Sjögren’s Syndrome and Ocular Inflammation: Pathophysiology, Clinical Manifestation and Mitigation Strategies
Abstract
1. Introduction
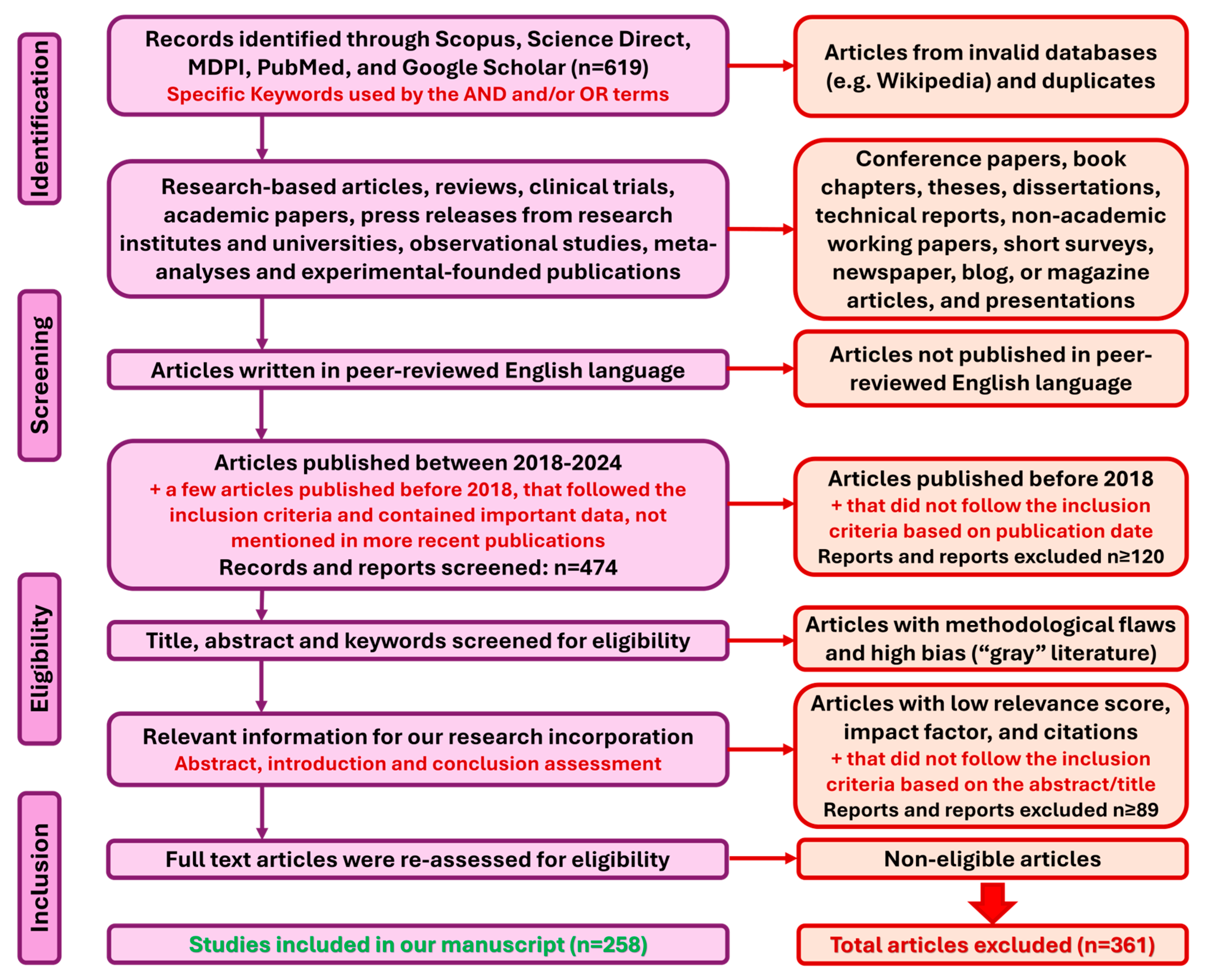
2. Materials and Methods
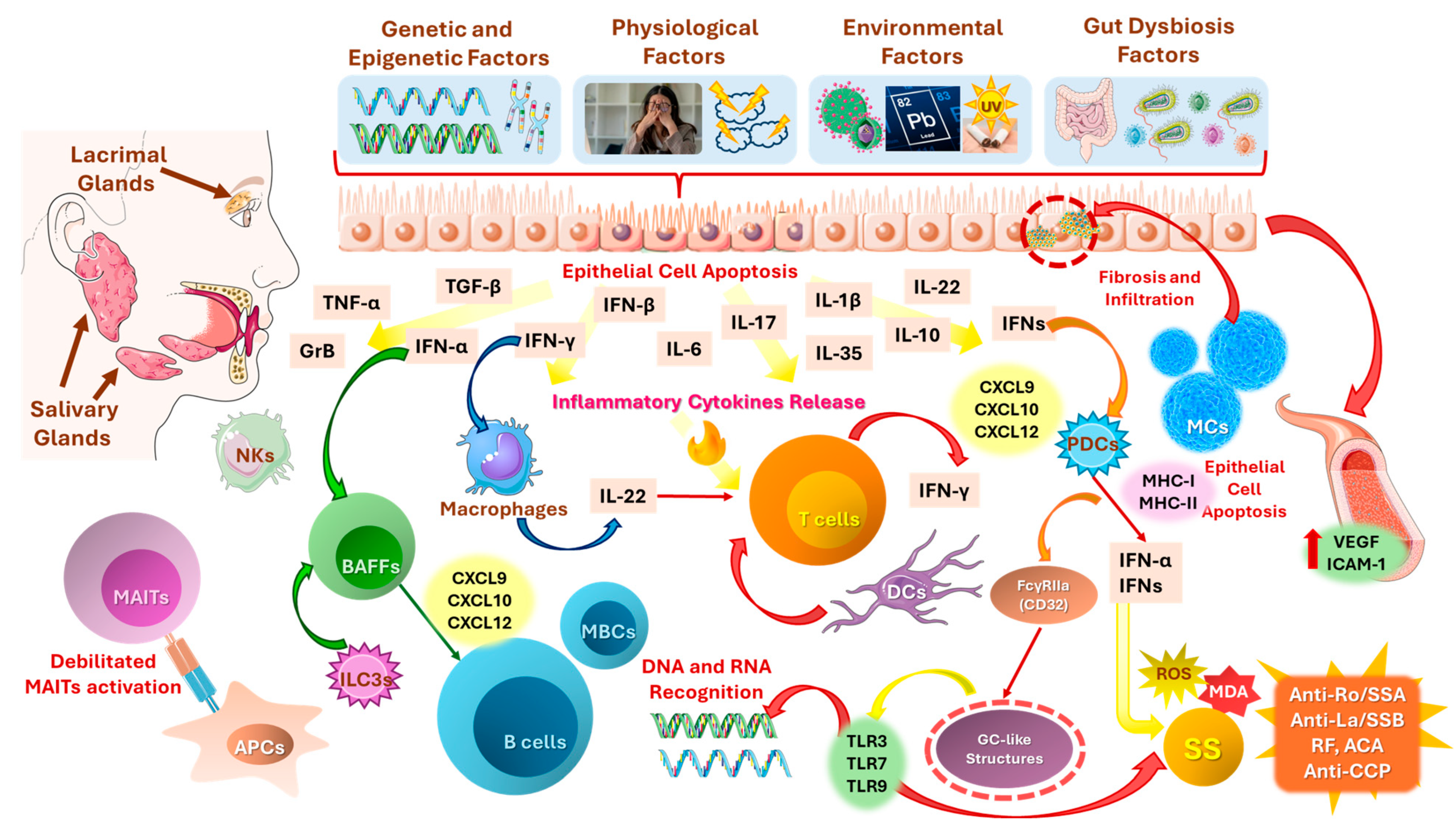
3. Pathophysiology of SS and Ocular Inflammation
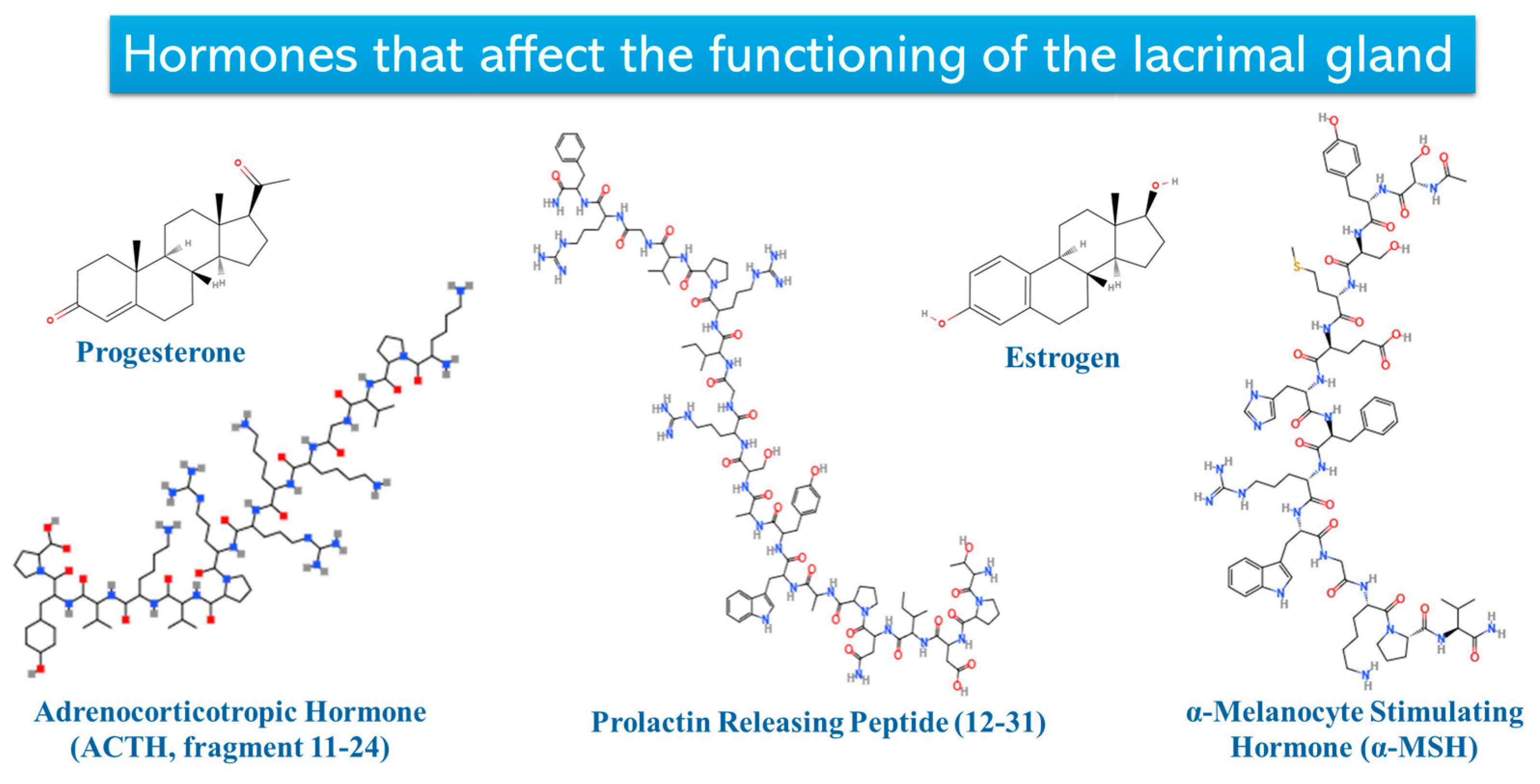
3.1. Lacrimal Glands
3.2. Autoimmune and Inflammatory Mechanisms
3.2.1. Type I Interferon System in Primary SS (pSS): An Interplay Between Innate Immunity, Inflammation, and pSS Exacerbation
3.2.2. Role of B-Cells in SS
3.3. Main Autoantibodies in SS: Their Role in Inflammation, Clinical Significance, and Diagnostic Value in SS
3.3.1. Anti-Ro/SSA Autoantibodies
3.3.2. Anti-La/SSB Autoantibodies
3.3.3. Rheumatoid Factor (Rf), Complement System, and Cryoglobulins
3.3.4. Cyclic Citrullinated Peptide Antibodies (Anti-CCP)
3.3.5. Anti-Centromere Antibody (ACA)
3.4. Genetic, Epigenetic, Environmental, and Physiological Factors Triggering SS Development
3.4.1. Genetic-Epigenetic Factors
microRNAs Implication
3.4.2. Psychological Factors
3.4.3. Environmental Factors
3.4.4. Role of Gut Dysbiosis and Gut-Lacrimal Axis
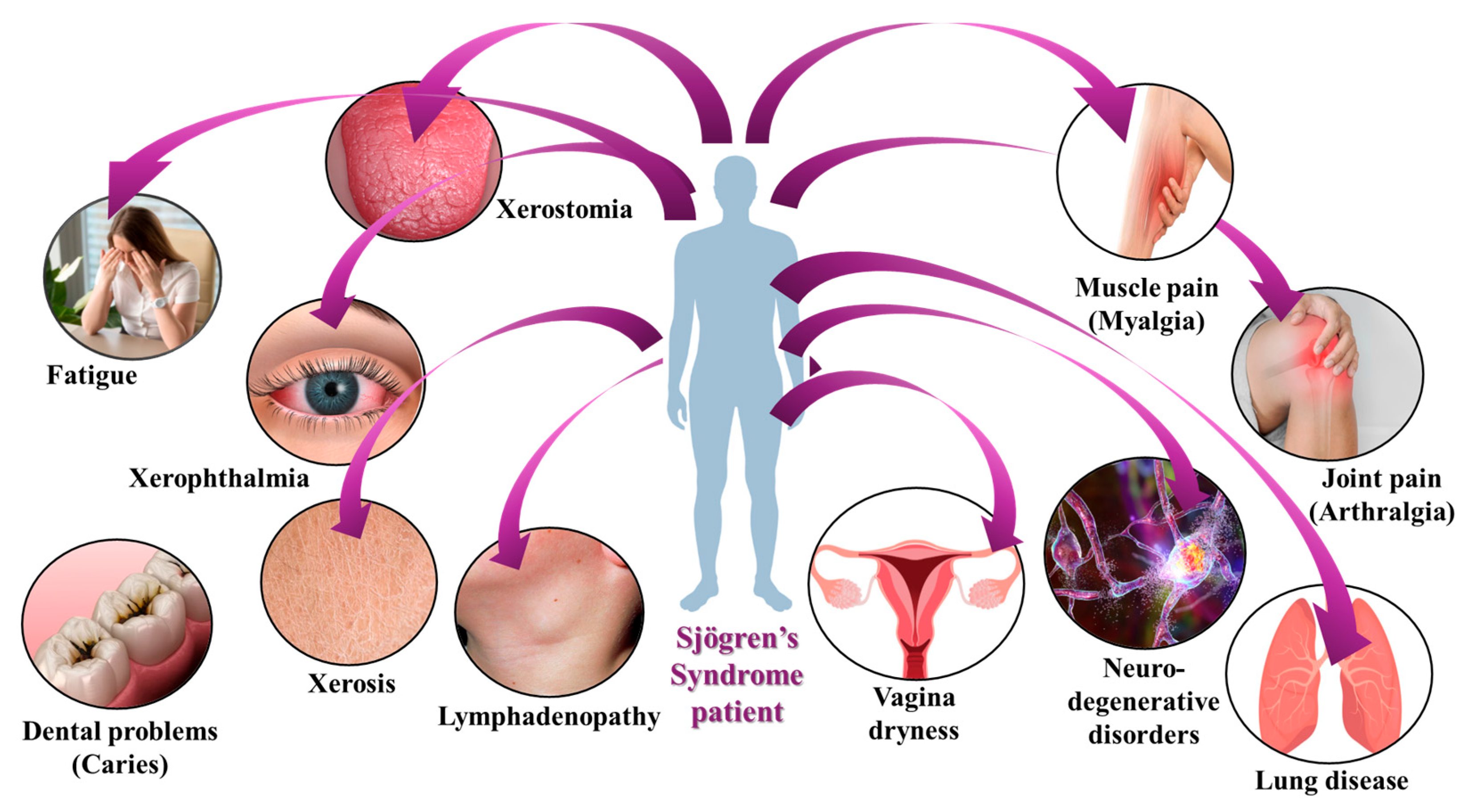
4. Clinical Manifestations of SS
4.1. Clinical Manifestations of SS: Sicca Symptoms and More
4.2. Dry Eye Diseases of SS
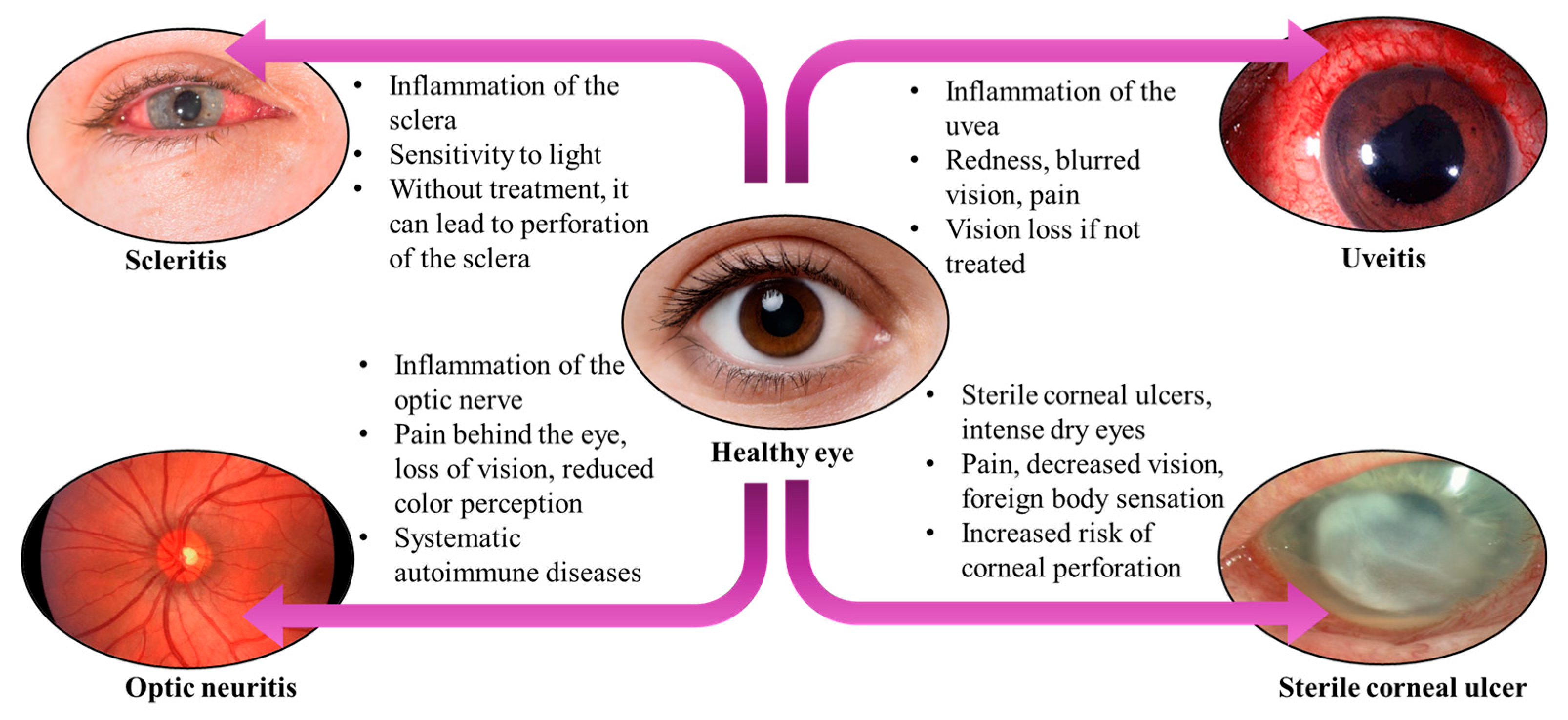
4.3. Ocular Inflammatory Complications
4.3.1. Sterile Corneal Ulcers
4.3.2. Scleritis
4.3.3. Optic Neuritis
4.3.4. Uveitis
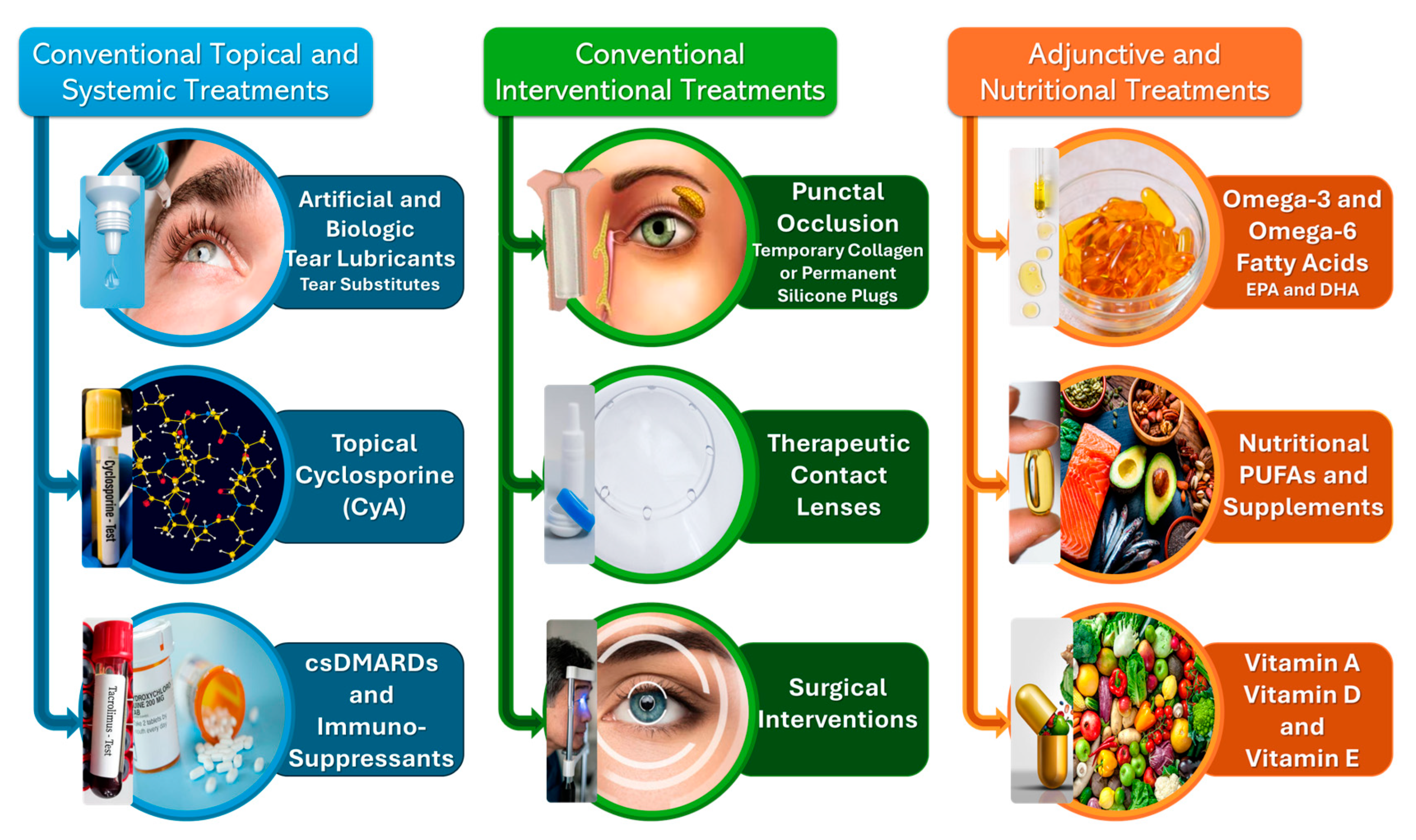
5. Common Conventional and Newly-Emerged Treatment and Mitigation Techniques for SS and DED Management
5.1. Conventional Topical and Systemic Treatments
5.1.1. Topical Lubricants: Artificial and Biologic Tear Substitutes
5.1.2. Topical Cyclosporine A (CyA) Immunosuppressive Treatment
5.1.3. Conventional Systemic Corticosteroids, Disease-Modifying Anti-Rheumatic Drugs (DMARDs) and Immunosuppressants
5.2. Conventional Interventional Procedures
5.2.1. Punctal Occlusion
5.2.2. Therapeutic Contact Lenses (CLs)
5.2.3. Surgical Interventions
5.3. Adjunctive and Nutritional Therapies
5.3.1. Oral Administration: Omega-3 and/or Omega-6 Fatty Acid Supplementation
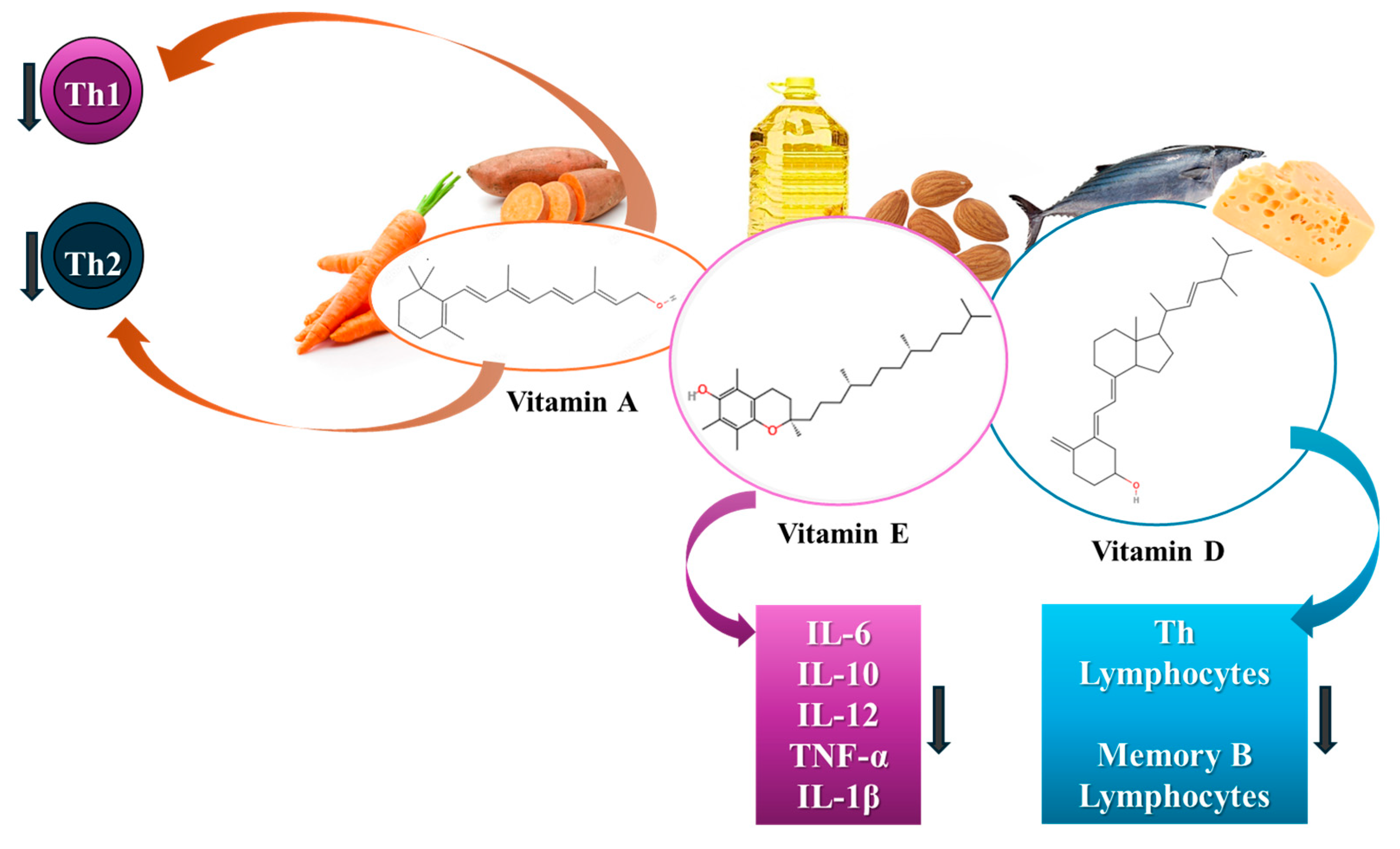
5.3.2. The Role of Vitamins in SS Mitigation
Vitamin A
Vitamin E
Vitamin D
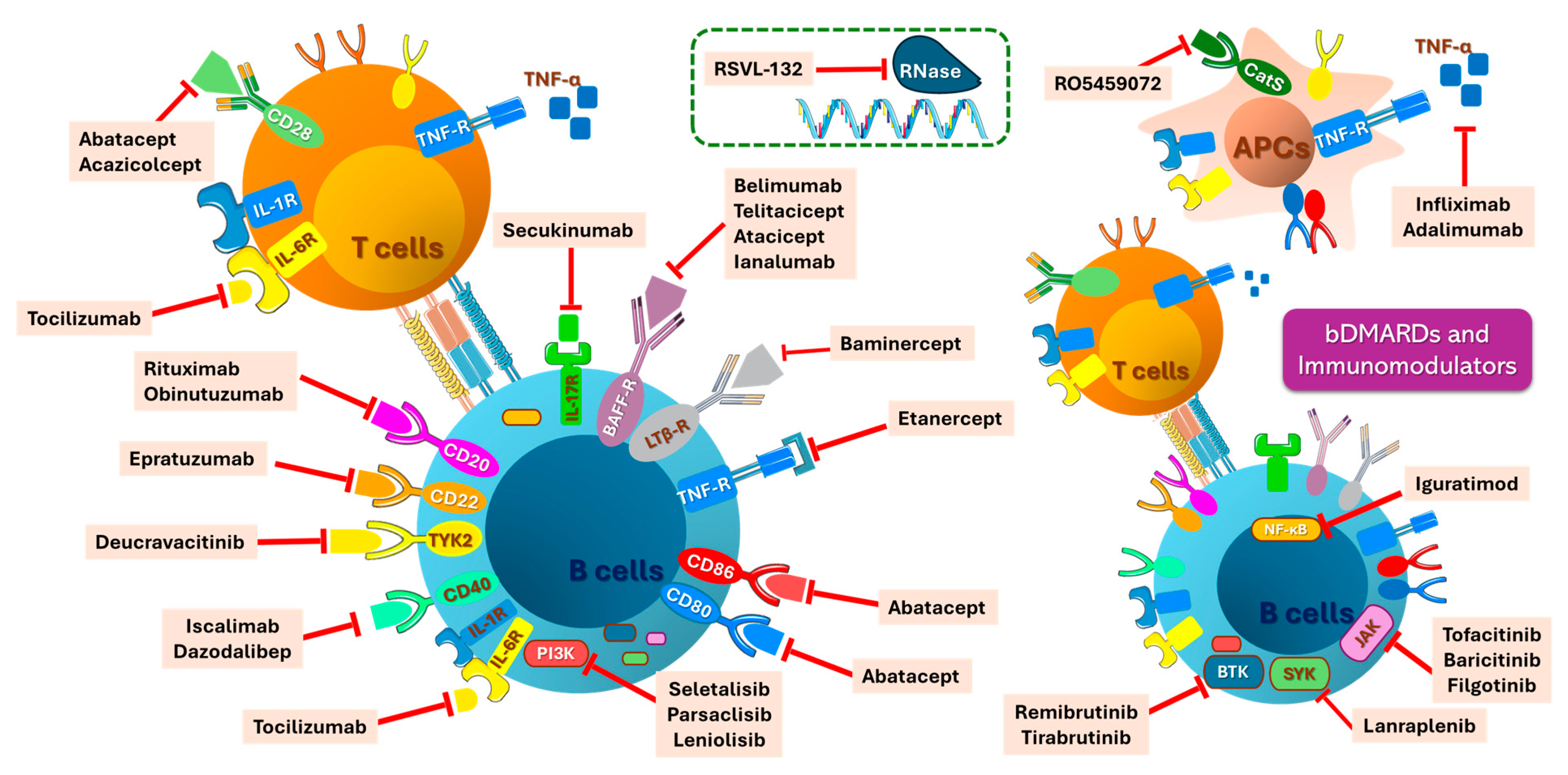
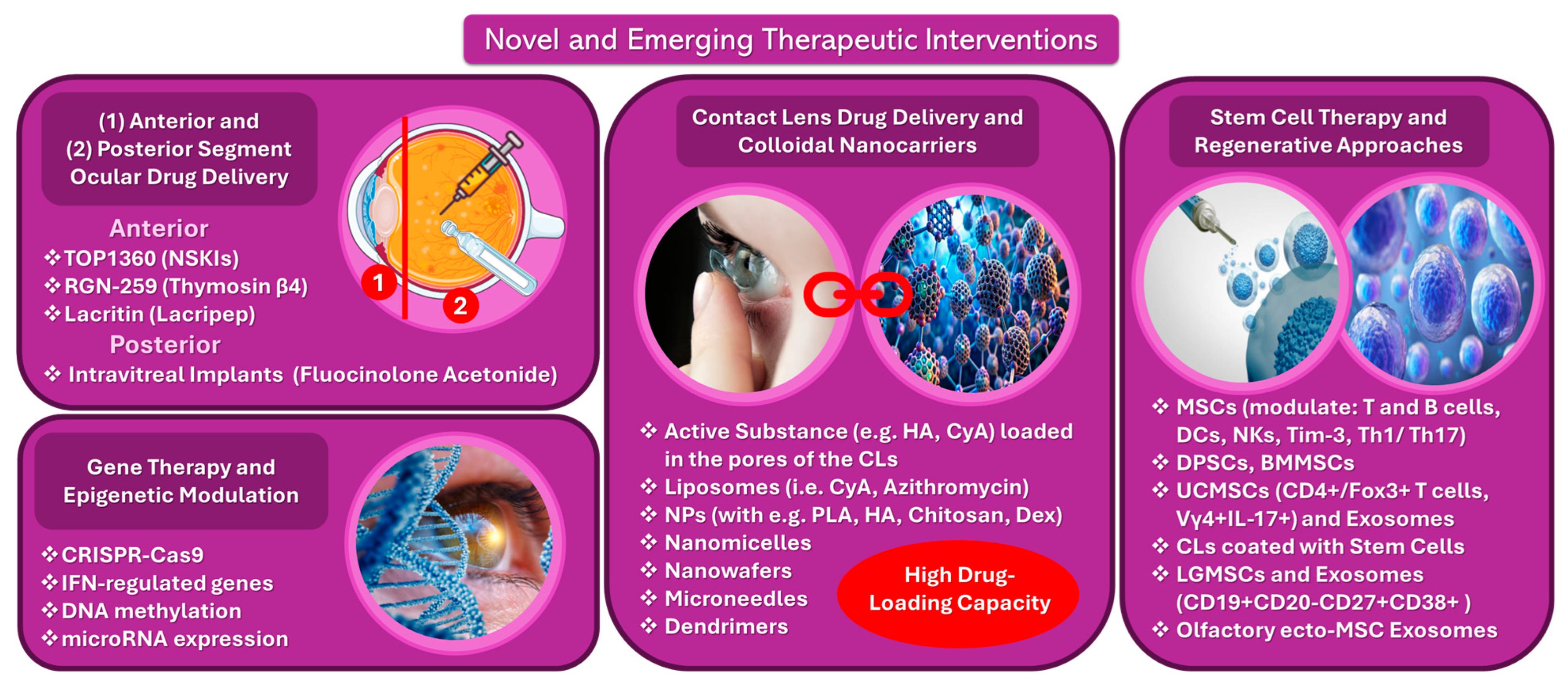
5.4. Novel and Emerging Therapies
5.4.1. Biologic Interventions, Targeted Therapies, and Immunomodulators
5.4.2. Anterior and Posterior Segment Ocular Drug-Delivery Technologies
5.4.3. Gene Therapy and Epigenetic Modulation
5.4.4. Contact Lens Drug-Delivery and Colloidal Nanocarriers
5.4.5. Stem Cell Therapy and Regenerative Approaches
6. Future Directions, Perspectives, and Research
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.L.; Nelson, R.J.; Sara, R.; Alberto, S. Sjögren Syndrome: New Insights in the Pathogenesis and Role of Nuclear Medicine. J. Clin. Med. 2022, 11, 5227. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Soares, R. Inflammation in Sjögren’s Syndrome: Cause or Consequence? Autoimmunity 2017, 50, 141–150. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Tseng, J.-C.; Leong, P.-Y.; Wang, Y.-H.; Wei, J.C.-C. Increased Risk of Sjögren’s Syndrome in Patients with Obsessive-Compulsive Disorder: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 5936. [Google Scholar] [CrossRef] [PubMed]
- Horai, Y.; Shimizu, T.; Nakamura, H.; Kawakami, A. Recent Advances in Pathogenesis, Diagnostic Imaging, and Treatment of Sjögren’s Syndrome. J. Clin. Med. 2024, 13, 6688. [Google Scholar] [CrossRef]
- Yamamoto, K. Pathogenesis of Sjögren’s Syndrome. Autoimmun. Rev. 2003, 2, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s Syndrome: A Systemic Autoimmune Disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef]
- Miyamoto, S.T.; Lendrem, D.W.; Ng, W.-F.; Hackett, K.L.; Valim, V. Managing Fatigue in Patients with Primary Sjögren’s Syndrome: Challenges and Solutions. Open Access Rheumatol. 2019, 11, 77–88. [Google Scholar] [CrossRef]
- Rizzo, C.; Grasso, G.; Destro Castaniti, G.M.; Ciccia, F.; Guggino, G. Primary Sjogren Syndrome: Focus on Innate Immune Cells and Inflammation. Vaccines 2020, 8, 272. [Google Scholar] [CrossRef]
- Chaigne, B.; Lasfargues, G.; Marie, I.; Hüttenberger, B.; Lavigne, C.; Marchand-Adam, S.; Maillot, F.; Diot, E. Primary Sjögren’s Syndrome and Occupational Risk Factors: A Case–Control Study. J. Autoimmun. 2015, 60, 80–85. [Google Scholar] [CrossRef]
- Parisis, D.; Chivasso, C.; Perret, J.; Soyfoo, M.S.; Delporte, C. Current State of Knowledge on Primary Sjögren’s Syndrome, an Autoimmune Exocrinopathy. J. Clin. Med. 2020, 9, 2299. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Z.; Qin, B.; Zhong, R. Primary Sjogren’s Syndrome and Malignancy Risk: A Systematic Review and Meta-Analysis. Ann. Rheum. Dis. 2014, 73, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Nishishinya, M.B.; Pereda, C.A.; Muñoz-Fernández, S.; Pego-Reigosa, J.M.; Rúa-Figueroa, I.; Andreu, J.-L.; Fernández-Castro, M.; Rosas, J.; Loza Santamaría, E. Identification of Lymphoma Predictors in Patients with Primary Sjögren’s Syndrome: A Systematic Literature Review and Meta-Analysis. Rheumatol. Int. 2015, 35, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Akpek, E.K.; Bunya, V.Y.; Saldanha, I.J. Sjögren’s Syndrome: More Than Just Dry Eye. Cornea 2019, 38, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Skarlis, C.; Raftopoulou, S.; Mavragani, C.P. Sjogren’s Syndrome: Recent Updates. J. Clin. Med. 2022, 11, 399. [Google Scholar] [CrossRef]
- Björk, A.; Mofors, J.; Wahren-Herlenius, M. Environmental Factors in the Pathogenesis of Primary Sjögren’s Syndrome. J. Intern. Med. 2020, 287, 475–492. [Google Scholar] [CrossRef]
- Aviña-Zubieta, J.A.; Jansz, M.; Sayre, E.C.; Choi, H.K. The Risk of Deep Venous Thrombosis and Pulmonary Embolism in Primary Sjögren Syndrome: A General Population-Based Study. J. Rheumatol. 2017, 44, 1184–1189. [Google Scholar] [CrossRef]
- Barrientos, R.T.; Godín, F.; Rocha-De-Lossada, C.; Soifer, M.; Sánchez-González, J.-M.; Moreno-Toral, E.; González, A.-L.; Zein, M.; Larco, P.; Mercado, C.; et al. Ophthalmological Approach for the Diagnosis of Dry Eye Disease in Patients with Sjögren’s Syndrome. Life 2022, 12, 1899. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Oliverio, G.W.; Aragona, E.; Inferrera, L.; Severo, A.A.; Alessandrello, F.; Spinella, R.; Postorino, E.I.; Aragona, P. Ophthalmologic Manifestations of Primary Sjögren’s Syndrome. Genes 2021, 12, 365. [Google Scholar] [CrossRef]
- Michaelov, E.; McKenna, C.; Ibrahim, P.; Nayeni, M.; Dang, A.; Mather, R. Sjögren’s Syndrome Associated Dry Eye: Impact on Daily Living and Adherence to Therapy. J. Clin. Med. 2022, 11, 2809. [Google Scholar] [CrossRef]
- Zoukhri, D. Effect of Inflammation on Lacrimal Gland Function. Exp. Eye Res. 2006, 82, 885–898. [Google Scholar] [CrossRef]
- Hyon, J.Y.; Lee, Y.J.; Yun, P.-Y. Management of Ocular Surface Inflammation in Sjögren Syndrome. Cornea 2007, 26, S13–S15. [Google Scholar] [CrossRef]
- Wu, K.Y.; Chen, W.T.; Chu-Bédard, Y.-K.; Patel, G.; Tran, S.D. Management of Sjogren’s Dry Eye Disease—Advances in Ocular Drug Delivery Offering a New Hope. Pharmaceutics 2023, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.I.; Rauz, S. The Eye and Inflammatory Rheumatic Diseases: The Eye and Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis. Best Pract. Res. Clin. Rheumatol. 2016, 30, 802–825. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; de Paiva, C.S. Managing Sjögren’s Syndrome and Non-Sjögren Syndrome Dry Eye with Anti-Inflammatory Therapy. Clin. Ophthalmol. 2014, 8, 1447–1458. [Google Scholar] [CrossRef]
- Harrell, C.R.; Volarevic, A.; Arsenijevic, A.; Djonov, V.; Volarevic, V. Targeted Therapy for Severe Sjogren’s Syndrome: A Focus on Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 13712. [Google Scholar] [CrossRef] [PubMed]
- Kalk, W.; Vissink, A.; Spijkervet, F.; Bootsma, H.; Kallenberg, C.; Amerongen, A. Sialometry and Sialochemistry: Diagnostic Tools for Sjögren’s Syndrome. Ann. Rheum. Dis. 2001, 60, 1110–1116. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Shiboski, C.H.; Shiboski, S.C.; Heidenreich, A.M.; Kitagawa, K.; Zhang, S.; Hamann, S.; Larkin, G.; McNamara, N.A.; Greenspan, J.S.; et al. A Simplified Quantitative Method for Assessing Keratoconjunctivitis Sicca from the Sjögren’s Syndrome International Registry. Am. J. Ophthalmol. 2010, 149, 405–415. [Google Scholar] [CrossRef]
