A Scoping Review of Clinical, Genetic, and Mechanistic Evidence Linking IL-6/IL-6R Signaling and Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- Population: Patients of any age receiving tocilizumab for any indication, or genetic/biological studies evaluating IL-6/IL-6R signaling in the context of T1DM.
- Exposure/Intervention: Administration of tocilizumab or genetically proxied IL-6R blockade.
- Outcomes: New-onset T1DM diagnosed by clinical criteria (e.g., hyperglycemia, diabetic ketoacidosis, islet autoantibodies) or β-cell functional decline.
- Study Design: Case reports, case series, randomized controlled trials (RCTs), Mendelian randomization (MR) studies, and mechanistic studies published as full-text articles.
- Language and Time Frame: Publications in English from 1 January 2005 to 10 April 2025.
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Charting and Extraction
- Study characteristics (authors, publication year, study design)
- Population demographics (age, sex, underlying conditions)
- Tocilizumab dosage, duration, and indication
- Diagnostic criteria and timing for T1DM onset
- Genetic or molecular findings related to IL-6/IL-6R signaling
- Key outcomes and mechanistic observations
2.6. Synthesis of Results
2.7. Risk of Bias and Critical Appraisal
2.8. Ethics and Data Availability
2.9. Generative AI Use
3. Results
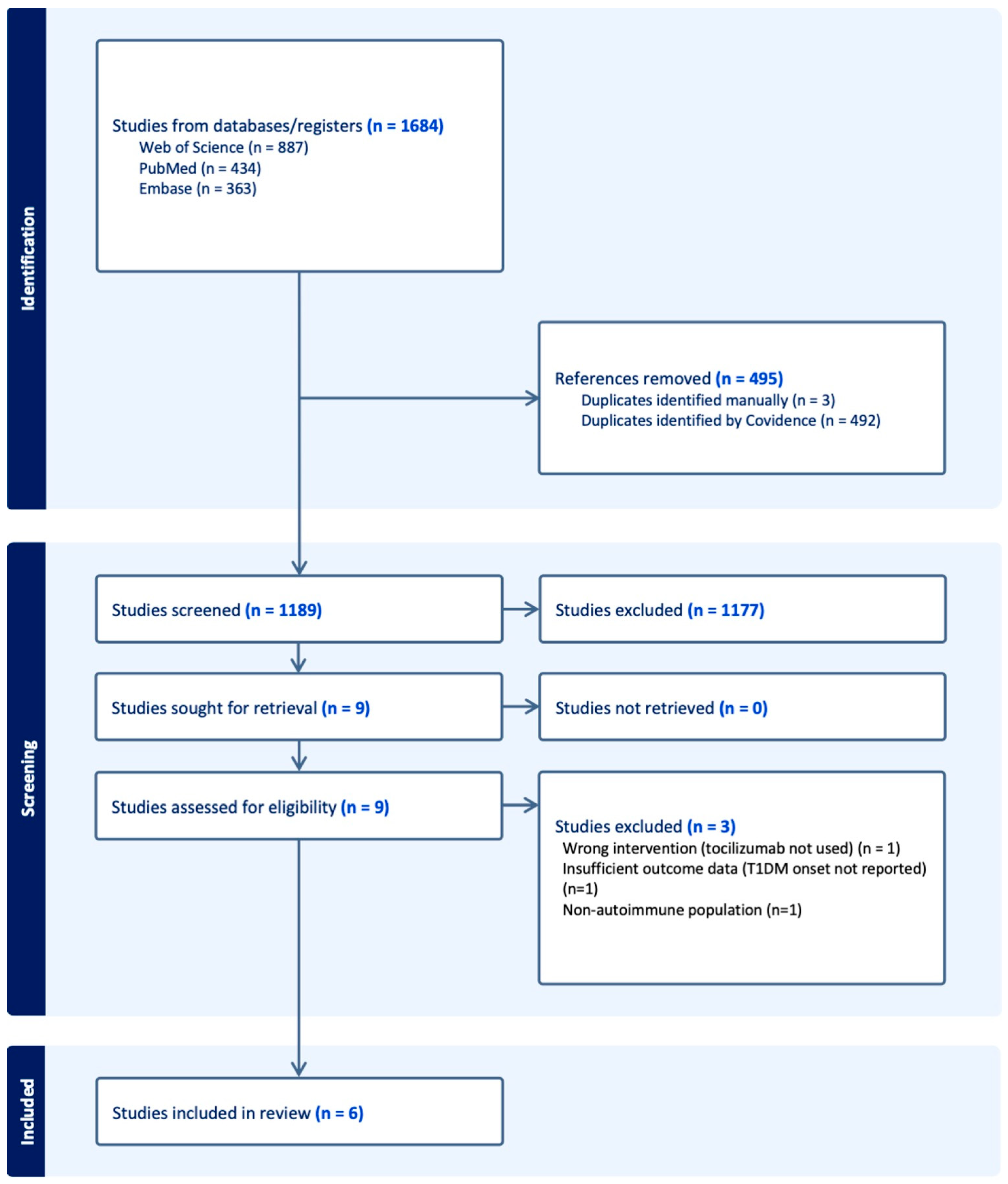
3.1. Study Selection
3.2. Study Characteristics
- Case report (n = 1): A single patient developing T1DM during tocilizumab therapy.
