Role of Interferon-Gamma (IFN-γ) in Pathophysiology and Management of Deep Vein Thrombosis
Abstract
1. Introduction
2. Role of Immune Cells in the Pathophysiology of DVT
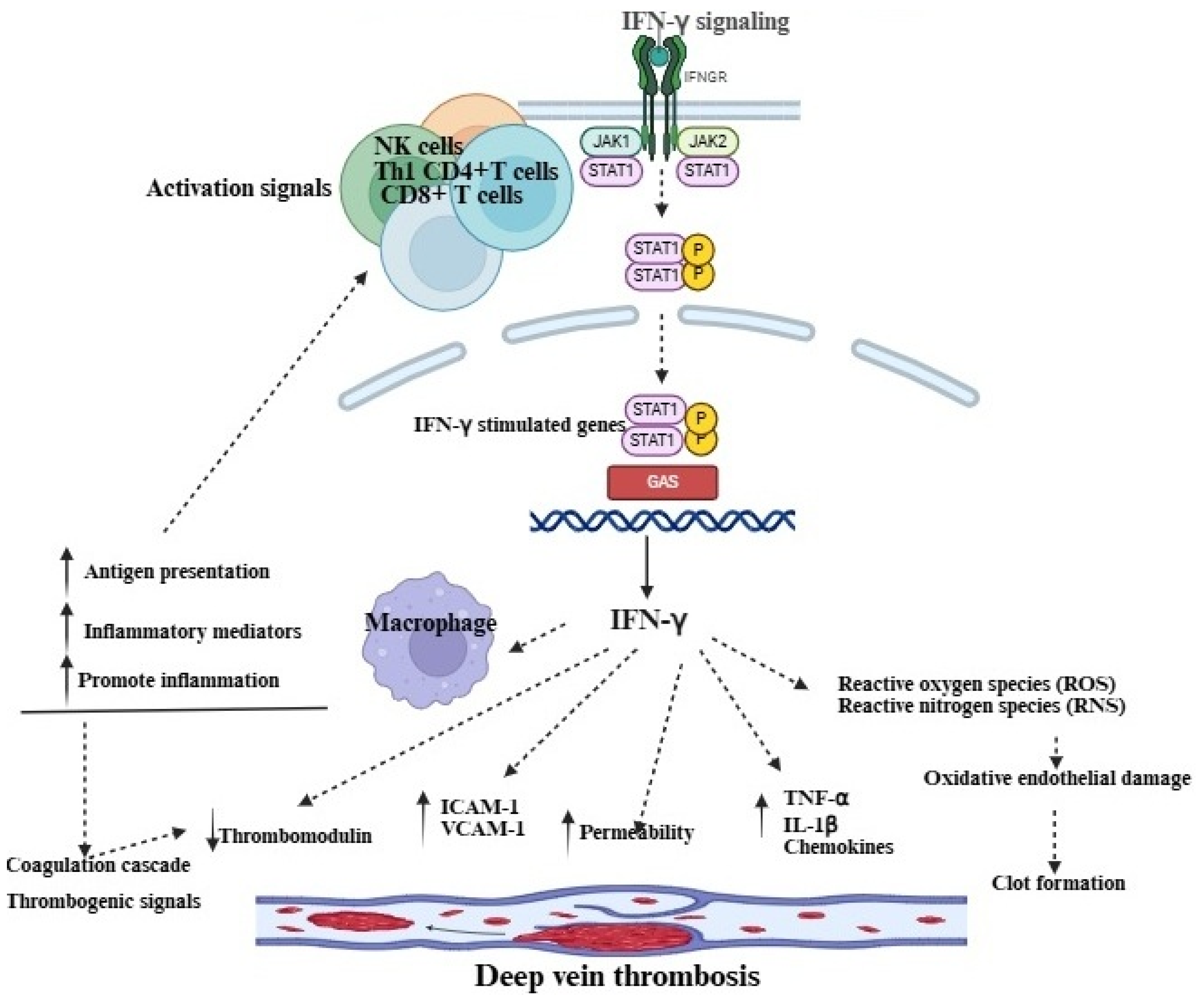
3. IFN-γ and Its Role in the Pathophysiology of DVT
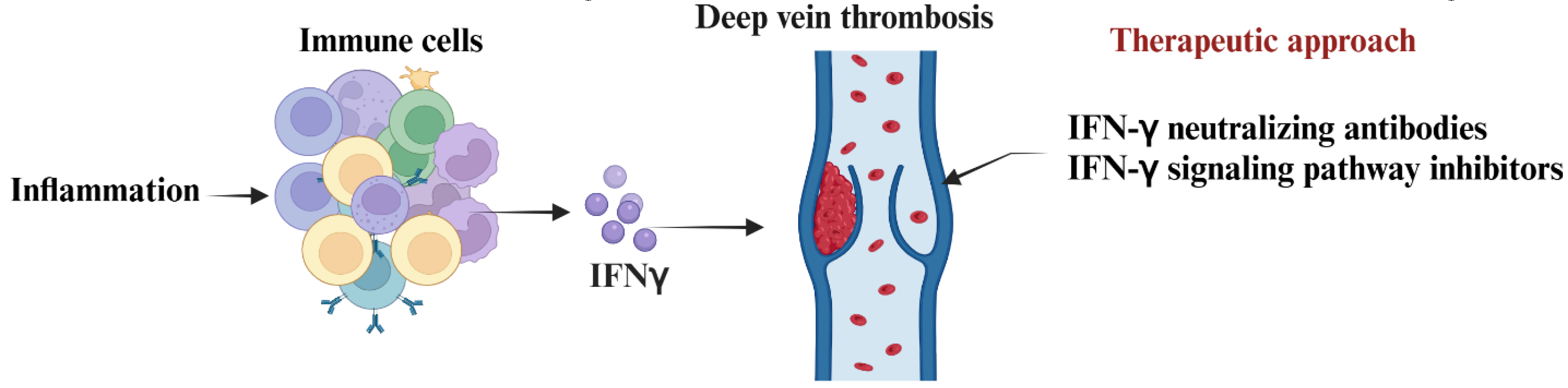
4. Targeting IFN-γ and Its Signaling to Advance Therapeutics of DVT
5. Role of Hypoxia in Inducing an Increase in IFN-γ or Directly Contributing to the Pathogenesis of DVT
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DVT | Deep vein thrombosis |
| IFN-γ | Interferon-gamma |
| NETs | Neutrophil extracellular traps |
| CRP | C-Reactive protein |
| TF | Tissue factor |
| NK | Natural killer |
| IL | Interleukin |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MIP | Macrophage inflammatory protein |
| ICAM | Intracellular adhesion molecule |
| IFNGR1/IFNGR2 | Interferon-gamma receptor 1/2 |
| JAK-STAT1 | Janus kinase-signal transducer and activator of transcription |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| VCAM | Vascular cell adhesion molecule |
| P38 MAPK | p38 mitogen-activated protein kinase |
| GTP | Guanosine triphosphate |
| HIFs | Hypoxia-inducible factors |
| PAI-1 | Plasminogen activator inhibitor-1 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
References
- Duffy, S. Understanding patient assessment and treatment in deep vein thrombosis. Nurs. Stand. 2022, 37, 71–75. [Google Scholar] [CrossRef]
- Waheed, S.M.; Kudaravalli, P.; Hotwagner, D.T. Deep Vein Thrombosis; StatPearls: St. Petersburg, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507708/ (accessed on 25 September 2025).