- Lin, H.; Yiu, S.C. Dry Eye Disease: A Review of Diagnostic Approaches and Treatments. Saudi J. Ophthalmol. 2014, 28, 173–181. [Google Scholar] [CrossRef]
- Bjordal, O.; Norheim, K.B.; Rødahl, E.; Jonsson, R.; Omdal, R. Primary Sjögren’s Syndrome and the Eye. Surv. Ophthalmol. 2020, 65, 119–132. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, F.; Cavazzana, I.; Andreoli, L.; Tincani, A. The 2016 Classification Criteria for Primary Sjogren’s Syndrome: What’s New? BMC Med. 2017, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zhou, X.; Yu, S.; Liu, R.; Quek, C.W.N.; Yu, H.; Tay, R.Y.K.; Lin, X.; Feng, Y. The Future of Targeted Treatment of Primary Sjögren’s Syndrome: A Focus on Extra-Glandular Pathology. Int. J. Mol. Sci. 2022, 23, 14135. [Google Scholar] [CrossRef]
- Priani, D.; Muhiddin, H.S.; Sirajuddin, J.; Eka, H.B.; Bahar, B.; Bukhari, A. Effectiveness of Topical Cyclosporin-A 0.1% Compared to Combined Topical Cyclosporin-A 0.1% with Topical Sodium Hyaluronate on Interleukin-6 Levels in the Tears of Patients with Dry Eye Disease. Vision 2023, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Radić, M.; Kolak, E.; Đogaš, H.; Gelemanović, A.; Bučan Nenadić, D.; Vučković, M.; Radić, J. Vitamin D and Sjögren’s Disease: Revealing the Connections—A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 497. [Google Scholar] [CrossRef]
- Kassan, S.S.; Moutsopoulos, H.M. Clinical Manifestations and Early Diagnosis of Sjögren Syndrome. Arch. Intern. Med. 2004, 164, 1275–1284. [Google Scholar] [CrossRef]
- Yura, Y.; Hamada, M. Outline of Salivary Gland Pathogenesis of Sjögren’s Syndrome and Current Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 11179. [Google Scholar] [CrossRef]
- D’Souza, S.; Vaidya, T.; Nair, A.P.; Shetty, R.; Kumar, N.R.; Bisht, A.; Panigrahi, T.; J, T.S.; Khamar, P.; Dickman, M.M.; et al. Altered Ocular Surface Health Status and Tear Film Immune Profile Due to Prolonged Daily Mask Wear in Health Care Workers. Biomedicines 2022, 10, 1160. [Google Scholar] [CrossRef]
- Schlegel, I.; De Goüyon Matignon de Pontourade, C.M.F.; Lincke, J.-B.; Keller, I.; Zinkernagel, M.S.; Zysset-Burri, D.C. The Human Ocular Surface Microbiome and Its Associations with the Tear Proteome in Dry Eye Disease. Int. J. Mol. Sci. 2023, 24, 14091. [Google Scholar] [CrossRef]
- Hughes, G.K.; Miszkiel, K.A. Imaging of the Lacrimal Gland. Semin. Ultrasound CT MRI 2006, 27, 476–491. [Google Scholar] [CrossRef]
- Muntean, D.D.; Bădărînză, M.; Ștefan, P.A.; Lenghel, M.L.; Rusu, G.M.; Csutak, C.; Coroian, P.A.; Lupean, R.A.; Fodor, D. The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 10051. [Google Scholar] [CrossRef]
- Conrady, C.D.; Joos, Z.P.; Patel, B.C.K. Review: The Lacrimal Gland and Its Role in Dry Eye. J. Ophthalmol. 2016, 2016, 7542929. [Google Scholar] [CrossRef] [PubMed]
- Hat, K.; Planinić, A.; Ježek, D.; Kaštelan, S. Expression of Androgen and Estrogen Receptors in the Human Lacrimal Gland. Int. J. Mol. Sci. 2023, 24, 5609. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kahale, F.; Naderi, A.; Surico, P.L.; Yin, J.; Dohlman, T.; Chen, Y.; Dana, R. Therapeutic Effects of Stimulating the Melanocortin Pathway in Regulating Ocular Inflammation and Cell Death. Biomolecules 2024, 14, 169. [Google Scholar] [CrossRef]
- Delcroix, V.; Mauduit, O.; Yang, M.; Srivastava, A.; Umazume, T.; de Paiva, C.S.; Shestopalov, V.I.; Dartt, D.A.; Makarenkova, H.P. Lacrimal Gland Epithelial Cells Shape Immune Responses through the Modulation of Inflammasomes and Lipid Metabolism. Int. J. Mol. Sci. 2023, 24, 4309. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C. What Causes Dryness in Sjögren’s Syndrome Patients and How Can It Be Targeted? Expert. Rev. Clin. Immunol. 2014, 10, 425–427. [Google Scholar] [CrossRef]
- Fan, Q.; Yan, R.; Li, Y.; Lu, L.; Liu, J.; Li, S.; Fu, T.; Xue, Y.; Liu, J.; Li, Z. Exploring Immune Cell Diversity in the Lacrimal Glands of Healthy Mice: A Single-Cell RNA-Sequencing Atlas. Int. J. Mol. Sci. 2024, 25, 1208. [Google Scholar] [CrossRef]
- Murashima, A.d.A.B.; Sant’Ana, A.M.S.; Faustino-Barros, J.F.; Machado Filho, E.B.; da Silva, L.C.M.; Fantucci, M.Z.; Módulo, C.M.; Chahud, F.; Garcia, D.M.; Rocha, E.M. Exorbital Lacrimal Gland Ablation and Regrafting Induce Inflammation but Not Regeneration or Dry Eye. Int. J. Mol. Sci. 2024, 25, 8318. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Peck, A.B. Unraveling the Pathophysiology of Sjogren Syndrome-Associated Dry Eye Disease. Ocul. Surf. 2009, 7, 11–27. [Google Scholar] [CrossRef]
- Lemos, C.N.; da Silva, L.E.C.M.; Faustino, J.F.; Fantucci, M.Z.; Murashima, A.d.A.B.; Adriano, L.; Alves, M.; Rocha, E.M. Oxidative Stress in the Protection and Injury of the Lacrimal Gland and the Ocular Surface: Are There Perspectives for Therapeutics? Front. Cell Dev. Biol. 2022, 10, 824726. [Google Scholar] [CrossRef]
- Nakamura, H.; Shimizu, T.; Takagi, Y.; Takahashi, Y.; Horai, Y.; Nakashima, Y.; Sato, S.; Shiraishi, H.; Nakamura, T.; Fukuoka, J.; et al. Reevaluation for Clinical Manifestations of HTLV-I-Seropositive Patients with Sjögren’s Syndrome. BMC Musculoskelet. Disord. 2015, 16, 335. [Google Scholar] [CrossRef]
- Yoo, S.-A.; Kwok, S.-K.; Kim, W.-U. Proinflammatory Role of Vascular Endothelial Growth Factor in the Pathogenesis of Rheumatoid Arthritis: Prospects for Therapeutic Intervention. Mediat. Inflamm. 2008, 2008, 129873. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Lim, G.; Lee, R.; Tong, L. A Systematic Review of Tear Vascular Endothelial Growth Factor and External Eye Diseases. Int. J. Mol. Sci. 2024, 25, 1369. [Google Scholar] [CrossRef] [PubMed]
- Nordmark, G.; Alm, G.V.; Rönnblom, L. Mechanisms of Disease: Primary Sjögren’s Syndrome and the Type I Interferon System. Nat. Clin. Pract. Rheumatol. 2006, 2, 262–269. [Google Scholar] [CrossRef]
- Del Papa, N.; Minniti, A.; Lorini, M.; Carbonelli, V.; Maglione, W.; Pignataro, F.; Montano, N.; Caporali, R.; Vitali, C. The Role of Interferons in the Pathogenesis of Sjögren’s Syndrome and Future Therapeutic Perspectives. Biomolecules 2021, 11, 251. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, J.; Chen, W. Type I Interferons in the Pathogenesis and Treatment of Sjögren’s Syndrome: An Update. Rheumatology 2022, 9, 59–69. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of Type-I- and Type-II-Interferon-Mediated Signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Rönnblom, L.; Eloranta, M.-L. The Interferon Signature in Autoimmune Diseases. Curr. Opin. Rheumatol. 2013, 25, 248–253. [Google Scholar] [CrossRef]
- Bedei, I.A.; Kniess, D.; Keil, C.; Wolter, A.; Schenk, J.; Sachs, U.J.; Axt-Fliedner, R. Monitoring of Women with Anti-Ro/SSA and Anti-La/SSB Antibodies in Germany—Status Quo and Intensified Monitoring Concepts. J. Clin. Med. 2024, 13, 1142. [Google Scholar] [CrossRef]
- Parisis, D.; Sarrand, J.; Cabrol, X.; Delporte, C.; Soyfoo, M.S. Clinical Profile of Patients with Primary Sjögren’s Syndrome with Non-Identified Antinuclear Autoantibodies. Diagnostics 2024, 14, 935. [Google Scholar] [CrossRef]
- Massa, C.; Wang, Y.; Marr, N.; Seliger, B. Interferons and Resistance Mechanisms in Tumors and Pathogen-Driven Diseases—Focus on the Major Histocompatibility Complex (MHC) Antigen Processing Pathway. Int. J. Mol. Sci. 2023, 24, 6736. [Google Scholar] [CrossRef] [PubMed]
- Bhujel, B.; Oh, S.-H.; Kim, C.-M.; Yoon, Y.-J.; Chung, H.-S.; Ye, E.-A.; Lee, H.; Kim, J.-Y. Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review. Bioengineering 2024, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Honing, D.Y.; Luiten, R.M.; Matos, T.R. Regulatory T Cell Dysfunction in Autoimmune Diseases. Int. J. Mol. Sci. 2024, 25, 7171. [Google Scholar] [CrossRef]
- Lapenta, C.; Gabriele, L.; Santini, S.M. IFN-Alpha-Mediated Differentiation of Dendritic Cells for Cancer Immunotherapy: Advances and Perspectives. Vaccines 2020, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, M.; Shikama, Y.; Furukawa, M.; Arakaki, R.; Ishimaru, N.; Matsushita, K. Chemokines Up-Regulated in Epithelial Cells Control Senescence-Associated T Cell Accumulation in Salivary Glands of Aged and Sjögren’s Syndrome Model Mice. Int. J. Mol. Sci. 2021, 22, 2302. [Google Scholar] [CrossRef]
- Witas, R.; Gupta, S.; Nguyen, C.Q. Contributions of Major Cell Populations to Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 3057. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ture, H.Y.; Lee, E.J.; Jang, J.A.; Kim, G.; Nam, E.J. Syndecan-1 Plays a Role in the Pathogenesis of Sjögren’s Disease by Inducing B-Cell Chemotaxis through CXCL13–Heparan Sulfate Interaction. Int. J. Mol. Sci. 2024, 25, 9375. [Google Scholar] [CrossRef]
