The Role of IL28B Polymorphism in Regulating Innate and Adaptive Immunity Against Viral Infection Among Allogenic Stem Cells Transplant Recipients
Abstract
1. Introduction
2. Bone Marrow Transplantation and Immune Reconstitution
3. IL28B Polymorphisms and Viral Infections in the General Population and Solid Organ Transplant (SOT)
4. IL28B in Bone Marrow Transplant Patient Recipients
5. Effect of IL28B Polymorphisms on Interferon Production and Signaling
6. Clinical Implications of IL28B Polymorphism
7. Predictive Value of IL28B Genotyping in Donor/Recipient Matching in Allogenic-HSCT
8. Potential Clinical and Predictive Role of IL28B in HSCT
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, S.Y.; Lee, H.J.; Lee, D.G. Infectious complications after hematopoietic stem cell transplantation: Current status and future perspectives in Korea. Korean J. Intern. Med. 2018, 33, 256–276. [Google Scholar] [CrossRef]
- Ljungman, P. Viral infection after hematopoietic stem cell transplantation. Curr. Opin. Hematol. 2024, 31, 270–274. [Google Scholar] [CrossRef]
- Ferdjallah, A.; Young, J.-A.H.; MacMillan, M.L. A Review of Infections After Hematopoietic Cell Transplantation Requiring PICU Care: Transplant Timeline Is Key. Front. Pediatr. 2021, 9, 634449. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.R.; Pouch, S.M.; Scully, B. Infections in Allogeneic Stem Cell Transplantation. In Principles and Practice of Transplant Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2018; pp. 209–226. [Google Scholar] [CrossRef]
- Stern, L.; Withers, B.; Avdic, S.; Gottlieb, D.; Abendroth, A.; Blyth, E.; Slobedman, B. Human Cytomegalovirus Latency and Reactivation in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Front. Microbiol. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Zerr, D.M.; Boeckh, M.; Delaney, C.; Martin, P.J.; Xie, H.; Adler, A.L.; Huang, M.-L.; Corey, L.; Leisenring, W.M. HHV-6 reactivation and associated sequelae after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Gentile, G.; Antonelli, G. HBV Reactivation in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Narrative Review. Viruses 2019, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.A.; Chong, P.P.; De Lima, M.; Friedman, M.S.; Giralt, S.; Hammond, S.P.; Kiel, P.J.; Masur, H.; McDonald, G.B.; Wingard, J.R.; et al. Hepatitis C Virus Infection among Hematopoietic Cell Transplant Donors and Recipients: American Society for Blood and Marrow Transplantation Task Force Recommendations. Biol. Blood Marrow Transplant. 2015, 21, 1870–1882. [Google Scholar] [CrossRef]
- Mendoza, M.A.; Imlay, H. Polyomaviruses After Allogeneic Hematopoietic Stem Cell Transplantation. Viruses 2025, 17, 403. [Google Scholar] [CrossRef]
- Inazawa, N.; Hori, T.; Nojima, M.; Saito, M.; Igarashi, K.; Yamamoto, M.; Shimizu, N.; Yoto, Y.; Tsutsumi, H.J. Virus reactivations after autologous hematopoietic stem cell transplantation detected by multiplex PCR assay. J. Med. Virol. 2017, 89, 358–362. [Google Scholar] [CrossRef]