- Navarrete, S.; Solar, C.; Tapia, R.; Pereira, J.; Fuentes, E.; Palomo, I. Pathophysiology of deep vein thrombosis. Clin. Exp. Med. 2023, 23, 645–654. [Google Scholar] [CrossRef]
- Kesieme, E.; Kesieme, C.; Jebbin, N.; Irekpita, E.; Dongo, A. Deep vein thrombosis: A clinical review. J. Blood. Med. 2011, 2, 59–69. [Google Scholar] [CrossRef]
- Bonner, L.; Johnson, J. Deep vein thrombosis: Diagnosis and treatment. Nurs Stand. 2014, 28, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.; Pastores, S.M. Venous Thromboembolic Events in Cancer Immunotherapy: A Narrative Review. J. Clin. Med. 2025, 14, 4926. [Google Scholar] [CrossRef]
- Roopkumar, J.; Kim, A.S.; Bicky, T.; Hobbs, B.P.; Khorana, A.A. Venous Thromboembolism in Cancer Patients Receiving Immunotherapy. Blood 2018, 132, 2510. [Google Scholar] [CrossRef]
- Linkins, L.A.; Takach Lapner, S. Review of D-dimer testing: Good, Bad, and Ugly. Int. J. Lab. Hematol. 2017, 39, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.C.; Eikelboom, J.W.; Douketis, J.D.; Hankey, G.J. Deep vein thrombosis: Update on diagnosis and management. Med. J. Aust. 2019, 210, 516–524. [Google Scholar] [CrossRef]
- Schulman, S.; Makatsariya, A.; Khizroeva, J.; Bitsadze, V.; Kapanadze, D. The Basic Principles of Pathophysiology of Venous Thrombosis. Int. J. Mol. Sci. 2024, 25, 11447. [Google Scholar] [CrossRef]
- Budnik, I.; Brill, A. Immune Factors in Deep Vein Thrombosis Initiation. Trends Immunol. 2018, 39, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Hangge, P.; Albadawi, H.; Wallace, A.; Shamoun, F.; Knuttien, M.G.; Naidu, S.; Oklu, R. Deep Vein Thrombosis: Pathogenesis, Diagnosis, and Medical Management. Cardiovasc. Diagn. Ther. 2017, 7, S276–S284. [Google Scholar] [CrossRef]
- Arnold, M.J. Venous Thromboembolism: Management Guidelines from the American Society of Hematology. Am. Fam. Physician 2021, 104, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Luther, N.; Shahneh, F.; Brähler, M.; Krebs, F.; Jäckel, S.; Subramaniam, S.; Stanger, C.; Schönfelder, T.; Kleis-Fischer, B.; Reinhardt, C.; et al. Innate Effector-Memory T-Cell Activation Regulates Post-Thrombotic Vein Wall Inflammation and Thrombus Resolution. Circ. Res. 2016, 119, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Nitta, D.; Mitani, H.; Ishimura, R.; Moriya, M.; Fujimoto, Y.; Ishiwata, S.; Yamaguchi, T.; Ohno, M. Deep vein thrombosis risk stratification. Int. Heart J. 2013, 54, 166–170. [Google Scholar] [CrossRef]
- Rayes, J.; Brill, A. Hot under the clot: Venous thrombogenesis is an inflammatory process. Blood 2024, 144, 477–489. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Johnson, T.A.; Duru, N.; Buzza, M.S.; Pawar, N.R.; Sarkar, R.; Antalis, T.M. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front. Immunol. 2019, 10, 1348. [Google Scholar] [CrossRef]
- Qi, N.; Lyu, Z.; Huang, L.; Zhao, Y.; Zhang, W.; Zhou, X.; Zhang, Y.; Cui, J. Investigating the dual causative pathways linking immune cells and venous thromboembolism via Mendelian randomization analysis. Thromb. J. 2025, 23, 8. [Google Scholar] [CrossRef]
- Poredos, P.; Jezovnik, M.K. Endothelial Dysfunction and Venous Thrombosis. Angiology 2018, 69, 564–567. [Google Scholar] [CrossRef]
- Cronkite, D.A.; Strutt, T.M. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J. Immunol. Res. 2018, 2018, 1467538. [Google Scholar] [CrossRef]