- Bu, J.; Liu, Y.; Zhang, R.; Lin, S.; Zhuang, J.; Sun, L.; Zhang, L.; He, H.; Zong, R.; Wu, Y.; et al. Potential New Target for Dry Eye Disease—Oxidative Stress. Antioxidants 2024, 13, 422. [Google Scholar] [CrossRef]
- Peng, J.; Feinstein, D.; DeSimone, S.; Gentile, P. A Review of the Tear Film Biomarkers Used to Diagnose Sjogren’s Syndrome. Int. J. Mol. Sci. 2024, 25, 10380. [Google Scholar] [CrossRef]
- Dammak, A.; Pastrana, C.; Martin-Gil, A.; Carpena-Torres, C.; Peral Cerda, A.; Simovart, M.; Alarma, P.; Huete-Toral, F.; Carracedo, G. Oxidative Stress in the Anterior Ocular Diseases: Diagnostic and Treatment. Biomedicines 2023, 11, 292. [Google Scholar] [CrossRef]
- De Oliveira, F.R.; Fantucci, M.Z.; Adriano, L.; Valim, V.; Cunha, T.M.; Louzada-Junior, P.; Rocha, E.M. Neurological and Inflammatory Manifestations in Sjögren’s Syndrome: The Role of the Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2018, 19, 3953. [Google Scholar] [CrossRef] [PubMed]
- Módis, L.V.; Aradi, Z.; Horváth, I.F.; Bencze, J.; Papp, T.; Emri, M.; Berényi, E.; Bugán, A.; Szántó, A. Central Nervous System Involvement in Primary Sjögren’s Syndrome: Narrative Review of MRI Findings. Diagnostics 2023, 13, 14. [Google Scholar] [CrossRef]
- Mihai, A.; Chitimus, D.M.; Jurcut, C.; Blajut, F.C.; Opris-Belinski, D.; Caruntu, C.; Ionescu, R.; Caruntu, A. Comparative Analysis of Hematological and Immunological Parameters in Patients with Primary Sjögren’s Syndrome and Peripheral Neuropathy. J. Clin. Med. 2023, 12, 3672. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Han, M.; Zhu, X.; Xiao, F.; Huang, E.; Che, N.; Tang, X.; Zou, H.; Jiang, Q.; Lu, L. The Multiple Roles of B Cells in the Pathogenesis of Sjögren’s Syndrome. Front. Immunol. 2021, 12, 684999. [Google Scholar] [CrossRef]
- Bruno, D.; Tolusso, B.; Lugli, G.; Di Mario, C.; Petricca, L.; Perniola, S.; Bui, L.; Benvenuto, R.; Ferraccioli, G.; Alivernini, S.; et al. B-Cell Activation Biomarkers in Salivary Glands Are Related to Lymphomagenesis in Primary Sjögren’s Disease: A Pilot Monocentric Exploratory Study. Int. J. Mol. Sci. 2024, 25, 3259. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnezhad, L.; Shekarkar Azgomi, M.; La Manna, M.P.; Guggino, G.; Botta, C.; Dieli, F.; Caccamo, N. B-Cell Receptor Signaling Is Thought to Be a Bridge between Primary Sjogren Syndrome and Diffuse Large B-Cell Lymphoma. Int. J. Mol. Sci. 2023, 24, 8385. [Google Scholar] [CrossRef]
- Ambrus, J.L.; Suresh, L.; Peck, A. Multiple Roles for B-Lymphocytes in Sjogren’s Syndrome. J. Clin. Med. 2016, 5, 87. [Google Scholar] [CrossRef]
- Stergiou, I.E.; Poulaki, A.; Voulgarelis, M. Pathogenetic Mechanisms Implicated in Sjögren’s Syndrome Lymphomagenesis: A Review of the Literature. J. Clin. Med. 2020, 9, 3794. [Google Scholar] [CrossRef]
- Stern, M.E.; Schaumburg, C.S.; Dana, R.; Calonge, M.; Niederkorn, J.Y.; Pflugfelder, S.C. Autoimmunity at the Ocular Surface: Pathogenesis and Regulation. Mucosal Immunol. 2010, 3, 425–442. [Google Scholar] [CrossRef]
- Reyes, J.L.; Vannan, D.T.; Eksteen, B.; Avelar, I.J.; Rodríguez, T.; González, M.I.; Mendoza, A.V. Innate and Adaptive Cell Populations Driving Inflammation in Dry Eye Disease. Mediat. Inflamm. 2018, 2018, 2532314. [Google Scholar] [CrossRef]
- Michée-Cospolite, M.; Boudigou, M.; Grasseau, A.; Simon, Q.; Mignen, O.; Pers, J.-O.; Cornec, D.; Le Pottier, L.; Hillion, S. Molecular Mechanisms Driving IL-10- Producing B Cells Functions: STAT3 and c-MAF as Underestimated Central Key Regulators? Front. Immunol. 2022, 13, 818814. [Google Scholar] [CrossRef]
- Chivasso, C.; Sarrand, J.; Perret, J.; Delporte, C.; Soyfoo, M.S. The Involvement of Innate and Adaptive Immunity in the Initiation and Perpetuation of Sjögren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 658. [Google Scholar] [CrossRef]
- Blinova, V.G.; Vasilyev, V.I.; Rodionova, E.B.; Zhdanov, D.D. The Role of Regulatory T Cells in the Onset and Progression of Primary Sjögren’s Syndrome. Cells 2023, 12, 1359. [Google Scholar] [CrossRef]
- Vílchez-Oya, F.; Balastegui Martin, H.; García-Martínez, E.; Corominas, H. Not All Autoantibodies Are Clinically Relevant. Classic and Novel Autoantibodies in Sjögren’s Syndrome: A Critical Review. Front. Immunol. 2022, 13, 1003054. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, G.; Gury, A.; Jeannin, P.; Chevailler, A.; Lozac’h, P.; Reynier, P.; Lavigne, C.; Lacout, C.; Vinatier, E. Discordant Predictions of Extraglandular Involvement in Primary Sjögren’s Syndrome According to the Anti-SSA/Ro60 Antibodies Detection Assay in a Cohort Study. J. Clin. Med. 2022, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Veenbergen, S.; Kozmar, A.; van Daele, P.L.A.; Schreurs, M.W.J. Autoantibodies in Sjögren’s Syndrome and Its Classification Criteria. J. Transl. Autoimmun. 2022, 5, 100138. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Tzioufas, A.G. A Comprehensive Review of Autoantibodies in Primary Sjögren’s Syndrome: Clinical Phenotypes and Regulatory Mechanisms. J. Autoimmun. 2014, 51, 67–74. [Google Scholar] [CrossRef]
- Mahla, R.S.; Jones, E.L.; Dustin, L.B. Ro60—Roles in RNA Processing, Inflammation, and Rheumatic Autoimmune Diseases. Int. J. Mol. Sci. 2024, 25, 7705. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, A.; Kurien, B.T.; Scofield, R.H. Autoantibodies in Sjögren’s Syndrome. Rheum. Dis. Clin. N. Am. 2016, 42, 419–434. [Google Scholar] [CrossRef]
- Tiwari, V.; Jandu, J.S.; Bergman, M.J. Rheumatoid Factor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Chen, M.; Daha, M.R.; Kallenberg, C.G.M. The Complement System in Systemic Autoimmune Disease. J. Autoimmun. 2010, 34, J276–J286. [Google Scholar] [CrossRef]
- Itty, S.; Pulido, J.S.; Bakri, S.J.; Baratz, K.H.; Matteson, E.L.; Hodge, D.O. Anti-Cyclic Citrullinated Peptide, Rheumatoid Factor, and Ocular Symptoms Typical of Rheumatoid Arthritis. Trans. Am. Ophthalmol. Soc. 2008, 106, 75–81, discussion 81–83. [Google Scholar] [PubMed]
- Kim, S.-M.; Park, E.; Lee, J.-H.; Lee, S.-H.; Kim, H.-R. The Clinical Significance of Anti-Cyclic Citrullinated Peptide Antibody in Primary Sjögren Syndrome. Rheumatol. Int. 2012, 32, 3963–3967. [Google Scholar] [CrossRef]
- Wu, K.Y.; Serhan, O.; Faucher, A.; Tran, S.D. Advances in Sjögren’s Syndrome Dry Eye Diagnostics: Biomarkers and Biomolecules beyond Clinical Symptoms. Biomolecules 2024, 14, 80. [Google Scholar] [CrossRef]
- Lee, Y.J. Is the Anti-Centromere Antibody a Marker for a Distinct Subset of Polyautoimmunity in Sjögren’s Syndrome? Korean J. Intern. Med. 2021, 36, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nishihata, S.; Nakamura, H.; Takagi, Y.; Sumi, M.; Kawakami, A. Anti-Centromere Antibody Positivity Is an Independent Variable Associated with Salivary Gland Ultrasonography Score in Sjögren’s Syndrome. Sci. Rep. 2024, 14, 5303. [Google Scholar] [CrossRef]
- Cha, S.; Mona, M.; Lee, K.E.; Kim, D.H.; Han, K. MicroRNAs in Autoimmune Sjögren’s Syndrome. Genom. Inform. 2018, 16, e19. [Google Scholar] [CrossRef] [PubMed]
- Pauley, K.M.; Stewart, C.M.; Gauna, A.E.; Dupre, L.C.; Kuklani, R.; Chan, A.L.; Pauley, B.A.; Reeves, W.H.; Chan, E.K.L.; Cha, S. Altered miR-146a Expression in Sjögren’s Syndrome and Its Functional Role in Innate Immunity. Eur. J. Immunol. 2011, 41, 2029–2039. [Google Scholar] [CrossRef]
- Xu, W.-D.; Feng, S.-Y.; Huang, A.-F. Role of miR-155 in Inflammatory Autoimmune Diseases: A Comprehensive Review. Inflamm. Res. 2022, 71, 1501–1517. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Zhang, L.; Zhao, M.; Huang, H. Decreased microRNA-181a and -16 Expression Levels in the Labial Salivary Glands of Sjögren Syndrome Patients. Exp. Ther. Med. 2018, 15, 426–432. [Google Scholar] [CrossRef]
- Jin, L.; Dai, M.; Li, C.; Wang, J.; Wu, B. Risk Factors for Primary Sjögren’s Syndrome: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2023, 42, 327–338. [Google Scholar] [CrossRef]
- Li, X.X.; Maitiyaer, M.; Tan, Q.; Huang, W.H.; Liu, Y.; Liu, Z.P.; Wen, Y.Q.; Zheng, Y.; Chen, X.; Chen, R.L.; et al. Emerging Biologic Frontiers for Sjogren’s Syndrome: Unveiling Novel Approaches with Emphasis on Extra Glandular Pathology. Front. Pharmacol. 2024, 15, 1377055. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius, G.E.; Björk, A.; Wahren-Herlenius, M. Genetics and Epigenetics of Primary Sjögren Syndrome: Implications for Future Therapies. Nat. Rev. Rheumatol. 2023, 19, 288–306. [Google Scholar] [CrossRef] [PubMed]