- Ogonek, J.; Kralj Juric, M.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Sommereyns, C.; Paul, S.; Staeheli, P.; Michiels, T. IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008, 4, e1000017. [Google Scholar] [CrossRef]
- Radtke, S.; Enstrom, M.; Pande, D.; Duke, E.R.; Cardozo-Ojeda, E.F.; Madhu, R.; Owen, S.; Kanestrom, G.; Cui, M.; Perez, A.M.; et al. Stochastic fate decisions of HSCs after transplantation: Early contribution, symmetric expansion, and pool formation. Blood 2023, 142, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Teshima, T.; Boelens, J.J.; Matsuoka, K.-I. Novel insights into GVHD and immune reconstitution after allogeneic hematopoietic cell transplantation. Blood Cell Ther. 2023, 6, 42–48. [Google Scholar] [CrossRef]
- Palmer, J.M.; Rajasekaran, K.; Thakar, M.S.; Malarkannan, S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J. Cancer 2013, 4, 25–35. [Google Scholar] [CrossRef]
- Ito, M.; Fujino, M. Macrophage-mediated complications after stem cell transplantation. Pathol. Int. 2019, 69, 679–687. [Google Scholar] [CrossRef]
- Fischer, J.C.; Bscheider, M.; Göttert, S.; Thiele Orberg, E.; Combs, S.E.; Bassermann, F.; Heidegger, S.; Haas, T.; Poeck, H. Type I interferon signaling before hematopoietic stem cell transplantation lowers donor T cell activation via reduced allogenicity of recipient cells. Sci. Rep. 2019, 9, 14955. [Google Scholar] [CrossRef]
- Booth, C.; Veys, P. T cell depletion in paediatric stem cell transplantation. Clin. Exp. Immunol. 2013, 172, 139–147. [Google Scholar] [CrossRef]
- Einsele, H.; Ljungman, P.; Boeckh, M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020, 135, 1619–1629. [Google Scholar] [CrossRef]
- Epstein, D.J.; Otoukesh, S.; Shahid, Z.; Dadwal, S.S. Infectious Disease Considerations in Chronic Graft-versus-Host Disease and Transplantation Survivors. Transplant. Cell. Ther. 2024, 30, S534–S547. [Google Scholar] [CrossRef] [PubMed]
- Mohei, H.; Kellampalli, U.; Vlasova-St Louis, I. Immune reconstitution disorders: Spotlight on interferons. Int. J. Biomed. Investig. 2019, 2, 119. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.C.; Sridhar, P.R.; Baldridge, M.T. Differential roles of interferons in innate responses to mucosal viral infections. Trends Immunol. 2021, 42, 1009–1023. [Google Scholar] [CrossRef]
- Lazear, H.M.; Nice, T.J.; Diamond, M.S. Interferon-λ: Immune Functions at Barrier Surfaces and Beyond. Immunity 2015, 43, 15–28. [Google Scholar] [CrossRef]
- Charlton, M.R.; Thompson, A.; Veldt, B.J.; Watt, K.; Tillmann, H.; Poterucha, J.J.; Heimbach, J.K.; Goldstein, D.; McHutchison, J. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology 2011, 53, 317–324. [Google Scholar] [CrossRef]
- O’Brien, T.R.; Jackson, S.S. What Have We Learned from Studies of IFN-λ Variants and Hepatitis C Virus Infection? J. Interferon Cytokine Res. 2019, 39, 618–626. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nishida, N.; Sugiyama, M.; Kurosaki, M.; Matsuura, K.; Sakamoto, N.; Nakagawa, M.; Korenaga, M.; Hino, K.; Hige, S.; et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 2009, 41, 1105–1109. [Google Scholar] [CrossRef]