- Kim, Y.; Greenleaf, W.J.; Bendall, S.C. Systems biology approaches to unravel lymphocyte subsets and function. Curr. Opin. Immunol. 2023, 82, 102323. [Google Scholar] [CrossRef]
- Alhabibi, A.M.; Wahab, M.A.; Sakr, A.K.; Abd El-Hamid, S.M.; Zakaria, M.Y.; Althoqapy, A.A.; El Sayed, H.M.E.; Kasim, S.A.; Ibrahim, H.F.; Saleh, O.I.; et al. The Diagnostic Utility of Natural Killer Cell Subsets in Deep Vein Thrombosis. Vasc. Health Risk Manag. 2023, 19, 779–787. [Google Scholar] [CrossRef]
- Duan, Q.; Gong, Z.; Song, H.; Wang, L.; Yang, F.; Lv, W.; Song, Y. Symptomatic venous thromboembolism is a disease related to infection and immune dysfunction. Int. J. Med. Sci. 2012, 9, 453–461. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Shen, Y.; Song, H.; Gong, Z.; Wang, L. Compromised natural killer cells in pulmonary embolism. Int. J. Clin. Exp. Pathol. 2015, 8, 8244–8251. [Google Scholar] [PubMed]
- Bertin, F.R.; Rys, R.N.; Mathieu, C.; Laurance, S.; Lemarié, C.A.; Blostein, M.D. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J. Thromb. Haemost. 2019, 17, 403–414. [Google Scholar] [CrossRef]
- Becker, C.; Reinhardt, C. Unexpected role of natural killer cell-derived interferon-γ as a driver of NETosis and DVT. J. Thromb. Haemost. 2019, 17, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Kopitar-Jerala, N. The Role of Interferons in Inflammation and Inflammasome Activation. Front. Immunol. 2017, 8, 873. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11. [Google Scholar] [CrossRef]
- Sica, A.; Dorman, L.; Viggiano, V.; Cippitelli, M.; Ghosh, P.; Rice, N.; Young, H.A. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J. Biol. Chem. 1997, 272, 30412–30420. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [CrossRef]
- Kannan, Y.; Yu, J.; Raices, R.M.; Seshadri, S.; Wei, M.; Caligiuri, M.A.; Wewers, M.D. IκBζ augments IL-12– and IL-18–mediated IFN-γ production in human NK cells. Blood 2011, 117, 2855–2863. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Escalante, N.K.; Jude, K.M.; Sotolongo Bellon, J.; Su, L.; Horton, T.M.; Tsutsumi, N.; Berardinelli, S.J.; Haltiwanger, R.S.; Piehler, J.; et al. Structure of the IFNγ receptor complex guides design of biased agonists. Nature 2019, 567, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The Two Faces of Interferon-γ in Cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Mullen, A.C.; Martins, G.A.; Krawczyk, C.M.; Hutchins, A.S.; Zediak, V.P.; Banica, M.; DiCioccio, C.B.; Gross, D.A.; Mao, C.A.; et al. Control of Effector CD8+ T Cell Function by the Transcription Factor Eomesodermin. Science 2003, 302, 1041–1043. [Google Scholar] [CrossRef]
- Ng, C.T.; Fong, L.Y.; Abdullah, M.N.H. Interferon-gamma (IFN-γ): Reviewing its mechanisms and signaling pathways on the regulation of endothelial barrier function. Cytokine 2023, 166, 156208. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Inui, M.; Mukaida, N.; Kondo, T. Absence of IFN-γ accelerates thrombus resolution through enhanced MMP-9 and VEGF expression in mice. J. Clin. Investig. 2011, 121, 2911–2920. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Manni, S.; Del Bufalo, F.; Merli, P.; Silvestris, D.A.; Guercio, M.; Caruso, S.; Reddel, S.; Iaffaldano, L.; Pezzella, M.; Di Cecca, S.; et al. Neutralizing IFNγ improves safety without compromising efficacy of CAR-T cell therapy in B-cell malignancies. Nat. Commun. 2023, 14, 3423. [Google Scholar] [CrossRef]