- St Clair, E.W.; Baer, A.N.; Ng, W.-F.; Noaiseh, G.; Baldini, C.; Tarrant, T.K.; Papas, A.; Devauchelle-Pensec, V.; Wang, L.; Xu, W.; et al. CD40 Ligand Antagonist Dazodalibep in Sjögren’s Disease: A Randomized, Double-Blinded, Placebo-Controlled, Phase 2 Trial. Nat. Med. 2024, 30, 1583–1592. [Google Scholar] [CrossRef]
- Mariette, X.; Barone, F.; Baldini, C.; Bootsma, H.; Clark, K.L.; De Vita, S.; Gardner, D.H.; Henderson, R.B.; Herdman, M.; Lerang, K.; et al. A Randomized, Phase II Study of Sequential Belimumab and Rituximab in Primary Sjögren’s Syndrome. JCI Insight 2022, 7, e163030. [Google Scholar] [CrossRef]
- Nakamura, H.; Shimizu, T.; Kawakami, A. Role of Viral Infections in the Pathogenesis of Sjögren’s Syndrome: Different Characteristics of Epstein-Barr Virus and HTLV-1. J. Clin. Med. 2020, 9, 1459. [Google Scholar] [CrossRef]
- Otsuka, K.; Sato, M.; Tsunematsu, T.; Ishimaru, N. Virus Infections Play Crucial Roles in the Pathogenesis of Sjögren’s Syndrome. Viruses 2022, 14, 1474. [Google Scholar] [CrossRef] [PubMed]
- Bandinelli, F.; Pagano, M.; Vallecoccia, M.S. Post-COVID-19 and Post-COVID-19 Vaccine Arthritis, Polymyalgia Rheumatica and Horton’s Arteritis: A Single-Center Assessment of Clinical, Serological, Genetic, and Ultrasonographic Biomarkers. J. Clin. Med. 2023, 12, 7563. [Google Scholar] [CrossRef]
- Shen, Y.; Voigt, A.; Goranova, L.; Abed, M.; Kleiner, D.E.; Maldonado, J.O.; Beach, M.; Pelayo, E.; Chiorini, J.A.; Craft, W.F.; et al. Evidence of a Sjögren’s Disease-like Phenotype Following COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- Bakhsh, R.; Dairi, K.; Almadabgy, E.; Albiladi, A.; Gamal, L.; Almatrafi, D.; AlShariff, F.; Alsefri, A. New Onset of Neuro-Sjögren’s Syndrome Nine Months After the Third COVID-19 Vaccine Dose: A Case Report. Cureus 2024, 16, e69562. [Google Scholar] [CrossRef]
- Sharif, K.; Amital, H. Heavy Metals in Autoimmune Diseases: Too Much Noise in Autoimmunity. In Autoimmune Disorders; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 201–223. ISBN 978-1-119-85843-0. [Google Scholar]
- Lee, C.-P.; Hsu, P.-Y.; Su, C.-C. Increased Prevalence of Sjogren’s Syndrome in Where Soils Contain High Levels of Chromium. Sci. Total Environ. 2019, 657, 1121–1126. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, S.; Jin, H.; Fan, C.; Liao, K.; Zhang, S.; Xue, J. Relationship Between Perfluoroalkyl Acids in Human Serum and Sjogren’s Syndrome: A Case–Control Study of Populations in Hangzhou, China. Toxics 2024, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.; Turesson, C.; Mandl, T.; Jacobsson, L.; Theander, E. Cigarette Smoking and the Risk of Primary Sjögren’s Syndrome: A Nested Case Control Study. Arthritis Res. Ther. 2017, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Mieliauskaitė, D.; Kontenis, V. Insights into Microbiota in Sjögren’s Syndrome. Medicina 2023, 59, 1661. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Xia, W. Causal Relationship Between Sjögren’s Syndrome and Gut Microbiota: A Two-Sample Mendelian Randomization Study. Biomedicines 2024, 12, 2378. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Laborda-Illanes, A.; Plaza-Andrades, I.; Membrillo del Pozo, A.; Villarrubia Cuadrado, A.; Rodríguez Calvo de Mora, M.; Leiva-Gea, I.; Sanchez-Alcoholado, L.; Queipo-Ortuño, M.I. Connection between the Gut Microbiome, Systemic Inflammation, Gut Permeability and FOXP3 Expression in Patients with Primary Sjögren’s Syndrome. Int. J. Mol. Sci. 2020, 21, 8733. [Google Scholar] [CrossRef]
- Psianou, K.; Panagoulias, I.; Papanastasiou, A.D.; de Lastic, A.-L.; Rodi, M.; Spantidea, P.I.; Degn, S.E.; Georgiou, P.; Mouzaki, A. Clinical and Immunological Parameters of Sjögren’s Syndrome. Autoimmun. Rev. 2018, 17, 1053–1064. [Google Scholar] [CrossRef]
- Sandhya, P.; Jeyaseelan, L.; Scofield, R.H.; Danda, D. Clinical Characteristics and Outcome of Primary Sjogren’s Syndrome: A Large Asian Indian Cohort. Open Rheumatol. J. 2014, 9, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lodba, A.; Ancuta, C.; Tatarciuc, D.; Ghiorghe, A.; Lodba, L.-O.; Iordache, C. Comparative Analysis of Glandular and Extraglandular Manifestations in Primary and Secondary Sjögren’s Syndrome: A Study in Two Academic Centers in North-East Romania. Diagnostics 2024, 14, 2367. [Google Scholar] [CrossRef]
- Mathews, P.M.; Hahn, S.; Hessen, M.; Kim, J.; Grader-Beck, T.; Birnbaum, J.; Baer, A.N.; Akpek, E.K. Ocular Complications of Primary Sjögren Syndrome in Men. Am. J. Ophthalmol. 2015, 160, 447–452.e1. [Google Scholar] [CrossRef]
- Virdee, S.; Greenan-Barrett, J.; Ciurtin, C. A Systematic Review of Primary Sjögren’s Syndrome in Male and Paediatric Populations. Clin. Rheumatol. 2017, 36, 2225–2236. [Google Scholar] [CrossRef]
- La Rocca, G.; Ferro, F.; Sambataro, G.; Elefante, E.; Fonzetti, S.; Fulvio, G.; Navarro, I.C.; Mosca, M.; Baldini, C. Primary-Sjögren’s-Syndrome-Related Interstitial Lung Disease: A Clinical Review Discussing Current Controversies. J. Clin. Med. 2023, 12, 3428. [Google Scholar] [CrossRef] [PubMed]
- Flament, T.; Bigot, A.; Chaigne, B.; Henique, H.; Diot, E.; Marchand-Adam, S. Pulmonary Manifestations of Sjögren’s Syndrome. Eur. Respir. Rev. 2016, 25, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Z.; Qiu, M.; Ye, Q. Risk Factors for Primary Sjögren Syndrome-Associated Interstitial Lung Disease. J. Thorac. Dis. 2018, 10, 2108–2117. [Google Scholar] [CrossRef]
- Stojan, G.; Baer, A.N.; Danoff, S.K. Pulmonary Manifestations of Sjögren’s Syndrome. Curr. Allergy Asthma Rep. 2013, 13, 354–360. [Google Scholar] [CrossRef]
- Enomoto, Y.; Takemura, T.; Hagiwara, E.; Iwasawa, T.; Okudela, K.; Yanagawa, N.; Baba, T.; Sakai, F.; Fukuda, Y.; Nagaoka, S.; et al. Features of Usual Interstitial Pneumonia in Patients with Primary Sjögren’s Syndrome Compared with Idiopathic Pulmonary Fibrosis. Respir. Investig. 2014, 52, 227–235. [Google Scholar] [CrossRef][Green Version]
- Kim, J.-Y.; Park, S.-H.; Kim, S.-K.; Hyun, D.-S.; Kum, Y.S.; Jung, K.-J.; Choe, J.-Y. Lymphocytic Interstitial Pneumonia in Primary Sjögren’s Syndrome: A Case Report. Korean J. Intern. Med. 2011, 26, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Young, L. Lymphocytic Interstitial Pneumonia as a Manifestation of SLE and Secondary Sjogren’s Syndrome. BMJ Case Rep. 2013, 2013, bcr2013009598. [Google Scholar] [CrossRef]
- Ioannou, S.; Toya, S.P.; Tomos, P.; Tzelepis, G.E. Cryptogenic Organizing Pneumonia Associated with Primary Sjogren’s Syndrome. Rheumatol. Int. 2008, 28, 1053–1055. [Google Scholar] [CrossRef]
- Centala, S.; Park, J.H.; Girnita, D. Sjogren’s Syndrome Presenting with Solely Cutaneous Features. Diagnostics 2021, 11, 1260. [Google Scholar] [CrossRef]
- Ilzkovitz, M.; Kayembe, E.E.; Geers, C.; Pozdzik, A. Kidney Stones, Proteinuria and Renal Tubular Metabolic Acidosis: What Is the Link? Healthcare 2022, 10, 836. [Google Scholar] [CrossRef]
- Bouchalova, K.; Flögelova, H.; Horak, P.; Civrny, J.; Mlcak, P.; Pink, R.; Michalek, J.; Camborova, P.; Mikulkova, Z.; Kriegova, E. Juvenile Primary Sjögren Syndrome in a 15-Year-Old Boy with Renal Involvement: A Case Report and Review of the Literature. Diagnostics 2024, 14, 258. [Google Scholar] [CrossRef]
- Garcia, D.M.; Reis de Oliveira, F.; Módulo, C.M.; Faustino, J.; Barbosa, A.P.; Alves, M.; Rocha, E.M. Is Sjögren’s Syndrome Dry Eye Similar to Dry Eye Caused by Other Etiologies? Discriminating Different Diseases by Dry Eye Tests. PLoS ONE 2018, 13, e0208420. [Google Scholar] [CrossRef]
- Saldanha, I.J.; Bunya, V.Y.; McCoy, S.S.; Makara, M.; Baer, A.N.; Akpek, E.K. Ocular Manifestations and Burden Related to Sjögren Syndrome: Results of a Patient Survey. Am. J. Ophthalmol. 2020, 219, 40–48. [Google Scholar] [CrossRef]
- Friedlaender, M.H. Ocular Manifestations of Sjögren’s Syndrome: Keratoconjunctivitis Sicca. Rheum. Dis. Clin. N. Am. 1992, 18, 591–608. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.H.; Yoon, C.H.; Kim, M.K.; Oh, J.Y. Infectious Keratitis in Patients with Ocular Sjögren’s Syndrome. Korean J. Ophthalmol. 2022, 36, 407–412. [Google Scholar] [CrossRef]
- Singh, S.; Chaudhary, S.; Das, A.V.; Basu, S. Presentation, Aetiology and Outcomes of Corneal Ulceration in Sjogren’s Syndrome. Eye 2023, 37, 3217–3220. [Google Scholar] [CrossRef]
- Dart, J.K. Predisposing Factors in Microbial Keratitis: The Significance of Contact Lens Wear. Br. J. Ophthalmol. 1988, 72, 926–930. [Google Scholar] [CrossRef]
- Trojacka, E.; Izdebska, J.; Szaflik, J.; Przybek-Skrzypecka, J. The Ocular Microbiome: Micro-Steps Towards Macro-Shift in Targeted Treatment? A Comprehensive Review. Microorganisms 2024, 12, 2232. [Google Scholar] [CrossRef]