- Ge, D.; Fellay, J.; Thompson, A.J.; Simon, J.S.; Shianna, K.V.; Urban, T.J.; Heinzen, E.L.; Qiu, P.; Bertelsen, A.H.; Muir, A.J.; et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009, 461, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, V.; Moldovan, M.; Ahlenstiel, G.; Berg, T.; Weltman, M.; Abate, M.L.; Bassendine, M.; Spengler, U.; Dore, G.J.; Powell, E.; et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 2009, 41, 1100–1104. [Google Scholar] [CrossRef]
- Thomas, D.L.; Thio, C.L.; Martin, M.P.; Qi, Y.; Ge, D.; O’Huigin, C.; Kidd, J.; Kidd, K.; Khakoo, S.I.; Alexander, G.; et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009, 461, 798–801. [Google Scholar] [CrossRef]
- O’Brien, T.R.; Prokunina-Olsson, L.; Donnelly, R.P. IFN-λ4: The paradoxical new member of the interferon lambda family. J Interferon Cytokine Res. 2014, 34, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Prokunina-Olsson, L.; Muchmore, B.; Tang, W.; Pfeiffer, R.M.; Park, H.; Dickensheets, H.; Hergott, D.; Porter-Gill, P.; Mumy, A.; Kohaar, I.; et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 2013, 45, 164–171. [Google Scholar] [CrossRef]
- Martin, M.P.; Qi, Y.; Goedert, J.J.; Hussain, S.K.; Kirk, G.D.; Keith Hoots, W.; Buchbinder, S.; Carrington, M.; Thio, C.L. IL28B Polymorphism Does Not Determine Outcomes of Hepatitis B Virus or HIV Infection. J. Infect. Dis. 2010, 202, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Fang, L.; Pan, H.; Shi, J. Association between IL28B Polymorphisms and Outcomes of Hepatitis B Virus Infection: A meta-analysis. BMC Med. Genet. 2020, 21, 88. [Google Scholar] [CrossRef]
- Nattermann, J.; Vogel, M.; Nischalke, H.D.; Danta, M.; Mauss, S.; Stellbrink, H.J.; Baumgarten, A.; Mayr, C.; Bruno, R.; Tural, C.; et al. Genetic variation in IL28B and treatment-induced clearance of hepatitis C virus in HIV-positive patients with acute and chronic hepatitis C. J. Infect. Dis. 2011, 203, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Borivoje, S.; Svetlana, S.; Milan, H.M.; Nela, Đ.; Olivera, M.; Filip, M.; Milenko, S.; Srbislav, P. IL28B Genetic Variations in Patients with Recurrent Herpes Simplex Keratitis. Medicina 2019, 55, 642. [Google Scholar] [CrossRef]
- Yin, Y.; Favoreel, H.W. Herpesviruses and the Type III Interferon System. Virol. Sin. 2021, 36, 577–587. [Google Scholar] [CrossRef]
- Manuel, O.; Wójtowicz, A.; Bibert, S.; Mueller, N.J.; van Delden, C.; Hirsch, H.H.; Steiger, J.; Stern, M.; Egli, A.; Garzoni, C.; et al. Influence of IFNL3/4 polymorphisms on the incidence of cytomegalovirus infection after solid-organ transplantation. J. Infect. Dis. 2015, 211, 906–914. [Google Scholar] [CrossRef]
- Chmelova, K.; Frankova, S.; Jirsa, M.; Neroldova, M.; Sticova, E.; Merta, D.; Senkerikova, R.; Trunecka, P.; Spicak, J.; Sperl, J. IL28B rs12979860 T allele protects against CMV disease in liver transplant recipients in the post-prophylaxis and late period. Transpl. Infect. Dis. 2019, 21, e13124. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Levin, A.; Santer, D.M.; Joyce, M.; O’Shea, D.; Thomas, B.S.; Lisboa, L.F.; Barakat, K.; Bhat, R.; Fischer, K.P.; et al. Immunomodulatory Function of Interleukin 28B During Primary Infection With Cytomegalovirus. J. Infect. Dis. 2014, 210, 717–727. