–810. [Google Scholar] [CrossRef]
- Park, I.; Kim, M.; Choe, K.; Song, E.; Seo, H.; Hwang, Y.; Ahn, J.; Lee, S.-H.; Lee, J.H.; Jo, Y.H.; et al. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur. Respir. J. 2019, 53, 1800786. [Google Scholar] [CrossRef]
- Bajt, M.L.; Farhood, A.; Jaeschke, H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1188–G1195. [Google Scholar] [CrossRef]
- Doerschuk, C.M. Mechanisms of Leukocyte Sequestration in Inflamed Lungs. Microcirculation 2001, 8, 71–88. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; McAvoy, E.F.; Lam, F.; Gill, V.; de la Motte, C.; Savani, R.C.; Kubes, P. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J. Exp. Med. 2008, 205, 915–927. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.S.C.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.E.S.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef]
- Liu, S.; Su, X.; Pan, P.; Zhang, L.; Hu, Y.; Tan, H.; Wu, D.; Liu, B.; Li, H.; Li, H.; et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016, 6, 37252. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef]
- Soehnlein, O.; Oehmcke, S.; Rothfuchs, A.G.; Frithiof, R.; van Rooijen, N.; Mörgelin, M.; Herwald, H.; Lindbom, L. Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur. Respir. J. 2008, 32, 405–412. [Google Scholar] [CrossRef]
- Ginsburg, I.; Korem, M.; Koren, E.; Varani, J. Pro-inflammatory agents released by pathogens, dying host cells, and neutrophils act synergistically to destroy host tissues: A working hypothesis. J. Inflamm. Res. 2019, 12, 35–47. [Google Scholar] [CrossRef]
- Chan, L.; Karimi, N.; Morovati, S.; Alizadeh, K.; Kakish, J.E.; Vanderkamp, S.; Fazel, F.; Napoleoni, C.; Alizadeh, K.; Mehrani, Y.; et al. The Roles of Neutrophils in Cytokine Storms. Viruses 2021, 13, 2318. [Google Scholar] [CrossRef]
- Kasama, T.; Miwa, Y.; Isozaki, T.; Odai, T.; Adachi, M.; Kunkel, S.L. Neutrophil-derived cytokines: Potential therapeutic targets in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 273–279. [Google Scholar] [CrossRef]
- Tateda, K.; Moore, T.A.; Newstead, M.W.; Tsai, W.C.; Zeng, X.; Deng, J.C.; Chen, G.; Reddy, R.; Yamaguchi, K.; Standiford, T.J. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: Potential role of neutrophils as immunoregulatory cells. Infect. Immun. 2001, 69, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Pravda, J. Metabolic theory of septic shock. World J. Crit. Care Med. 2014, 3, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pravda, J. Hydrogen peroxide and disease: Towards a unified system of pathogenesis and therapeutics. Mol. Med. 2020, 26, 41. [Google Scholar] [CrossRef]
- Margotti, W.; Goldim, M.P.d.S.; Machado, R.S.; Bagio, E.; Dacoregio, C.; Bernades, G.; Lanzzarin, E.; Stork, S.; Cidreira, T.; Denicol, T.L.; et al. Oxidative stress in multiple organs after sepsis in elderly rats. Exp. Gerontol. 2022, 160, 111705. [Google Scholar] [CrossRef] [PubMed]
- Morad, H.; Luqman, S.; Tan, C.H.; Swann, V.; McNaughton, P.A. TRPM2 ion channels steer neutrophils towards a source of hydrogen peroxide. Sci. Rep. 2021, 11, 9339. [Google Scholar] [CrossRef]
- Thomas, R.C.; Bath, M.F.; Stover, C.M.; Lambert, D.G.; Thompson, J.P. Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system. Peptides 2014, 61, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tong, C.S.W.; Zhao, L.; Zhang, Y.; Nicholls, J.M.; Rainer, T.H. Intraperitoneal versus intranasal administration of lipopolysaccharide in causing sepsis severity in a murine model: A preliminary comparison. Lab. Anim. Res. 2024, 40, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; He, X.; Yang, F.; Han, S.; Qin, A.; Wu, G.; Liu, M.; Li, Z.; Wang, J.; et al. ING4 alleviated lipopolysaccharide-induced inflammation by regulating the NF-κB pathway via a direct interaction with SIRT1. Immunol. Cell Biol. 2020, 98, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Morad, H.O.J.; Luqman, S.; Pinto, L.G.; Cunningham, K.P.; Vilar, B.; Clayton, G.; Shankar-Hari, M.; McNaughton, P.A. Artemisinin inhibits neutrophil and macrophage chemotaxis, cytokine production and NET release. Sci. Rep. 2022, 12, 11078. [Google Scholar] [CrossRef]