- Ni, N.; Srinivasan, M.; McLeod, S.D.; Acharya, N.R.; Lietman, T.M.; Rose-Nussbaumer, J. Use of Adjunctive Topical Corticosteroids in Bacterial Keratitis. Curr. Opin. Ophthalmol. 2016, 27, 353–357. [Google Scholar] [CrossRef]
- Jan, R.-L.; Ho, C.-H.; Wang, J.-J.; Tseng, S.-H.; Chang, Y.-S. Associations between Sjögren Syndrome, Sociodemographic Factors, Comorbid Conditions, and Scleritis in a Taiwanese Population-Based Study. J. Pers. Med. 2022, 12, 105. [Google Scholar] [CrossRef]
- Nagy, G.; Czirják, L.; Kumánovics, G. Patients with Systemic Sclerosis with and without Overlap Syndrome Show Similar Microvascular Abnormalities. Diagnostics 2021, 11, 1606. [Google Scholar] [CrossRef]
- Wakefield, D.; Di Girolamo, N.; Thurau, S.; Wildner, G.; McCluskey, P. Scleritis: Immunopathogenesis and Molecular Basis for Therapy. Prog. Retin. Eye Res. 2013, 35, 44–62. [Google Scholar] [CrossRef]
- Bak, E.; Yang, H.K.; Hwang, J.-M. Optic Neuropathy Associated with Primary Sjögren’s Syndrome: A Case Series. Optom. Vis. Sci. 2017, 94, 519–526. [Google Scholar] [CrossRef]
- Tang, W.-Q.; Wei, S.-H. Primary Sjögren’s Syndrome Related Optic Neuritis. Int. J. Ophthalmol. 2013, 6, 888–891. [Google Scholar] [CrossRef]
- Kaufhold, F.; Zimmermann, H.; Schneider, E.; Ruprecht, K.; Paul, F.; Oberwahrenbrock, T.; Brandt, A.U. Optic Neuritis Is Associated with Inner Nuclear Layer Thickening and Microcystic Macular Edema Independently of Multiple Sclerosis. PLoS ONE 2013, 8, e71145. [Google Scholar] [CrossRef]
- Wu, K.Y.; Kulbay, M.; Tanasescu, C.; Jiao, B.; Nguyen, B.H.; Tran, S.D. An Overview of the Dry Eye Disease in Sjögren’s Syndrome Using Our Current Molecular Understanding. Int. J. Mol. Sci. 2023, 24, 1580. [Google Scholar] [CrossRef]
- Srivastava, A.; Makarenkova, H.P. Innate Immunity and Biological Therapies for the Treatment of Sjögren’s Syndrome. Int. J. Mol. Sci. 2020, 21, 9172. [Google Scholar] [CrossRef]
- Akpek, E.K.; Lindsley, K.B.; Adyanthaya, R.S.; Swamy, R.; Baer, A.N.; McDonnell, P.J. Treatment of Sjögren’s Syndrome–Associated Dry Eye: An Evidence-Based Review. Ophthalmology 2011, 118, 1242–1252. [Google Scholar] [CrossRef]
- Almulhim, A. Therapeutic Targets in the Management of Dry Eye Disease Associated with Sjögren’s Syndrome: An Updated Review of Current Insights and Future Perspectives. J. Clin. Med. 2024, 13, 1777. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Lee, W.-Y.; Kim, Y.; Hong, Y. A Meta-Analysis of the Efficacy of Hyaluronic Acid Eye Drops for the Treatment of Dry Eye Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 2383. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, Chemokine, and Growth Factor Profile of Platelet-Rich Plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Metheetrairut, C.; Ngowyutagon, P.; Tunganuntarat, A.; Khowawisetsut, L.; Kittisares, K.; Prabhasawat, P. Comparison of Epitheliotrophic Factors in Platelet-Rich Plasma versus Autologous Serum and Their Treatment Efficacy in Dry Eye Disease. Sci. Rep. 2022, 12, 8906. [Google Scholar] [CrossRef]
- Franchini, M.; Cruciani, M.; Mengoli, C.; Marano, G.; Capuzzo, E.; Pati, I.; Masiello, F.; Veropalumbo, E.; Pupella, S.; Vaglio, S.; et al. Serum Eye Drops for the Treatment of Ocular Surface Diseases: A Systematic Review and Meta-Analysis. Blood Transfus. 2019, 17, 200–209. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Hodge, C.; Hoque, M.; Petsoglou, C.; Sutton, G. Human Platelets and Derived Products in Treating Ocular Surface Diseases—A Systematic Review. Clin. Ophthalmol. 2020, 14, 3195–3210. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Angelina, A.; Marrone, M.; Stark, W.J.; Akpek, E.K. Autologous Serum Eye Drops for Dry Eye. Cochrane Database Syst. Rev. 2017, 2, CD009327. [Google Scholar] [CrossRef]
- Wang, K.; Shi, L.; Yu, Z.; Deng, Z.; He, A.; Li, S.; Liu, L. Cyclosporine A Suppresses the Activation of the Th17 Cells in Patients with Primary Sjögren’s Syndrome. Iran. J. Allergy Asthma Immunol. 2015, 14, 198–207. [Google Scholar]
- de Paiva, C.S.; Pflugfelder, S.C.; Ng, S.M.; Akpek, E.K. Topical Cyclosporine A Therapy for Dry Eye Syndrome. Cochrane Database Syst. Rev. 2019, 9, CD010051. [Google Scholar] [CrossRef]
- Jung, H.H.; Ji, Y.S.; Sung, M.S.; Kim, K.K.; Yoon, K.C. Long-Term Outcome of Treatment with Topical Corticosteroids for Severe Dry Eye Associated with Sjögren’s Syndrome. Chonnam Med. J. 2015, 51, 26–32. [Google Scholar] [CrossRef]
- Yang, D.-H.; Wang, Y.-H.; Pan, L.-F.; Wei, J.C.-C. Cardiovascular Protection of Hydroxychloroquine in Patients with Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 3469. [Google Scholar] [CrossRef]
- Avalos-Salgado, F.A.; Gonzalez-Lopez, L.; Gonzalez-Vazquez, S.; Ponce-Guarneros, J.M.; Santiago-Garcia, A.P.; Amaya-Cabrera, E.L.; Arellano-Cervantes, R.; Gutiérrez-Aceves, J.A.; Alcaraz-Lopez, M.F.; Nava-Valdivia, C.A.; et al. Risk Factors Associated with Adverse Events Leading to Methotrexate Withdrawal in Elderly Rheumatoid Arthritis Patients: A Retrospective Cohort Study. J. Clin. Med. 2024, 13, 1863. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Moutsopoulos, H.M. Sjögren’s Syndrome: Old and New Therapeutic Targets. J. Autoimmun. 2020, 110, 102364. [Google Scholar] [CrossRef] [PubMed]
- Kiripolsky, J.; McCabe, L.G.; Kramer, J.M. Innate Immunity in Sjögren’s Syndrome. Clin. Immunol. 2017, 182, 4–13. [Google Scholar] [CrossRef]
- Lee, A.S.; Scofield, R.H.; Hammitt, K.M.; Gupta, N.; Thomas, D.E.; Moua, T.; Ussavarungsi, K.; Clair, E.W.S.; Meehan, R.; Dunleavy, K.; et al. Consensus Guidelines for Evaluation and Management of Pulmonary Disease in Sjögren’s. CHEST 2021, 159, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, J. Mycophenolate for the Treatment of Primary Sjögren’s Syndrome. J. Transl. Int. Med. 2020, 8, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yan, Q.; Gong, Y.; Cai, C.-S.; Wu, J.; Yuan, X.-Y.; Long, X.-M. Tacrolimus Therapy in Primary Sjögren’s Syndrome with Refractory Immune Thrombocytopenia: A Retrospective Study. Clin. Exp. Rheumatol. 2022, 40, 2268–2274. [Google Scholar] [CrossRef]
- Ahn, H.; Ji, Y.W.; Jun, I.; Kim, T.; Lee, H.K.; Seo, K.Y. Comparison of Treatment Modalities for Dry Eye in Primary Sjögren’s Syndrome. J. Clin. Med. 2022, 11, 463. [Google Scholar] [CrossRef]
- Romano, V.; Romano, D.; Semeraro, P.; Forbice, E.; Iaria, A.; Pizzolante, T.; Frassi, M.; Franceschini, F.; Semeraro, F. Therapeutic Hyper-CL Soft Contact Lens in Sjögren’s Syndrome. Am. J. Ophthalmol. Case Rep. 2022, 28, 101685. [Google Scholar] [CrossRef]
- Nunziata, S.; Petrini, D.; Dell’Anno, S.; Barone, V.; Coassin, M.; Di Zazzo, A. Customized Scleral Lenses: An Alternative Tool for Severe Dry Eye Disease—A Case Series. J. Clin. Med. 2024, 13, 3935. [Google Scholar] [CrossRef]
- Qiu, S.X.; Fadel, D.; Hui, A. Scleral Lenses for Managing Dry Eye Disease in the Absence of Corneal Irregularities: What Is the Current Evidence? J. Clin. Med. 2024, 13, 3838. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zheng, Q.; Zhu, Y.; Wang, H.; Ma, H.; Jhanji, V.; Chen, W. Comparative Evaluation of Silicone Hydrogel Contact Lenses and Autologous Serum for Management of Sjögren Syndrome-Associated Dry Eye. Cornea 2015, 34, 1072–1078. [Google Scholar] [CrossRef]
- Castrejón-Morales, C.Y.; Granados-Portillo, O.; Cruz-Bautista, I.; Ruiz-Quintero, N.; Manjarrez, I.; Lima, G.; Hernández-Ramírez, D.F.; Astudillo-Angel, M.; Llorente, L.; Hernández-Molina, G. Omega-3 and Omega-6 Fatty Acids in Primary Sjögren’s Syndrome: Clinical Meaning and Association with Inflammation. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 34–39. [Google Scholar] [PubMed]
- da Nave, C.B.; Pereira, P.; Silva, M.L. The Effect of Polyunsaturated Fatty Acid (PUFA) Supplementation on Clinical Manifestations and Inflammatory Parameters in Individuals with Sjögren’s Syndrome: A Literature Review of Randomized Controlled Clinical Trials. Nutrients 2024, 16, 3786. [Google Scholar] [CrossRef]
- Szodoray, P.; Horvath, I.F.; Papp, G.; Barath, S.; Gyimesi, E.; Csathy, L.; Kappelmayer, J.; Sipka, S.; Duttaroy, A.K.; Nakken, B.; et al. The Immunoregulatory Role of Vitamins A, D and E in Patients with Primary Sjögren’s Syndrome. Rheumatology 2010, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hyon, J.Y.; Han, S.B. Dry Eye Disease and Vitamins: A Narrative Literature Review. Appl. Sci. 2022, 12, 4567. [Google Scholar] [CrossRef]
- Nesvold, M.B.; Jensen, J.L.; Hove, L.H.; Singh, P.B.; Young, A.; Palm, Ø.; Frost Andersen, L.; Carlsen, M.H.; Iversen, P.O. Dietary Intake, Body Composition, and Oral Health Parameters among Female Patients with Primary Sjögren’s Syndrome. Nutrients 2018, 10, 866. [Google Scholar] [CrossRef]