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Corrales, I.; Arias, M.; Campistol, J.M.; Giménez, E.; Crespo, J.; López-Oliva, M.O.; Beneyto, I.; Martín-Moreno, P.L.; Llamas-Fuente, F.; et al. Association Between Individual and Combined SNPs in Genes Related to Innate Immunity and Incidence of CMV Infection in Seropositive Kidney Transplant Recipients. Am. J. Transplant. 2015, 15, 1323–1335. [Google Scholar] [CrossRef]
- Araújo, A.; Sgorlon, G.; Aguiar, L.E.; Cidrão, M.H.M.C.; Teixeira, K.S.; Villalobos Salcedo, J.M.; Passos-Silva, A.M.; Vieira, D. Influence of polymorphic variations of IFNL, HLA, and IL-6 genes in severe cases of COVID-19. Exp. Biol. Med. 2023, 248, 787–797. [Google Scholar] [CrossRef]
- Cakal, B.; Cavus, B.; Atasoy, A.; Altunok, D.; Poda, M.; Bulakci, M.; Gulluoglu, M.; Demirci, M.; Sener, L.T.; Arslan, A.B. The effects of IL28B rs12979860 and rs8099917 polymorphism on hepatitis B infection. North. Clin. Istanb. 2022, 9, 439. [Google Scholar] [CrossRef]
- Guedes de Sá, K.S.; Amoras, E.d.S.G.; Conde, S.R.S.d.S.; Queiroz, M.A.F.; Cayres-Vallinoto, I.M.V.; Ishak, R.; Vallinoto, A.C.R. Intrahepatic TLR3 and IFNL3 expressions are associated with stages of fibrosis in chronic hepatitis C. Viruses 2021, 13, 1103. [Google Scholar] [CrossRef]
- Credle, J.J.; Gunn, J.; Sangkhapreecha, P.; Monaco, D.R.; Zheng, X.A.; Tsai, H.-J.; Wilbon, A.; Morgenlander, W.R.; Dong, Y.; Jayaraman, S. Neutralizing IFNL3 autoantibodies in severe COVID-19 identified using molecular indexing of proteins by self-assembly. bioRxiv 2021. [Google Scholar] [CrossRef]
- Grimaudo, S.; Amodio, E.; Pipitone, R.M.; Maida, C.M.; Pizzo, S.; Prestileo, T.; Tramuto, F.; Sardina, D.; Vitale, F.; Casuccio, A. PNPLA3 and TLL-1 polymorphisms as potential predictors of disease severity in patients with COVID-19. Front. Cell Dev. Biol. 2021, 9, 627914. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Gloss, B.S.; Liddle, C.; George, J.; Ahlenstiel, G. Interferon-λ3 exacerbates the inflammatory response to microbial ligands: Implications for SARS-CoV-2 pathogenesis. J. Inflamm. Res. 2021, 14, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Sajadi, M.M.; Shakeri, N.; Talwani, R.; Howell, C.D.; Pakyz, R.; Redfield, R.R.; Parsa, A. IL28B genotype does not correlate with HIV control in African Americans. Clin. Transl. Sci. 2011, 4, 282–284. [Google Scholar] [CrossRef]
- Bravo, D.; Solano, C.; Giménez, E.; Remigia, M.J.; Corrales, I.; Amat, P.; Navarro, D. Effect of the IL28B Rs12979860 C/T polymorphism on the incidence and features of active cytomegalovirus infection in allogeneic stem cell transplant patients. J. Med. Virol. 2014, 86, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Akay, E.; Patel, M.; Conibear, T.; Chaggar, T.; Haque, T. Interleukin 28B gene polymorphisms and Epstein-Barr virus-associated lymphoproliferative diseases. Intervirology 2014, 57, 112–115. [Google Scholar] [CrossRef]