- Swamydas, M.; Luo, Y.; Dorf, M.E.; Lionakis, M.S. Isolation of Mouse Neutrophils. Curr. Protoc. Immunol. 2015, 110, 3.20.1–3.20.15. [Google Scholar] [CrossRef]
- Pinto, L.G.; Talbot, J.; Peres, R.S.; Franca, R.F.; Ferreira, S.H.; Ryffel, B.; Aves-Filho, J.C.F.; Figueiredo, F.; Cunha, T.M.; Cunha, F.Q. Joint production of IL-22 participates in the initial phase of antigen-induced arthritis through IL-1β production. Arthritis Res. Ther. 2015, 17, 235. [Google Scholar] [CrossRef]
- Appell, C.R.; Jiwan, N.C.; Wang, R.; Shen, C.L.; Luk, H.Y. Ginger Supplementation Attenuated Mitochondrial Fusion and Improved Skeletal Muscle Size in Type 2 Diabetic Rats. In Vivo 2024, 38, 73–81. [Google Scholar] [CrossRef]
- Kashyap, N.; Islam, M.; Kaur, H.; Tiwari, D.; Begum, A.; Bose, M.; Das, C.R.; Saikia, A.K.; Kalita, P.; Bose, P.D.; et al. Oxidative stress—A key determinant of complications and negative outcome in hepatitis E virus infected pregnancies: A comprehensive account involving cases from northeast India. J. Med. Virol. 2023, 95, e28576. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, H.; Tan, S.; Dong, Q.; Fan, X.; Wang, Y.; Zhang, H.; He, J. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp. Hematol. Oncol. 2022, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Creagh, E.M. Caspase-4 and -5 Biology in the Pathogenesis of Inflammatory Bowel Disease. Front. Pharmacol. 2022, 13, 919567. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Aspects Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Surabhi, S.; Jachmann, L.H.; Shumba, P.; Burchhardt, G.; Hammerschmidt, S.; Siemens, N. Hydrogen Peroxide Is Crucial for NLRP3 Inflammasome-Mediated IL-1β Production and Cell Death in Pneumococcal Infections of Bronchial Epithelial Cells. J. Innate Immun. 2021, 14, 192. [Google Scholar] [CrossRef]
- Benjamim, C.F.; Silva, J.S.; Fortes, Z.B.; Oliveira, M.A.; Ferreira, S.H.; Cunha, F.Q. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect. Immun. 2002, 70, 3602–3610. [Google Scholar] [CrossRef]
- Arraes, S.M.A.; Freitas, M.S.; da Silva, S.V.; Neto, H.A.d.P.; Alves-Filho, J.C.; Martins, M.A.; Basile-Filho, A.; Tavares-Murta, B.M.; Barja-Fidalgo, C.; Cunha, F.Q. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood 2006, 108, 2906–2913. [Google Scholar] [CrossRef]
- Yoo, S.K.; Starnes, T.W.; Deng, Q.; Huttenlocher, A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 2011, 480, 109–112. [Google Scholar] [CrossRef]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Klyubin, I.V.; Kirpichnikova, K.M.; Gamaley, I.A. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur. J. Cell Biol. 1996, 70, 347–351. [Google Scholar] [PubMed]
- Yamamoto, S.; Shimizu, S.; Kiyonaka, S.; Takahashi, N.; Wajima, T.; Hara, Y.; Negoro, T.; Hiroi, T.; Kiuchi, Y.; Okada, T.; et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008, 14, 738–747. [Google Scholar] [CrossRef]
- Lipcsey, M.; Bergquist, M.; Sirén, R.; Larsson, A.; Huss, F.; Pravda, J.; Furebring, M.; Sjölin, J.; Janols, H. Urine Hydrogen Peroxide Levels and Their Relation to Outcome in Patients with Sepsis, Septic Shock, and Major Burn Injury. Biomedicines 2022, 10, 848. [Google Scholar] [CrossRef]
- Nethery, D.; Callahan, L.A.; Stofan, D.; Mattera, R.; DiMarco, A.; Supinski, G. PLA2 dependence of diaphragm mitochondrial formation of reactive oxygen species. J. Appl. Physiol. 2000, 89, 72–80. [Google Scholar] [CrossRef]
- Fink, M.P. Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: Potential benefits of resuscitation with Ringer’s ethyl pyruvate solution. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Pierro, A.; Spitz, L.; Eaton, S. Cardiac and renal mitochondria respond differently to hydrogen peroxide in suckling rats. J. Surg. Res. 2003, 113, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Willmann, K.; Moita, L.F. Physiologic disruption and metabolic reprogramming in infection and sepsis. Cell Metab. 2024, 36, 927–946. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.; Zhang, Z.; Trautz, B.; Rieß, F.; Görlach, A. Cross Talk Between p22phox and ATF4 in the Endothelial Unfolded Protein Response. Antioxid. Redox Signal 2019, 30, 40–55. [Google Scholar] [CrossRef]