- Trotta, M.C.; Herman, H.; Balta, C.; Rosu, M.; Ciceu, A.; Mladin, B.; Gesualdo, C.; Lepre, C.C.; Russo, M.; Petrillo, F.; et al. Oral Administration of Vitamin D3 Prevents Corneal Damage in a Knock-Out Mouse Model of Sjögren’s Syndrome. Biomedicines 2023, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M.; Shoenfeld, Y. Vitamin D and Autoimmune Rheumatic Diseases. Biomolecules 2023, 13, 709. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Churov, A.V.; Starodubtseva, I.A.; Beloyartsev, D.F.; Kovyanova, T.I.; Sukhorukov, V.N.; Orekhov, N.A. Vitamin D in Primary Sjogren’s Syndrome (pSS) and the Identification of Novel Single-Nucleotide Polymorphisms Involved in the Development of pSS-Associated Diseases. Diagnostics 2024, 14, 2035. [Google Scholar] [CrossRef]
- Verstappen, G.M.; van Nimwegen, J.F.; Vissink, A.; Kroese, F.G.M.; Bootsma, H. The Value of Rituximab Treatment in Primary Sjögren’s Syndrome. Clin. Immunol. 2017, 182, 62–71. [Google Scholar] [CrossRef]
- Berardicurti, O.; Pavlich, V.; Cola, I.D.; Ruscitti, P.; Benedetto, P.D.; Navarini, L.; Marino, A.; Cipriani, P.; Giacomelli, R. Long-Term Safety of Rituximab in Primary Sjögren’s Syndrome: The Experience of a Single Centre. J. Rheumatol. 2021, 49, 171–175. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, X.Y.; Jin, X.; Yang, Z.; Xu, J. Rituximab Therapy for Primary Sjögren’s Syndrome. Front. Pharmacol. 2021, 12, 731122. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Seror, R.; Quartuccio, L.; Baron, G.; Salvin, S.; Fabris, M.; Desmoulins, F.; Nocturne, G.; Ravaud, P.; De Vita, S. Efficacy and Safety of Belimumab in Primary Sjögren’s Syndrome: Results of the BELISS Open-Label Phase II Study. Ann. Rheum. Dis. 2015, 74, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rivas, N.; Sang-Park, H.; Díaz del Campo, P.; Fernández-Castro, M.; Corominas, H.; Andreu, J.L.; Navarro-Compán, V. Efficacy of Belimumab in Primary Sjögren’s Syndrome: A Systematic Review. Reumatol. Clin. 2021, 17, 170–174. [Google Scholar] [CrossRef]
- Kaegi, C.; Steiner, U.C.; Wuest, B.; Crowley, C.; Boyman, O. Systematic Review of Safety and Efficacy of Belimumab in Treating Immune-Mediated Disorders. Allergy 2021, 76, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Pontarini, E.; Fabris, M.; Quartuccio, L.; Cappeletti, M.; Calcaterra, F.; Roberto, A.; Curcio, F.; Mavilio, D.; Della Bella, S.; De Vita, S. Treatment with Belimumab Restores B Cell Subsets and Their Expression of B Cell Activating Factor Receptor in Patients with Primary Sjogren’s Syndrome. Rheumatology 2015, 54, 1429–1434. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, R.; Li, Z.; Qin, D.; Wang, X. Novel and Potential Future Therapeutic Options in Sjögren’s Syndrome. Heliyon 2024, 10, e38803. [Google Scholar] [CrossRef]
- Baer, A.N.; Gottenberg, J.-E.; St Clair, E.W.; Sumida, T.; Takeuchi, T.; Seror, R.; Foulks, G.; Nys, M.; Mukherjee, S.; Wong, R.; et al. Efficacy and Safety of Abatacept in Active Primary Sjögren’s Syndrome: Results of a Phase III, Randomised, Placebo-Controlled Trial. Ann. Rheum. Dis. 2021, 80, 339–348. [Google Scholar] [CrossRef]
- de Wolff, L.; van Nimwegen, J.F.; Mossel, E.; van Zuiden, G.S.; Stel, A.J.; Majoor, K.I.; Olie, L.; Los, L.I.; Vissink, A.; Spijkervet, F.K.L.; et al. Long-Term Abatacept Treatment for 48 Weeks in Patients with Primary Sjögren’s Syndrome: The Open-Label Extension Phase of the ASAP-III Trial. Semin. Arthritis Rheum. 2022, 53, 151955. [Google Scholar] [CrossRef]
- Tsuboi, H.; Toko, H.; Honda, F.; Abe, S.; Takahashi, H.; Yagishita, M.; Hagiwara, S.; Ohyama, A.; Kondo, Y.; Nakano, K.; et al. Abatacept Ameliorates Both Glandular and Extraglandular Involvements in Patients with Sjögren’s Syndrome Associated with Rheumatoid Arthritis: Findings from an Open-Label, Multicentre, 1-Year, Prospective Study: The ROSE (Rheumatoid Arthritis with Orencia Trial Toward Sjögren’s Syndrome Endocrinopathy) and ROSE II Trials. Mod. Rheumatol. 2023, 33, 160–168. [Google Scholar] [CrossRef]
- Gottenberg, J.-E.; Dörner, T.; Bootsma, H.; Devauchelle-Pensec, V.; Bowman, S.J.; Mariette, X.; Bartz, H.; Oortgiesen, M.; Shock, A.; Koetse, W.; et al. Efficacy of Epratuzumab, an Anti-CD22 Monoclonal IgG Antibody, in Systemic Lupus Erythematosus Patients With Associated Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 763–773. [Google Scholar] [CrossRef]
- St Clair, E.W.; Baer, A.N.; Wei, C.; Noaiseh, G.; Parke, A.; Coca, A.; Utset, T.O.; Genovese, M.C.; Wallace, D.J.; McNamara, J.; et al. The Clinical Efficacy and Safety of Baminercept, a Lymphotoxin-β Receptor Fusion Protein, in Primary Sjögren’s Syndrome: Results from a Randomized, Double-Blind, Placebo-Controlled Phase II Trial. Arthritis Rheumatol. 2018, 70, 1470–1480. [Google Scholar] [CrossRef]
- Fisher, B.A.; Szanto, A.; Ng, W.-F.; Bombardieri, M.; Posch, M.G.; Papas, A.S.; Farag, A.M.; Daikeler, T.; Bannert, B.; Kyburz, D.; et al. Assessment of the Anti-CD40 Antibody Iscalimab in Patients with Primary Sjögren’s Syndrome: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Proof-of-Concept Study. Lancet Rheumatol. 2020, 2, e142–e152. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.A.; Mariette, X.; Papas, A.; Grader-Beck, T.; Bootsma, H.; Ng, W.-F.; van Daele, P.L.A.; Finzel, S.; Noaiseh, G.; Elgueta, S.; et al. Safety and Efficacy of Subcutaneous Iscalimab (CFZ533) in Two Distinct Populations of Patients with Sjögren’s Disease (TWINSS): Week 24 Results of a Randomised, Double-Blind, Placebo-Controlled, Phase 2b Dose-Ranging Study. Lancet 2024, 404, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.J.; Fox, R.; Dörner, T.; Mariette, X.; Papas, A.; Grader-Beck, T.; Fisher, B.A.; Barcelos, F.; De Vita, S.; Schulze-Koops, H.; et al. Safety and Efficacy of Subcutaneous Ianalumab (VAY736) in Patients with Primary Sjögren’s Syndrome: A Randomised, Double-Blind, Placebo-Controlled, Phase 2b Dose-Finding Trial. Lancet 2022, 399, 161–171. [Google Scholar] [CrossRef]
- Dörner, T.; Posch, M.G.; Li, Y.; Petricoul, O.; Cabanski, M.; Milojevic, J.M.; Kamphausen, E.; Valentin, M.-A.; Simonett, C.; Mooney, L.; et al. Treatment of Primary Sjögren’s Syndrome with Ianalumab (VAY736) Targeting B Cells by BAFF Receptor Blockade Coupled with Enhanced, Antibody-Dependent Cellular Cytotoxicity. Ann. Rheum. Dis. 2019, 78, 641–647. [Google Scholar] [CrossRef]
- Xu, D.; Fang, J.; Zhang, S.; Huang, C.; Huang, C.; Qin, L.; Li, X.; Chen, M.; Liu, X.; Liu, Y.; et al. Efficacy and Safety of Telitacicept in Primary Sjögren’s Syndrome: A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial. Rheumatology 2024, 63, 698–705. [Google Scholar] [CrossRef]
- Horai, Y.; Kurushima, S.; Shimizu, T.; Nakamura, H.; Kawakami, A. A Review of the Impact of Sjögren’s Syndrome and/or the Presence of Anti-Ro/SS-A Antibodies on Therapeutic Strategies for Rheumatoid Arthritis. J. Clin. Med. 2025, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Zhang, J.; Lin, Y.; Chen, W.; Fan, X.; Zhang, D. Pathogenesis and Treatment of Sjogren’s Syndrome: Review and Update. Front. Immunol. 2023, 14, 1127417. [Google Scholar] [CrossRef]
- Justet, A.; Ottaviani, S.; Dieudé, P.; Taillé, C. Tocilizumab for Refractory Organising Pneumonia Associated with Sjögren’s Disease. BMJ Case Rep. 2015, 2015, bcr2014209076. [Google Scholar] [CrossRef]
- Felten, R.; Devauchelle-Pensec, V.; Seror, R.; Duffau, P.; Saadoun, D.; Hachulla, E.; Pierre Yves, H.; Salliot, C.; Perdriger, A.; Morel, J.; et al. Interleukin 6 Receptor Inhibition in Primary Sjögren Syndrome: A Multicentre Double-Blind Randomised Placebo-Controlled Trial. Ann. Rheum. Dis. 2021, 80, 329–338. [Google Scholar] [CrossRef]
- Juarez, M.; Diaz, N.; Johnston, G.I.; Nayar, S.; Payne, A.; Helmer, E.; Cain, D.; Williams, P.; Devauchelle-Pensec, V.; Fisher, B.A.; et al. A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Proof-of-Concept Study of Oral Seletalisib in Primary Sjögren’s Syndrome. Rheumatology 2021, 60, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Scuron, M.D.; Fay, B.L.; Connell, A.J.; Oliver, J.; Smith, P.A. The PI3Kδ Inhibitor Parsaclisib Ameliorates Pathology and Reduces Autoantibody Formation in Preclinical Models of Systemic Lupus Erythematosus and Sjögren’s Syndrome. Int. Immunopharmacol. 2021, 98, 107904. [Google Scholar] [CrossRef] [PubMed]