- Dvir, R.; Paloschi, V.; Canducci, F.; Dell’Antonio, G.; Racca, S.; Caldara, R.; Pantaleo, G.; Clementi, M.; Secchi, A. IL28B rs12979860 genotype as a predictor marker of progression to BKVirus Associated nephropathy, after kidney transplantation. Sci. Rep. 2017, 7, 6746. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, S.; Guan, C.; Liu, S.; Zhang, H. Type III interferon, age and IFNL gene single nucleotide polymorphisms determine the characteristics of H1N1 influenza infection. Front. Immunol. 2025, 16, 1592841. [Google Scholar] [CrossRef]
- Corrales, I.; Solano, C.; Amat, P.; Giménez, E.; de la Cámara, R.; Nieto, J.; López, J.; García-Noblejas, A.; Piñana, J.L.; Navarro, D. IL28B genetic variation and cytomegalovirus-specific T-cell immunity in allogeneic stem cell transplant recipients. J. Med. Virol. 2017, 89, 685–695. [Google Scholar] [CrossRef]
- Annibali, O.; Piccioni, L.; Tomarchio, V.; Circhetta, E.; Sarlo, C.; Franceschini, L.; Cantonetti, M.; Rizzo, E.; Angeletti, S.; Tirindelli, M.C.; et al. Impact of IFN lambda 3/4 single nucleotide polymorphisms on the cytomegalovirus reactivation in autologous stem cell transplant patients. PLoS ONE 2018, 13, e0200221. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, S.M.; Wang, Y.; Wang, T.; Onabajo, O.O.; Banday, A.R.; Obajemu, A.; Karaesman, E.; Sucheston-Campbell, L.; Hahn, T.; Sees, J.A.; et al. Association of donor IFNL4 genotype and non-relapse mortality after unrelated donor myeloablative haematopoietic stem-cell transplantation for acute leukaemia: A retrospective cohort study. Lancet. Haematol. 2020, 7, e715–e723. [Google Scholar] [CrossRef] [PubMed]
- Coto-Llerena, M.; Lepore, M.; Spagnuolo, J.; Di Blasi, D.; Calabrese, D.; Suslov, A.; Bantug, G.; Duong, F.H.; Terracciano, L.M.; De Libero, G.; et al. Interferon lambda 4 can directly activate human CD19(+) B cells and CD8(+) T cells. Life Sci. Alliance 2021, 4. [Google Scholar] [CrossRef]
- Afzal, M.S.J.W.J.o.H. Predictive potential of IL-28B genetic testing for interferon based hepatitis C virus therapy in Pakistan: Current scenario and future perspective. J. World J. Hepatol. 2016, 8, 1116. [Google Scholar] [CrossRef]
- Sezgin, E.; An, P.; Winkler, C.A. Host Genetics of Cytomegalovirus Pathogenesis. Front. Genet. 2019, 10, 616. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Cervera, C.; Linares, L.; Suárez, B.; Llopis, J.; Sanclemente, G.; Casadó-Llombart, S.; Fernández-Ruiz, M.; Fariñas, M.C.; Cantisan, S.; et al. Polygenic Innate Immunity Score to Predict the Risk of Cytomegalovirus Infection in CMV D+/R− Transplant Recipients. A Prospective Multicenter Cohort Study. Front. Immunol. 2022, 13, 897912. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.T.; Kim, A.Y. IL28B polymorphisms as a pretreatment predictor of response to HCV treatment. Infect. Dis. Clin. N. Am. 2012, 26, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Dickensheets, H.; O’Brien, T.R. Interferon-lambda and therapy for chronic hepatitis C virus infection. Trends Immunol. 2011, 32, 443–450. [Google Scholar] [CrossRef]
- Chronopoulou, S.; Tsochantaridis, I. Interferon Lambda: The Next Frontier in Antiviral Therapy? Pharmaceuticals 2025, 18, 785. [Google Scholar] [CrossRef]