- Randomized controlled trial (n = 1): A multicenter trial evaluating tocilizumab for β-cell preservation in new-onset T1DM.
- Mendelian randomization studies (n = 3): Genetic analyses assessing the impact of IL-6R blockade or expression on T1DM risk.
- Mechanistic study (n = 1): Immunological investigation of IL-6 responsiveness in T1DM patients.
3.3. Risk of Bias Within Studies
3.4. Results of Individual Studies and Synthesis
3.4.1. Clinical Association Between Tocilizumab Therapy and the Onset of T1DM
- Case Report
3.4.2. Randomized Controlled Trial
3.4.3. Genetic and Mechanistic Evidence Linking IL-6R Signaling to T1DM Risk
3.4.4. Mechanistic Immunology Study
- IL-6-induced phosphorylation of STAT3 and STAT1 was significantly higher in CD4+ and CD8+ T cells from T1DM patients.
- This enhanced signaling was specific to IL-6 stimulation (not observed with IL-10 or IL-27).
- Increased IL-6 responsiveness correlated with elevated surface expression of IL-6R on T cells.
- Expression of the IL-6R sheddase ADAM17 was reduced in T1DM patients, contributing to the accumulation of surface IL-6R.
4. Discussion
4.1. Summary of Main Findings
4.2. Comparison with Previous Literature
4.3. Clinical Implications
4.4. Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T1DM | Type 1 diabetes mellitus |
| IL-6 | Interleukin-6 |
| IL-6R | Interleukin-6 receptor |
| TCZ | Tocilizumab |
| MR | Mendelian randomization |
| RCT | Randomized controlled trial |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| HLA | Human leukocyte antigen |
| ADAM17 | A disintegrin and metalloprotease 17 |
References
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422. [Google Scholar] [CrossRef]
- Sugandh, F.N.; Chandio, M.; Raveena, F.N.; Kumar, L.; Karishma, F.N.; Khuwaja, S.; Memon, U.A.; Bai, K.; Kashif, M.; Varrassi, G.; et al. Advances in the Management of Diabetes Mellitus: A Focus on Personalized Medicine. Cureus 2023, 15, e43697. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, J.; Li, L.; Lan, Y.; Liang, Y. Cytokines in Type 1 Diabetes: Mechanisms of Action and Immunotherapeutic Targets. Clin. Transl. Immunol. 2020, 9, e1122. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to Targeting a Complex Cytokine in Critical Illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef]
- Schnell, A.; Littman, D.R.; Kuchroo, V.K. TH17 Cell Heterogeneity and Its Role in Tissue Inflammation. Nat. Immunol. 2023, 24, 19–29. [Google Scholar] [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the Interface of Human Health and Disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Ohsugi, Y.; Kishimoto, T. Tocilizumab, a Humanized Anti-Interleukin-6 Receptor Antibody, for Treatment of Rheumatoid Arthritis. Open Access Rheumatol. 2011, 3, 19–29. [Google Scholar] [CrossRef]
- Favalli, E.G. Understanding the Role of Interleukin-6 (IL-6) in the Joint and Beyond: A Comprehensive Review of IL-6 Inhibition for the Management of Rheumatoid Arthritis. Rheumatol. Ther. 2020, 7, 473–516. [Google Scholar] [CrossRef]
- Greenbaum, C.J.; Serti, E.; Lambert, K.; Weiner, L.J.; Kanaparthi, S.; Lord, S.; Gitelman, S.E.; Wilson, D.M.; Gaglia, J.L.; Griffin, K.J.; et al. IL-6 Receptor Blockade Does Not Slow Beta Cell Loss in New-Onset Type 1 Diabetes. JCI Insight 2021, 6, e150074. [Google Scholar] [CrossRef] [PubMed]
- Hundhausen, C.; Roth, A.; Whalen, E.; Chen, J.; Schneider, A.; Long, S.A.; Wei, S.; Rawlings, R.; Kinsman, M.; Evanko, S.P.; et al. Enhanced T Cell Responses to IL-6 in Type 1 Diabetes Are Associated with Early Clinical Disease and Increased IL-6 Receptor Expression. Sci. Transl. Med. 2016, 8, 356ra119. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, M.; Jiang, D.; Su, Q.; Shi, J. The Role of Inflammation in Autoimmune Disease: A Therapeutic Target. Front. Immunol. 2023, 14, 1267091. [Google Scholar] [CrossRef]