- Vallurupalli, M.; Berliner, N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood 2019, 134, 1783–1786. [Google Scholar] [CrossRef]
- Goker Bagca, B.; Biray Avci, C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020, 54, 51–62. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Wang, M.; Meng, J.; Zhu, D.; Chen, L.; Xiao, Y.; Yi, D.; Shi, H.; Liu, H. Ruxolitinib targets JAK-STAT signaling to modulate neutrophil activation in refractory macrophage activation syndrome. Blood 2025, 146, 612–627. [Google Scholar] [CrossRef]
- Shah, R.R. Challenges, opportunities, and therapeutic potential of JAK inhibitors and their derived PROTACs (2022–2023). Expert Opin. Ther. Pat. 2025, 35, 571–582. [Google Scholar] [CrossRef]
- Ng, C.T.; Fong, L.Y.; Tan, J.J.; Abdullah, M.N.H. Endothelial barrier disruptive effect of IFN-Ƴ and TNF-α: Synergism of pro-inflammatory cytokines. Cytokine 2025, 190, 156922. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, W.; Li, C.; Zhao, R.; Lu, H.; Song, S.; Zhou, Y.; Hu, Y.; Shi, B.; Ge, J. Hypoxia-induced signaling in the cardiovascular system: Pathogenesis and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 431. [Google Scholar] [CrossRef]
- Gupta, N.; Zhao, Y.Y.; Evans, C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef]
- Closse, C.; Seigneur, M.; Renard, M.; Pruvost, A.; Dumain, P.; Belloc, F.; Boisseau, M.R. Influence of hypoxia and hypoxia-reoxygenation on endothelial P-selectin expression. Haemostasis 1996, 26, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Teacoe, I.; Olteanu, D.E.; Vaida-Voievod, C.; Clichici, A.; Sirbu, A.; Filip, G.A.; Clichici, S. Effects of different hypoxia degrees on endothelial cell cultures—Time course study. Mech. Ageing Dev. 2018, 172, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.; Rangasamy, T.; Guo, J.; Sugunan, S.; Meednu, N.; Packirisamy, G.; Shimoda, L.A.; Golding, A.; Semenza, G.; Georas, S.N. T-cell activation under hypoxic conditions enhances IFN-gamma secretion. Am. J. Respir. Cell Mol. Biol. 2010, 42, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Ojo, O.A.; Ding, H.; Mullen, L.J.; Xing, C.; Hossain, M.I.; Yassin, A.; Shi, V.Y.; Lewis, Z.; Podgorska, E.; et al. HIF1α-regulated glycolysis promotes activation-induced cell death and IFN-γ induction in hypoxic T cells. Nat. Commun. 2024, 15, 9394. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, A Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, K. Role of Interferon-Gamma (IFN-γ) in Pathophysiology and Management of Deep Vein Thrombosis. Immuno 2025, 5, 46. https://doi.org/10.3390/immuno5040046
Kaur K. Role of Interferon-Gamma (IFN-γ) in Pathophysiology and Management of Deep Vein Thrombosis. Immuno. 2025; 5(4):46. https://doi.org/10.3390/immuno5040046
Chicago/Turabian StyleKaur, Kawaljit. 2025. "Role of Interferon-Gamma (IFN-γ) in Pathophysiology and Management of Deep Vein Thrombosis" Immuno 5, no. 4: 46. https://doi.org/10.3390/immuno5040046
APA StyleKaur, K. (2025). Role of Interferon-Gamma (IFN-γ) in Pathophysiology and Management of Deep Vein Thrombosis. Immuno, 5(4), 46. https://doi.org/10.3390/immuno5040046