- Cook, L.; Diem, K.; Kim, W.; Scott, J.D.; Jerome, K.R. Allele-specific PCR for determination of IL28B genotype. J. Clin. Microbiol. 2012, 50, 4144–4146. [Google Scholar] [CrossRef][Green Version]
- Campos, C.F.; Leite, L.; Pereira, P.; Vaz, C.P.; Branca, R.; Campilho, F.; Freitas, F.; Ligeiro, D.; Marques, A.; Torrado, E.; et al. PTX3 Polymorphisms Influence Cytomegalovirus Reactivation After Stem-Cell Transplantation. Front. Immunol. 2019, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in Hematopoietic Stem Cell Transplant Recipients. Hematol. Oncol. Clin. N. Am. 2011, 25, 151–169. [Google Scholar] [CrossRef]
- Ganepola, S.; Gentilini, C.; Horvath, R.; Beham-Schmid, C.; Fritsch, G.; Lawitschka, A.; Worel, N. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007, 39, 293–299. [Google Scholar] [CrossRef]
- Teira, P.; Battiwalla, M.; Ramanathan, M.; Barrett, A.J.; Ahn, K.W.; Chen, M.; Eapen, M. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: A CIBMTR analysis. Blood 2016, 127, 2427–2438. [Google Scholar] [CrossRef]
- Broers, A.E.; van Bergen, C.A.; Langenhorst, J.B.; Gratama, J.W.; Lowenberg, B.; van Loon, A.M.; Cornelissen, J.J. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 2000, 95, 2240–2245. [Google Scholar] [CrossRef]
- Thiele, T.; Krüger, W.; Zimmermann, K.; Ittermann, T.; Wessel, A.; Steinmetz, I.; Dölken, G.; Greinacher, A. Transmission of cytomegalovirus (CMV) infection by leukoreduced blood products not tested for CMV antibodies: A single-center prospective study in high-risk patients undergoing allogeneic hematopoietic stem cell transplantation (CME). Transfusion 2011, 51, 2620–2626. [Google Scholar] [CrossRef]
- Sellar, R.S.; Peggs, K.S.; Osterwald, P.; Porter, D.L.; Rowntree, C.; Wang, X.N.; Amrolia, P.J. CMV promotes recipient T-cell immunity following reduced-intensity T-cell-depleted HSCT, significantly modulating chimerism status. Blood 2015, 125, 731–739. [Google Scholar] [CrossRef]
- Stevanovic, S.; Pasetto, A.; Helman, S.R.; Gartner, J.J.; Prickett, T.D.; Howie, B.; Rosenberg, S.A. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood 2013, 122, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.K.; Khanna, R.; Semple, K.J.; Messaoudi, I.; Munz, C.; Cooper, L.; Moss, D.J. Late diversification in the clonal composition of human cytomegalovirus-specific CD8+ T cells following allogeneic hemopoietic stem cell transplantation. Blood 2003, 102, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Hakki, M.; Styczynski, J.; Shimoni, A.; Winiarski, J.; Tridello, G.; Party, E.I.D.W. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: A study by the European group for blood and marrow transplantation. Clin. Infect. Dis. 2014, 59, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Henden, A.S.; Koyama, M.; Robb, R.J.; Forero, A.; Kuns, R.D.; Chang, K.; Ensbey, K.S.; Varelias, A.; Kazakoff, S.H.; Waddell, N. IFN-λ therapy prevents severe gastrointestinal graft-versus-host disease. Blood J. Am. Soc. Hematol. 2021, 138, 722–737. [Google Scholar] [CrossRef]