- Jones, B.E.; Maerz, M.D.; Buckner, J.H. IL-6: A Cytokine at the Crossroads of Autoimmunity. Curr. Opin. Immunol. 2018, 55, 9–14. [Google Scholar] [CrossRef]
- Roy, S.; Pokharel, P.; Piganelli, J.D. Decoding the Immune Dance: Unraveling the Interplay between Beta Cells and Type 1 Diabetes. Mol. Metab. 2024, 88, 101998. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.E.; Kaiser, E.K.; Lin, J.; Gill, D.; Koskenniemi, J.J.; Karhunen, V. Genetic Evidence for Efficacy of Targeting IL-2, IL-6 and TYK2 Signalling in the Prevention of Type 1 Diabetes: A Mendelian Randomisation Study. Diabetologia 2024, 67, 2667–2677. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Xiao, Z.; Zang, C.; Li, P.; Xiao, B.; Zhou, L. Exploring the Therapeutic Potential of Interleukin-6 Receptor Blockade in Autoimmune Diseases Using Drug Target Mendelian Randomization. Immunogenetics 2025, 77, 3. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Fukuyama, T.; Uchida, A.; Sagara, Y.; Nakano, Y.; Tamai, H.; Tojikubo, M.; Hiromatsu, Y.; Koga, N. Development of Type 1 Diabetes in a Patient Treated with Anti-Interleukin-6 Receptor Antibody for Rheumatoid Arthritis. J. Diabetes Investig. 2022, 13, 738–740. [Google Scholar] [CrossRef]
- Fu, C.; Wang, L.; Cai, W. IL6 Receptor Inhibitors: Exploring the Therapeutic Potential Across Multiple Diseases through Drug Target Mendelian Randomization. Front. Immunol. 2024, 15, 1452849. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting Findings from Mendelian Randomization Using the MR-Egger Method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Freitag, D.F.; Cutler, A.J.; Howson, J.M.M.; Rainbow, D.B.; Smyth, D.J.; Kaptoge, S.; Clarke, P.; Boreham, C.; Coulson, R.M. Functional IL6R 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases. PLoS Genet. 2013, 9, e1003444. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From Basic Biology to Selective Blockade of Pro-Inflammatory Activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 Biology into Effective Treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]
- Rose-John, S. Therapeutic Targeting of IL-6 Trans-Signaling. Cytokine 2021, 144, 155577. [Google Scholar] [CrossRef] [PubMed]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 Trans- and IL-6 Classic Signalling Is Determined by the Ratio of the IL-6 Receptor α to gp130 Expression: Fusing Experimental Insights and Dynamic Modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef]
- Ireland, S.J.; Monson, N.L.; Davis, L.S. Seeking Balance: Potentiation and Inhibition of Multiple Sclerosis Autoimmune Responses by IL-6 and IL-10. Cytokine 2015, 73, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Aubin, A.M.; Lombard-Vadnais, F.; Collin, R.; Aliesky, H.A.; McLachlan, S.M.; Lesage, S. The NOD Mouse Beyond Autoimmune Diabetes. Front. Immunol. 2022, 13, 874769. [Google Scholar] [CrossRef]
- Foster, T.P.; Bruggeman, B.S.; Haller, M.J. Emerging Immunotherapies for Disease Modification of Type 1 Diabetes. Drugs 2025, 85, 457–473. [Google Scholar] [CrossRef]
- Fontes, J.A.; Rose, N.R.; Čiháková, D. The Varying Faces of IL-6: From Cardiac Protection to Cardiac Failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.B. IL-6 and Type 1 Diabetes Mellitus: T Cell Responses and Increase in IL-6 Receptor Surface Expression. Ann. Transl. Med. 2017, 5, 16. [Google Scholar] [CrossRef]
- Bossi, F.; Bernardi, S.; Zauli, G.; Secchiero, P.; Fabris, B. TRAIL modulates the immune system and protects against the development of diabetes. J. Immunol. Res. 2015, 2015, 680749. [Google Scholar] [CrossRef]