- Lu, H.L.; Huang, X.Y.; Luo, Y.F.; Tan, W.P.; Chen, P.F.; Guo, Y.B. Activation of M1 macrophages plays a critical role in the initiation of acute lung injury. Biosci. Rep. 2018, 38, 20171555. [Google Scholar] [CrossRef]
- Cao, Q.; Harris, D.C.H.; Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology 2015, 30, 183–194. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, 709–725. [Google Scholar] [CrossRef]
- Hess, K.M.; Goad, J.A.; Arguin, P.M. Intravenous artesunate for the treatment of severe malaria. Ann. Pharmacother. 2010, 44, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Ndour, P.A.; Kendjo, E.; Larréché, S.; Taieb, A.; Henry, B.; Lebrun-Vignes, B.; Chambrion, C.; Argy, N.; Houzé, S.; et al. Intravenous Artesunate for the Treatment of Severe Imported Malaria: Implementation, Efficacy, and Safety in 1391 Patients. Clin. Infect. Dis. 2021, 73, 1795–1804. [Google Scholar] [CrossRef]
- Stenlo, M.; Silva, I.A.N.; Hyllén, S.; Bölükbas, D.A.; Niroomand, A.; Grins, E.; Ederoth, P.; Hallgren, O.; Pierre, L.; Wagner, D.E.; et al. Monitoring lung injury with particle flow rate in LPS- and COVID-19-induced ARDS. Physiol. Rep. 2021, 9, e14802. [Google Scholar] [CrossRef]
- Olwal, C.O.; Nganyewo, N.N.; Tapela, K.; Djomkam, L.A.; Owoicho, O.; Bediako, Y.; Duodu, S. Parallels in Sepsis and COVID-19 Conditions: Implications for Managing Severe COVID-19. Front. Immunol. 2021, 12, 602848. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Kurochkin, S.V. Lung lesions caused by COVID-19 in comparison with bacterial pneumonia and influenza pneumonia: Pathomorphological features. Kazan Med. J. 2021, 102, 703–715. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, W.; Zhang, S.; Wang, L.; Zhang, X. Artesunate attenuates inflammatory injury and inhibits the NF-κB pathway in a mouse model of cerebral ischemia. J. Int. Med. Res. 2021, 49, 03000605211053549. [Google Scholar] [CrossRef]
- Ho, W.E.; Cheng, C.; Peh, H.Y.; Xu, F.; Tannenbaum, S.R.; Ong, C.N.; Wong, W.F. Anti-malarial drug artesunate ameliorates oxidative lung damage in experimental allergic asthma. Free Radic. Biol. Med. 2012, 53, 498–507. [Google Scholar] [CrossRef]
- Golatkar, V.; Bhatt, L.K. Artesunate attenuates isoprenaline induced cardiac hypertrophy in rats via SIRT1 inhibiting NF-κB activation. Eur. J. Pharmacol. 2024, 977, 176709. [Google Scholar] [CrossRef]
- Silva, C.M.S.; Wanderley, C.W.S.; Veras, F.P.; Sonego, F.; Nascimento, D.C.; Gonçalves, A.V.; Martins, T.V.; Cólon, D.F.; Borges, V.F.; Brauer, V.S.; et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood 2021, 138, 2702–2713. [Google Scholar] [CrossRef] [PubMed]
- Alsabani, M.; Abrams, S.T.; Cheng, Z.; Morton, B.; Lane, S.; Alosaimi, S.; Yu, W.; Wang, G.; Toh, C.-H. Reduction of NETosis by targeting CXCR1/2 reduces thrombosis, lung injury, and mortality in experimental human and murine sepsis. Br. J. Anaesth. 2021, 128, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-H.; Jin, S.-G.; Fei, D.-S.; Kang, K.; Jiang, L.; Lian, Z.-Y.; Pan, S.-H.; Zhao, M.-R.; Zhao, M.-Y. Artesunate Protects Against Sepsis-Induced Lung Injury Via Heme Oxygenase-1 Modulation. Inflammation 2016, 39, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, J.; Pan, X.; Ding, G.; Cao, H.; Jiang, W.; Zheng, J.; Zhou, H. Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-α release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-κB activation. Int. Immunopharmacol. 2010, 10, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wang, J.; Chen, Q.; Wang, Z.; Li, D.; Jiang, N.; Ju, X. Artesunate ameliorates sepsis-induced acute lung injury by activating the mTOR/AKT/PI3K axis. Gene 2020, 759, 144969. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).