- Corrales, I.; Martínez-Laperche, C.; Bernal, T.; Buces, E.; López, P.; Serrano, D.; Solano, C. Incidence and dynamics of active cytomegalovirus infection in allogeneic stem cell transplant patients according to single nucleotide polymorphisms in donor and recipient CCR5, MCP-1, IL-10, and TLR9 genes. J. Med. Virol. 2015, 87, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ebid, A.I.M.; Ahmed, O.A.; Agwa, S.H.; Abdel-Motaleb, S.M.; Hagag, R.S. Impact of IL28B gene polymorphism on efficacy and safety of direct acting antivirals in hepatitis C Egyptian patients. Int. J. Clin. Pharm. 2020, 42, 1207–1216. [Google Scholar] [CrossRef]
- Yousry, A.; Abdel Aziz, M.S.; Shaker, O.G.; Omran, D.A.; El Neklawi, M.S. Impact of IL28B Polymorphism on the Response to Treatment of Hepatitis C with Interferon Based Therapy or Direct Acting Antivirals. Med. J. Cairo Univ. 2021, 89, 1333–1340. [Google Scholar]
- Takahashi, T. Interleukin 28B genetic polymorphism and hepatitis B virus infection. World J. Gastroenterol. 2014, 20, 12026–12030. [Google Scholar] [CrossRef]
- Gatselis, N.K.; Azariadis, K.; Lyberopoulou, A.; Dalekos, G.N. Programmed cell death-1 rs11568821 and interleukin-28B rs12979860 polymorphisms in autoimmune hepatitis. J. Transl. Autoimmun. 2021, 4, 100126. [Google Scholar] [CrossRef]
- Kim, S.U.; Song, K.J.; Chang, H.Y.; Shin, E.-C.; Park, J.Y.; Kim, D.Y.; Han, K.-H.; Chon, C.Y.; Ahn, S.H. Association between IL28B Polymorphisms and Spontaneous Clearance of Hepatitis B Virus Infection. PLoS ONE 2013, 8, e69166. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Kutalik, Z.; Descombes, P.; Cai, T.; Di Iulio, J.; Mueller, T.; Bochud, M.; Battegay, M.; Bernasconi, E.; Borovicka, J.; et al. Genetic Variation in IL28B Is Associated with Chronic Hepatitis C and Treatment Failure: A Genome-Wide Association Study. Gastroenterology 2010, 138, 1338–1345.e1337. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Sporea, I.; Şirli, R.; Neghină, A.M.; Popescu, A.; Străin, M. Role of Interleukin-28B Polymorphism as a Predictor of Sustained Virological Response in Patients with Chronic Hepatitis C Treated with Triple Therapy: A Systematic Review and Meta-Analysis. Clin. Drug Investig. 2013, 33, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, K.S.; El-Sayed, E.-S.M.; Ismail, R.S.; Zakarya, Z.M.; Helal, G.K. Association between interleukin 28B polymorphism and sustained virological response to sofosbuvir plus daclatasvir in chronic hepatitis C genotype 4 Egyptian patients. J. Clin. Pharm. Ther. 2021, 46, 942–949. [Google Scholar] [CrossRef]
- Jedlińska-Pijanowska, D.; Kasztelewicz, B.; Dobrzańska, A.; Dzierżanowska-Fangrat, K.; Jaworski, M.; Czech-Kowalska, J. Association between single nucleotide polymorphisms and viral load in congenital cytomegalovirus infection. J. Mother Child 2021, 24, 9–17. [Google Scholar] [CrossRef]
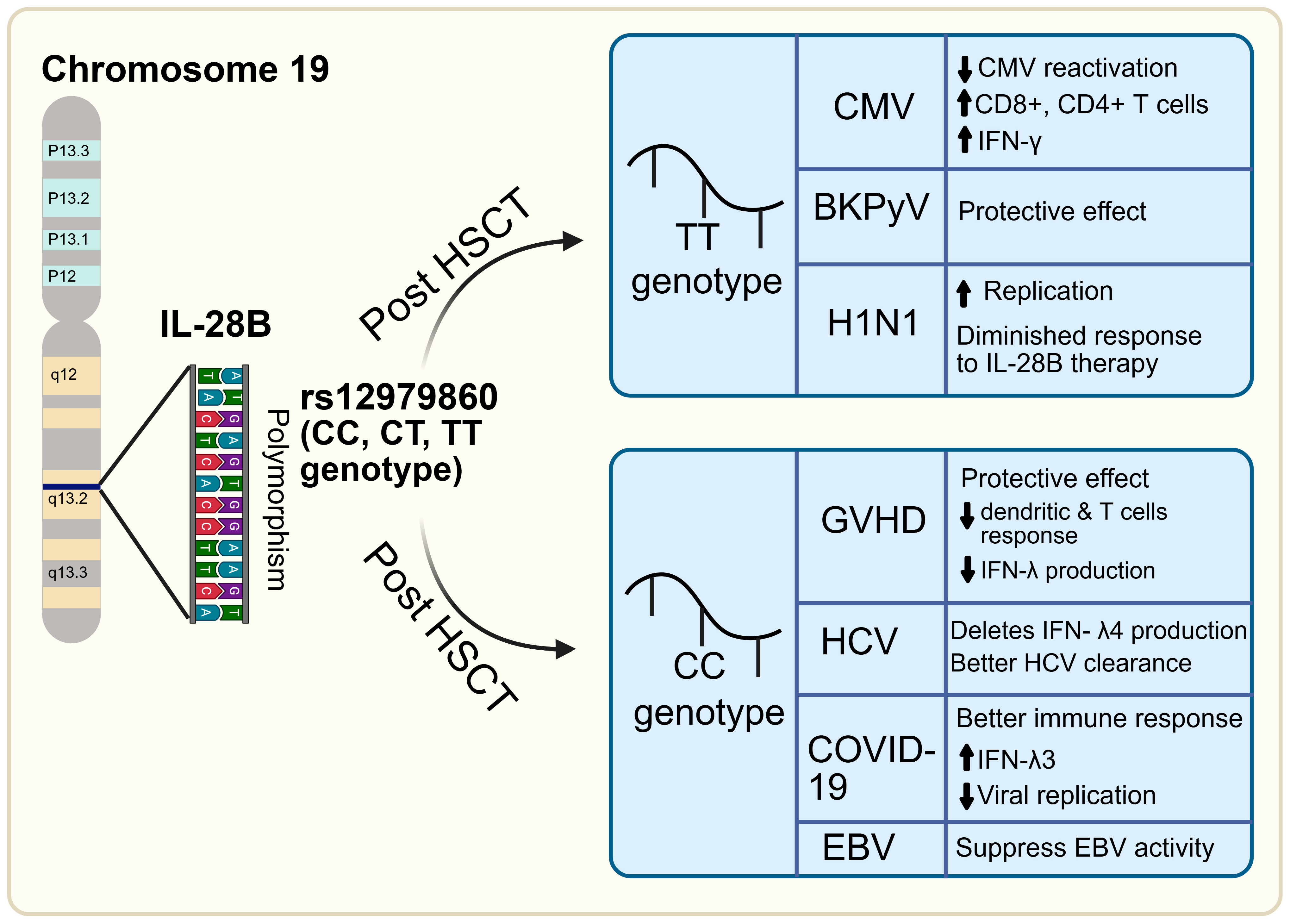
| Virus | Impact of IL-28B Polymorphism | Favorable Genotype | Clinical Setting | References |
|---|---|---|---|---|
| HCV | Strong: Predicts spontaneous and treatment-induced clearance | rs12979860 C/T | Population with IL28B CC allele (IFNL4-null) → better clearance | [30,31,32,33,34,35] |