- Ablasser, A.; Chen, Z.J. cGAS in action: Expanding roles in immunity and inflammation. Science 2019, 363, eaat8657. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Song, L.; Gao, X.; Chang, W.; Qin, X. Toll-like receptor 4 on islet β cells senses expression changes in high-mobility group box 1 and contributes to the initiation of type 1 diabetes. Exp. Mol. Med. 2012, 44, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, A.; Suarez-Pinzon, W.L. Role of Cytokines in the Pathogenesis of Autoimmune Diabetes Mellitus. Rev. Endocr. Metab. Disorders 2003, 4, 291–299. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Study Type | Population | Exposure/Intervention | Primary Outcome | Main Findings | Sample Size | Study Limitations |
|---|---|---|---|---|---|---|---|
| Hundhausen et al., 2016 [10] | Mechanistic Study | T1DM patients vs. healthy controls | IL-6 stimulation of PBMCs | IL-6-induced STAT3/STAT1 phosphorylation | T1DM patients exhibited enhanced IL-6 signaling responses | n = 25 T1DM, n = 20 controls | Small sample size; in vitro mechanistic findings may not reflect in vivo dynamics |
| Kawasaki et al., 2022 [16] | Case Report | 73-year-old woman with RA receiving tocilizumab | Tocilizumab 162mg SC every 2 weeks | Development of autoimmune T1DM (ketoacidosis) | T1DM onset 17 months after tocilizumab initiation | Single patient | Anecdotal; no causal inference possible |
| Greenbaum et al., 2021 [9] | Randomized Controlled Trial | Children and adolescents with new-onset T1DM | Tocilizumab IV monthly (7 doses) vs. placebo | Preservation of β-cell function (C-peptide AUC) | No significant effect of tocilizumab on C-peptide decline | n = 69 | Short follow-up; limited statistical power |
| Fu et al., 2024 [17] | Mendelian Randomization Study 1 | General population GWAS datasets | Genetically proxied IL-6R blockade | Association between IL-6R blockade and T1DM risk | No significant association (p > 0.05) | ~200,000 GWAS participants | Possible weak instruments; European ancestry only |
| Heikkilä et al., 2024 [14] | Mendelian Randomization Study 2 | GWAS datasets focused on IL6R expression and T1DM | Genetically proxied IL6R expression levels | Association between IL6R expression and T1DM risk | Higher IL6R expression increased T1DM risk (OR 1.98) | ~150,000 GWAS participants | Potential horizontal pleiotropy; limited trans-ethnic validation |
| Li et al., 2025 [15] | Mendelian Randomization Study 3 | GWAS datasets using CRP as IL-6R signaling proxy | Genetically proxied IL-6R blockade via CRP levels | Association between IL-6R blockade and T1DM risk | IL-6R blockade reduced T1DM risk (OR 0.410) | ~180,000 GWAS participants | Proxy SNPs may capture downstream effects, not IL-6R-specific |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, R.; Fujimori, T.; Sano, C.; Ichinose, K. A Scoping Review of Clinical, Genetic, and Mechanistic Evidence Linking IL-6/IL-6R Signaling and Type 1 Diabetes Mellitus. Immuno 2025, 5, 41. https://doi.org/10.3390/immuno5030041
Ohta R, Fujimori T, Sano C, Ichinose K. A Scoping Review of Clinical, Genetic, and Mechanistic Evidence Linking IL-6/IL-6R Signaling and Type 1 Diabetes Mellitus. Immuno. 2025; 5(3):41. https://doi.org/10.3390/immuno5030041
Chicago/Turabian StyleOhta, Ryuichi, Taichi Fujimori, Chiaki Sano, and Kunihiro Ichinose. 2025. "A Scoping Review of Clinical, Genetic, and Mechanistic Evidence Linking IL-6/IL-6R Signaling and Type 1 Diabetes Mellitus" Immuno 5, no. 3: 41. https://doi.org/10.3390/immuno5030041
APA StyleOhta, R., Fujimori, T., Sano, C., & Ichinose, K. (2025). A Scoping Review of Clinical, Genetic, and Mechanistic Evidence Linking IL-6/IL-6R Signaling and Type 1 Diabetes Mellitus. Immuno, 5(3), 41. https://doi.org/10.3390/immuno5030041