- Dörner, T.; Zeher, M.; Laessing, U.; Chaperon, F.; De Buck, S.; Hasselberg, A.; Valentin, M.-A.; Ma, S.; Cabanski, M.; Kalis, C.; et al. OP0250 A Randomised, Double-Blind Study to Assess the Safety, Tolerability and Preliminary Efficacy of Leniolisib (CDZ173) in Patients with Primary sjögren’s Syndrome. Ann. Rheum. Dis. 2018, 77, 174. [Google Scholar] [CrossRef]
- Dörner, T.; Kaul, M.; Szántó, A.; Tseng, J.-C.; Papas, A.S.; Pylvaenaeinen, I.; Hanser, M.; Abdallah, N.; Grioni, A.; Santos Da Costa, A.; et al. Efficacy and Safety of Remibrutinib, a Selective Potent Oral BTK Inhibitor, in Sjögren’s Syndrome: Results from a Randomised, Double-Blind, Placebo-Controlled Phase 2 Trial. Ann. Rheum. Dis. 2024, 83, 360–371. [Google Scholar] [CrossRef]
- Price, E.; Bombardieri, M.; Kivitz, A.; Matzkies, F.; Gurtovaya, O.; Pechonkina, A.; Jiang, W.; Downie, B.; Mathur, A.; Mozaffarian, A.; et al. Safety and Efficacy of Filgotinib, Lanraplenib and Tirabrutinib in Sjögren’s Syndrome: A Randomized, Phase 2, Double-Blind, Placebo-Controlled Study. Rheumatology 2022, 61, 4797–4808. [Google Scholar] [CrossRef]
- Zhao, Z.; Ye, C.; Dong, L. The Off-Label Uses Profile of Tofacitinib in Systemic Rheumatic Diseases. Int. Immunopharmacol. 2020, 83, 106480. [Google Scholar] [CrossRef]
- Barrera, M.-J.; Aguilera, S.; Castro, I.; Matus, S.; Carvajal, P.; Molina, C.; González, S.; Jara, D.; Hermoso, M.; González, M.-J. Tofacitinib Counteracts IL-6 Overexpression Induced by Deficient Autophagy: Implications in Sjögren’s Syndrome. Rheumatology 2021, 60, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Pu, J.; Wang, Y.; Wu, Z.; Liang, Y.; Song, J.; Pan, S.; Han, F.; Yang, L.; Xu, X.; et al. Tofacitinib in the Treatment of Primary Sjögren’s Syndrome-Associated Interstitial Lung Disease: Study Protocol for a Prospective, Randomized, Controlled and Open-Label Trial. BMC Pulm. Med. 2023, 23, 473. [Google Scholar] [CrossRef]
- Bai, W.; Yang, F.; Xu, H.; Wei, W.; Li, H.; Zhang, L.; Zhao, Y.; Shi, X.; Zhang, Y.; Zeng, X.; et al. A Multi-Center, Open-Label, Randomized Study to Explore Efficacy and Safety of Baricitinib in Active Primary Sjogren’s Syndrome Patients. Trials 2023, 24, 112. [Google Scholar] [CrossRef]
- Bang, C.-H.; Park, C.-J.; Kim, Y.-S. The Expanding Therapeutic Potential of Deucravacitinib Beyond Psoriasis: A Narrative Review. J. Clin. Med. 2025, 14, 1745. [Google Scholar] [CrossRef]
- Bentley, D.; Fisher, B.A.; Barone, F.; Kolb, F.A.; Attley, G. A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study on the Effects of a Cathepsin S Inhibitor in Primary Sjögren’s Syndrome. Rheumatology 2023, 62, 3644–3653. [Google Scholar] [CrossRef]
- Posada, J.; Valadkhan, S.; Burge, D.; Davies, K.; Tarn, J.; Casement, J.; Jobling, K.; Gallagher, P.; Wilson, D.; Barone, F.; et al. Improvement of Severe Fatigue Following Nuclease Therapy in Patients With Primary Sjögren’s Syndrome: A Randomized Clinical Trial. Arthritis Rheumatol. 2021, 73, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Ousler, G.; Torkildsen, G.; Walshe, C.; Fyfe, M.C.T.; Rowley, A.; Webber, S.; Sheppard, J.D.; Duggal, A. A Phase 2 Randomized, Double-Masked, Placebo-Controlled Study of Novel Nonsystemic Kinase Inhibitor TOP1630 for the Treatment of Dry Eye Disease. Clin. Ophthalmol. 2019, 13, 261–275. [Google Scholar] [CrossRef]
- Sosne, G.; Dunn, S.P.; Kim, C. Thymosin Β4 Significantly Improves Signs and Symptoms of Severe Dry Eye in a Phase 2 Randomized Trial. Cornea 2015, 34, 491. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.E.; Kleinman, H.K.; Sosne, G.; Ousler, G.W.; Kim, K.; Kang, S.; Yang, J. RGN-259 (Thymosin Β4) Improves Clinically Important Dry Eye Efficacies in Comparison with Prescription Drugs in a Dry Eye Model. Sci. Rep. 2018, 8, 10500. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of Topical Ophthalmic Drugs in the Treatment of Dry Eye Disease: A Systematic Literature Review. Ocul. Surf. 2019, 17, 412–423. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Gh., M.S.; Romano, J.; Dias Teixeira, K.L.; Struble, C.; Ryan, D.S.; Sia, R.K.; Kitt, J.P.; Harris, J.M.; Hsu, K.-L.; et al. Lacritin Proteoforms Prevent Tear Film Collapse and Maintain Epithelial Homeostasis. J. Biol. Chem. 2021, 296, 100070. [Google Scholar] [CrossRef]
- McNamara, N.A.; Ge, S.; Lee, S.M.; Enghauser, A.M.; Kuehl, L.; Chen, F.Y.-T.; Gallup, M.; McKown, R.L. Reduced Levels of Tear Lacritin Are Associated with Corneal Neuropathy in Patients with the Ocular Component of Sjögren’s Syndrome. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5237–5243. [Google Scholar] [CrossRef]
- Vijmasi, T.; Chen, F.Y.T.; Balasubbu, S.; Gallup, M.; McKown, R.L.; Laurie, G.W.; McNamara, N.A. Topical Administration of Lacritin Is a Novel Therapy for Aqueous-Deficient Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5401–5409. [Google Scholar] [CrossRef]
- Group, L.S. Lacripep for the Treatment of Primary Sjögren-Associated Ocular Surface Disease: Results of the First-In-Human Study. Cornea 2023, 42, 847–857. [Google Scholar] [CrossRef]
- Wasielica-Poslednik, J.; Pfeiffer, N.; Gericke, A. Fluocinolone Acetonide Intravitreal Implant as a Therapeutic Option for Severe Sjögren’s Syndrome-Related Keratopathy: A Case Report. J. Med. Case Rep. 2019, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Terceiro, L.E.L.; Blanchard, A.A.A.; Edechi, C.A.; Freznosa, A.; Triggs-Raine, B.; Leygue, E.; Myal, Y. Generation of Prolactin-Inducible Protein (Pip) Knockout Mice by CRISPR/Cas9-Mediated Gene Engineering. Can. J. Physiol. Pharmacol. 2022, 100, 86–91. [Google Scholar] [CrossRef]
- Mondal, H.; Kim, H.-J.; Mohanto, N.; Jee, J.-P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef]
- Chang, W.-H.; Liu, P.-Y.; Lin, M.-H.; Lu, C.-J.; Chou, H.-Y.; Nian, C.-Y.; Jiang, Y.-T.; Hsu, Y.-H.H. Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef] [PubMed]
- Garrós, N.; Mallandrich, M.; Beirampour, N.; Mohammadi, R.; Domènech, Ò.; Rodríguez-Lagunas, M.J.; Clares, B.; Colom, H. Baricitinib Liposomes as a New Approach for the Treatment of Sjögren’s Syndrome. Pharmaceutics 2022, 14, 1895. [Google Scholar] [CrossRef]
- Hofauer, B.; Kirschstein, L.; Graf, S.; Strassen, U.; Johnson, F.; Zhu, Z.; Knopf, A. Inhalative Treatment of Laryngitis Sicca in Patients with Sjögren’s Syndrome—A Pilot Study. J. Clin. Med. 2022, 11, 1081. [Google Scholar] [CrossRef]
- Beirampour, N.; Bustos-Salgado, P.; Garrós, N.; Mohammadi-Meyabadi, R.; Domènech, Ò.; Suñer-Carbó, J.; Rodríguez-Lagunas, M.J.; Kapravelou, G.; Montes, M.J.; Calpena, A.; et al. Formulation of Polymeric Nanoparticles Loading Baricitinib as a Topical Approach in Ocular Application. Pharmaceutics 2024, 16, 1092. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Shin, C.S.; Wang, C.; Pflugfelder, S.C.; Acharya, G.; De Paiva, C.S. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Song, H.B.; Lee, K.J.; Seo, I.H.; Lee, J.Y.; Lee, S.-M.; Kim, J.H.; Kim, J.H.; Ryu, W. Impact Insertion of Transfer-Molded Microneedle for Localized and Minimally Invasive Ocular Drug Delivery. J. Control. Release 2015, 209, 272–279. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan Nanoparticles: A New Vehicle for the Improvement of the Delivery of Drugs to the Ocular Surface. Application to Cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Lancina, M.G.; Yang, H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897–902. [Google Scholar] [CrossRef]
- Pérez-Carrión, M.D.; Posadas, I. Dendrimers in Neurodegenerative Diseases. Processes 2023, 11, 319. [Google Scholar] [CrossRef]
- Yavuz, B.; Bozdağ Pehlivan, S.; Sümer Bolu, B.; Nomak Sanyal, R.; Vural, İ.; Ünlü, N. Dexamethasone—PAMAM Dendrimer Conjugates for Retinal Delivery: Preparation, Characterization and in Vivo Evaluation. J. Pharm. Pharmacol. 2016, 68, 1010–1020. [Google Scholar] [CrossRef]
- Chihaby, N.; Orliaguet, M.; Le Pottier, L.; Pers, J.-O.; Boisramé, S. Treatment of Sjögren’s Syndrome with Mesenchymal Stem Cells: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10474. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, S.; Yang, G.; Zhu, R.; Li, Z.; Yao, G.; Chen, H.; Sun, L. Mesenchymal Stem Cell Transplantation Alleviates Sjögren’s Syndrome Symptoms by Modulating Tim-3 Expression. Int. Immunopharmacol. 2022, 111, 109152. [Google Scholar] [CrossRef]