| HBV | Minimal or no significant impact | _ | No consistent association; not predictive of outcome | [36,37] |
| HIV | Minimal (except in HIV/HCV co-infection) | CC allele in co-infection | Better outcomes observed only in HCV/HIV co-infected individuals | [38,51] |
| CMV | Moderate: Influences viremia/reactivation in transplant patients | TT (rs12979860) (IFNL4-producing genotype) | In solid organ and HSCT patients, TT allele is linked to lower CMV viremia and better control | [43,52] |
| EBV | Weak evidence | Possibly CC | Few studies; no strong association, but lower EBV DNA in CC genotype patient | [53] |
| BKPyV | Possible association: Linked to nephropathy risk in kidney transplant | TT allele | Studies in renal transplant patients suggest TT allele may protect against BK virus nephropathy; limited data in HSCT | [54] |
| Influenza (H1N1) | Experimental evidence only | Possibly TG/GG (rs8099917) and TT (rs12979860) allele | Animal models suggest that genotype affects viral replication and vaccine response | [43,55] |
| SARS-CoV-2 (COVID-19) | Possible association: Protection from severe COVID-19 | CC (rs12979860), AA (rs12980275 | Associated with resistance to infection and milder disease; TT genotype is linked to severe outcomes | [45,46,47,48,49,50] |
| Virus/Disease | Genotype | Effect | References |
|---|---|---|---|
| CMV | TT allele | ↑CMV-specific T cell response, lower incidence of CMV viremia | [52,56] |
| GVHD | CC allele | ↓Mortality | [58,59] |
| EBV | CC allele | ↓EBV DNA levels and reactivation | [53] |
| BKPyV | TT allele | ↓BKPyV-related nephropathy | [54] |
| H1N1 | TT and GG alleles | ↑H1N1 replication in mice models | [43,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltokhy, M.A.; Patel, B.; Curcic, M.; Alabi, F.; Modaresahmadi, S.; Eltoukhy, O.; Abdelmageed, E.G.; Radwan, S. The Role of IL28B Polymorphism in Regulating Innate and Adaptive Immunity Against Viral Infection Among Allogenic Stem Cells Transplant Recipients. Immuno 2025, 5, 38. https://doi.org/10.3390/immuno5030038
Eltokhy MA, Patel B, Curcic M, Alabi F, Modaresahmadi S, Eltoukhy O, Abdelmageed EG, Radwan S. The Role of IL28B Polymorphism in Regulating Innate and Adaptive Immunity Against Viral Infection Among Allogenic Stem Cells Transplant Recipients. Immuno. 2025; 5(3):38. https://doi.org/10.3390/immuno5030038
Chicago/Turabian StyleEltokhy, Mohamed A., Bhaumik Patel, Marina Curcic, Faizah Alabi, Shadan Modaresahmadi, Omar Eltoukhy, Esraa G. Abdelmageed, and Sahar Radwan. 2025. "The Role of IL28B Polymorphism in Regulating Innate and Adaptive Immunity Against Viral Infection Among Allogenic Stem Cells Transplant Recipients" Immuno 5, no. 3: 38. https://doi.org/10.3390/immuno5030038
APA StyleEltokhy, M. A., Patel, B., Curcic, M., Alabi, F., Modaresahmadi, S., Eltoukhy, O., Abdelmageed, E. G., & Radwan, S. (2025). The Role of IL28B Polymorphism in Regulating Innate and Adaptive Immunity Against Viral Infection Among Allogenic Stem Cells Transplant Recipients. Immuno, 5(3), 38. https://doi.org/10.3390/immuno5030038