- Surico, P.L.; Barone, V.; Singh, R.B.; Coassin, M.; Blanco, T.; Dohlman, T.H.; Basu, S.; Chauhan, S.K.; Dana, R.; Di Zazzo, A. Potential Applications of Mesenchymal Stem Cells in Ocular Surface Immune-Mediated Disorders. Surv. Ophthalmol. 2025, 70, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Matsumura-Kawashima, M.; Moriyama, M.; Kawado, T.; Nakamura, S. Dental Pulp-Derived Stem Cell-Conditioned Media Attenuates Secondary Sjögren’s Syndrome via Suppression of Inflammatory Cytokines in the Submandibular Glands. Regen. Ther. 2021, 16, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Matsumura-Kawashima, M.; Ogata, K.; Moriyama, M.; Murakami, Y.; Kawado, T.; Nakamura, S. Secreted Factors from Dental Pulp Stem Cells Improve Sjögren’s Syndrome via Regulatory T Cell-Mediated Immunosuppression. Stem Cell Res. Ther. 2021, 12, 182. [Google Scholar] [CrossRef]
- Wen, K.; Li, W.; Cheng, C.; Weige, X.; Jiaqi, C.; Shiyu, S.; Lingyan, H.; Hongwei, W.; Sijing, X. Human Dental Pulp Stem Cells Ameliorate the Imiquimod-Induced Psoriasis in Mice. Heliyon 2023, 9, e13337. [Google Scholar] [CrossRef]
- Genç, D.; Bulut, O.; Günaydin, B.; Göksu, M.; Düzgün, M.; Dere, Y.; Sezgin, S.; Aladağ, A.; Bülbül, A. Dental Follicle Mesenchymal Stem Cells Ameliorated Glandular Dysfunction in Sjögren’s Syndrome Murine Model. PLoS ONE 2022, 17, e0266137. [Google Scholar] [CrossRef]
- Yang, N.; Liu, X.; Chen, X.; Yu, S.; Yang, W.; Liu, Y. Stem Cells from Exfoliated Deciduous Teeth Transplantation Ameliorates Sjögren’s Syndrome by Secreting Soluble PD-L1. J. Leukoc. Biol. 2022, 111, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Wang, S.; Guo, J.; Guo, J.; Fu, J.; Ren, L.; An, Y.; He, J.; Li, Z. Human Umbilical Cord Mesenchymal Stem Cells Confer Potent Immunosuppressive Effects in Sjögren’s Syndrome by Inducing Regulatory T Cells. Mod. Rheumatol. 2021, 31, 186–196. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, W.; Liu, D.; Li, X.; Li, L.; Peng, L.; Xiong, Y.; Gan, H.; Ren, X. Human Umbilical Cord Mesenchymal Stem Cells Improve Disease Characterization of Sjogren’s Syndrome in NOD Mice through Regulation of Gut Microbiota and Treg/Th17 Cellular Immunity. Immun. Inflamm. Dis. 2024, 12, e1139. [Google Scholar] [CrossRef]
- Cong, Y.; Tang, X.; Wang, D.; Zhang, Z.; Huang, S.; Zhang, X.; Yao, G.; Sun, L. Umbilical Cord Mesenchymal Stem Cells Alleviate Sjögren’s Syndrome and Related Pulmonary Inflammation through Regulating Vγ4+ IL-17+ T Cells. Ann. Transl. Med. 2022, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.F.; Yong, D.W.W.; Manotosh, R. A Review of Contact Lens-Induced Limbal Stem Cell Deficiency. Biology 2023, 12, 1490. [Google Scholar] [CrossRef] [PubMed]
- Rossen, J.; Amram, A.; Milani, B.; Park, D.; Harthan, J.; Joslin, C.; McMahon, T.; Djalilian, A. Contact Lens-Induced Limbal Stem Cell Deficiency. Ocul. Surf. 2016, 14, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Ji, J.; Yao, K.; Fu, Q. Regenerative Treatment of Ophthalmic Diseases with Stem Cells: Principles, Progress, and Challenges. Adv. Ophthalmol. Pract. Res. 2024, 4, 52–64. [Google Scholar] [CrossRef]
- Keye, P.; Issleib, S.; Gier, Y.; Glegola, M.; Maier, P.; Böhringer, D.; Eberwein, P.; Reinhard, T. Visual and Ocular Surface Benefits of Mini-Scleral Contact Lenses in Patients with Chronic Ocular Graft-versus-Host Disease (GvHD). Sci. Rep. 2024, 14, 25254. [Google Scholar] [CrossRef]
- Liu, Y.; Song, S.; Liu, Y.; Fu, T.; Guo, Y.; Liu, R.; Chen, J.; Lin, Y.; Cheng, Y.; Li, Y.; et al. MSCohi-O Lenses for Long-Term Retention of Mesenchymal Stem Cells on Ocular Surface as a Therapeutic Approach for Chronic Ocular Graft-versus-Host Disease. Stem Cell Rep. 2023, 18, 2356–2369. [Google Scholar] [CrossRef]
- Møller-Hansen, M.; Larsen, A.-C.; Wiencke, A.K.; Terslev, L.; Siersma, V.; Andersen, T.T.; Hansen, A.E.; Bruunsgaard, H.; Haack-Sørensen, M.; Ekblond, A.; et al. Allogeneic Mesenchymal Stem Cell Therapy for Dry Eye Disease in Patients with Sjögren’s Syndrome: A Randomized Clinical Trial. Ocul. Surf. 2024, 31, 1–8. [Google Scholar] [CrossRef]
- Zhao, J.; An, Q.; Zhu, X.; Yang, B.; Gao, X.; Niu, Y.; Zhang, L.; Xu, K.; Ma, D. Research Status and Future Prospects of Extracellular Vesicles in Primary Sjögren’s Syndrome. Stem Cell Res. Ther. 2022, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Cheng, R.-J.; Wei, S.-X.; Xia, Z.-J.; Pu, Y.-Y.; Liu, Y. Advances in Mesenchymal Stem Cell-Derived Extracellular Vesicles Therapy for Sjogren’s Syndrome-Related Dry Eye Disease. Exp. Eye Res. 2023, 237, 109716. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xing, Y.; Gan, Y.; He, J.; Hua, H. Labial Gland-Derived Mesenchymal Stem Cells and Their Exosomes Ameliorate Murine Sjögren’s Syndrome by Modulating the Balance of Treg and Th17 Cells. Stem Cell Res. Ther. 2021, 12, 478. [Google Scholar] [CrossRef]
- Xing, Y.; Li, B.; He, J.; Hua, H. Labial Gland Mesenchymal Stem Cell Derived Exosomes-Mediated miRNA-125b Attenuates Experimental Sjogren’s Syndrome by Targeting PRDM1 and Suppressing Plasma Cells. Front. Immunol. 2022, 13, 871096. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Zheng, L.; Lu, Z.; Huang, J.; Pu, J.; Pan, S.; Zhang, M.; Liu, J.; Tang, J. Mesenchymal Stem Cells Negatively Regulate CD4+ T Cell Activation in Patients with Primary SjöGren Syndrome through the miRNA-125b and miRNA-155 TCR Pathway. Mol. Med. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Rui, K.; Hong, Y.; Zhu, Q.; Shi, X.; Xiao, F.; Fu, H.; Yin, Q.; Xing, Y.; Wu, X.; Kong, X.; et al. Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Murine Sjögren’s Syndrome by Modulating the Function of Myeloid-Derived Suppressor Cells. Cell Mol. Immunol. 2021, 18, 440–451. [Google Scholar] [CrossRef]

| Cytokines and Chemokines | Main Producer(s) | Effect(s)/Role(s) | Pathogenesis Association | Clinical Significance | References |
|---|---|---|---|---|---|
| IFN-γ | T cells Monocytes Macrophages | Pro-inflammatory regulation of MHC class I and II | Increases the expression of autoantigens, facilitating the immune response | Associated with increased T-cell infiltration | [54,61] |
| IL-1, IL-2, IL-6, TNF | T cells Monocytes Macrophages | Pro-inflammatory | They activate inflammatory pathways and contribute to the destruction of glandular cells. | Associated with increased dry eyes and dry mouth | [54,62] |
| IL-10, TGF-β | Τ cells | Anti-inflammatory | They regulate the immune response, reducing autoimmunity | Their reduced expression may contribute to autoimmunity | [54,63] |
| IFN-α | PDCs Monocytes | Antiviral, anti-cancer, pro-inflammatory | Triggers the production of autoantibodies and the release of chemokines | Associated with relapse and extraneous events | [54,55,64] |
| CXCL12 | Epithelial cells | Binds to CXCR4 on T cells and PDCs | Attracts immune cells to the area of the glands | Promotes chronic inflammation and fibrosis | [54,65] |
| CXCL13 | Activated and upgraded epithelium | Associated with CXCR5 in B cells | Promotes the accumulation of B cells and the formation of ectopic lymphocytes | Associated with the likelihood of developing lymphoma | [54,65] |
| CXCL9, CXCL10, CXCL11 | Upgraded epithelium | Associated with CXCR3 in T cells and PDCs | Enhances cell entry and maintenance in the inflammatory microenvironment | Can serve as a biomarker of disease activity | [37,54,65,66,67] |
| Breg Cell Derivative | Main Producer(s) | Effect(s)/Role(s) | References |
|---|---|---|---|
| IL-10 | Generated by nearly all immune cell types |
| [9,26,74,81] |
| IL-35 | Immunosuppressive cytokines |
| [26,74,82] |
| GrB | Perforin-induced apoptosis of target cells by a serine protease family |
| [19,74,83] |
| Differently Expressed miRNAs | Correlation with SS | References |
|---|---|---|
| miR-146a |
| [98] |
| miR-155 |
| [97,99] |
| miR-181a |
| [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlidis, K.; Adamantidi, T.; Maria, C.; Georgios, K.; Dania, V.; Krokidis, X.; Tsoupras, A. Sjögren’s Syndrome and Ocular Inflammation: Pathophysiology, Clinical Manifestation and Mitigation Strategies. Immuno 2025, 5, 24. https://doi.org/10.3390/immuno5030024
Pavlidis K, Adamantidi T, Maria C, Georgios K, Dania V, Krokidis X, Tsoupras A. Sjögren’s Syndrome and Ocular Inflammation: Pathophysiology, Clinical Manifestation and Mitigation Strategies. Immuno. 2025; 5(3):24. https://doi.org/10.3390/immuno5030024
Chicago/Turabian StylePavlidis, Konstantinos, Theodora Adamantidi, Chatzikamari Maria, Karamanis Georgios, Vasiliki Dania, Xenophon Krokidis, and Alexandros Tsoupras. 2025. "Sjögren’s Syndrome and Ocular Inflammation: Pathophysiology, Clinical Manifestation and Mitigation Strategies" Immuno 5, no. 3: 24. https://doi.org/10.3390/immuno5030024
APA StylePavlidis, K., Adamantidi, T., Maria, C., Georgios, K., Dania, V., Krokidis, X., & Tsoupras, A. (2025). Sjögren’s Syndrome and Ocular Inflammation: Pathophysiology, Clinical Manifestation and Mitigation Strategies. Immuno, 5(3), 24. https://doi.org/10.3390/immuno5030024










