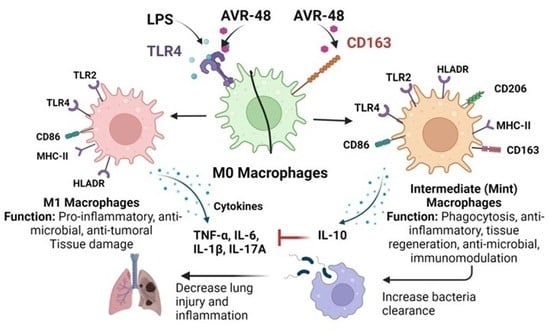
Chitin Derived Small Molecule AVR-48 Reprograms the Resting Macrophages to an Intermediate Phenotype and Decrease Pseudomonas aeruginosa Mouse Lung Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cells and Cell Lines
2.3. Bacteria
2.4. Chemicals and Reagents
2.5. Cell Viability Assay
2.5.1. Cytokine and CD163 Assay
2.5.2. IL-10 and IL-17A Detection in Lung Homogenates and Serum
2.6. Flow Cytometry Studies
2.6.1. Binding of AVR-48 to Splenic Monocytes/Macrophages
2.6.2. Binding of Biotinylated Conjugated AVR-48 to Splenic Monocytes/Macrophages
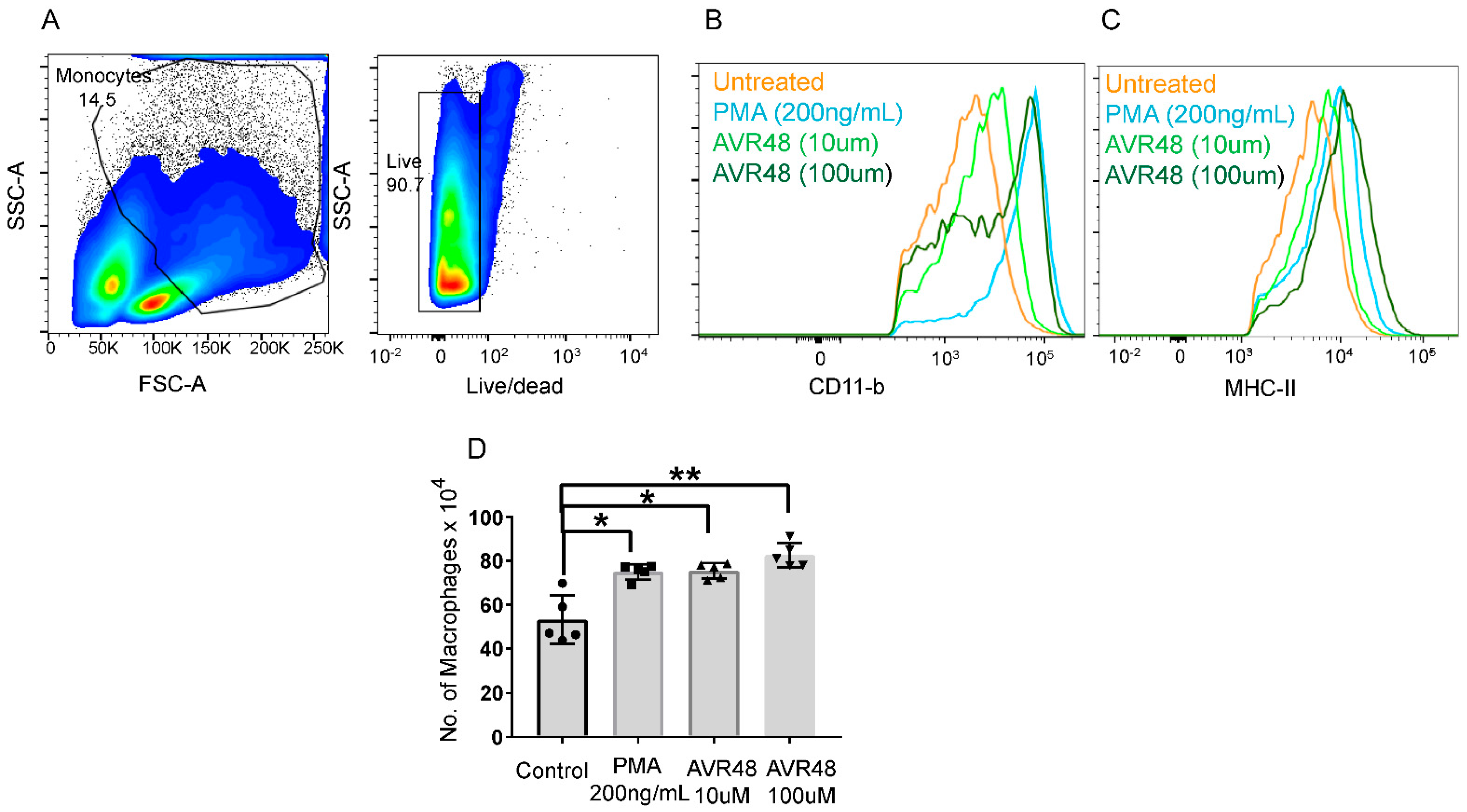
2.6.3. Quantification of Macrophages after AVR-48 Treatment to hPBMC Cells
2.7. Phagocytosis and Bacteria CFU Measurement Using THP-1 Cells
2.8. Combination MIC Assay
2.9. Pseudomonas aeruginosa Mouse Lung Infection
2.10. Quantification of Bacterial Load in Mouse Lung and Blood Samples
2.11. Statistical Analysis
3. Results
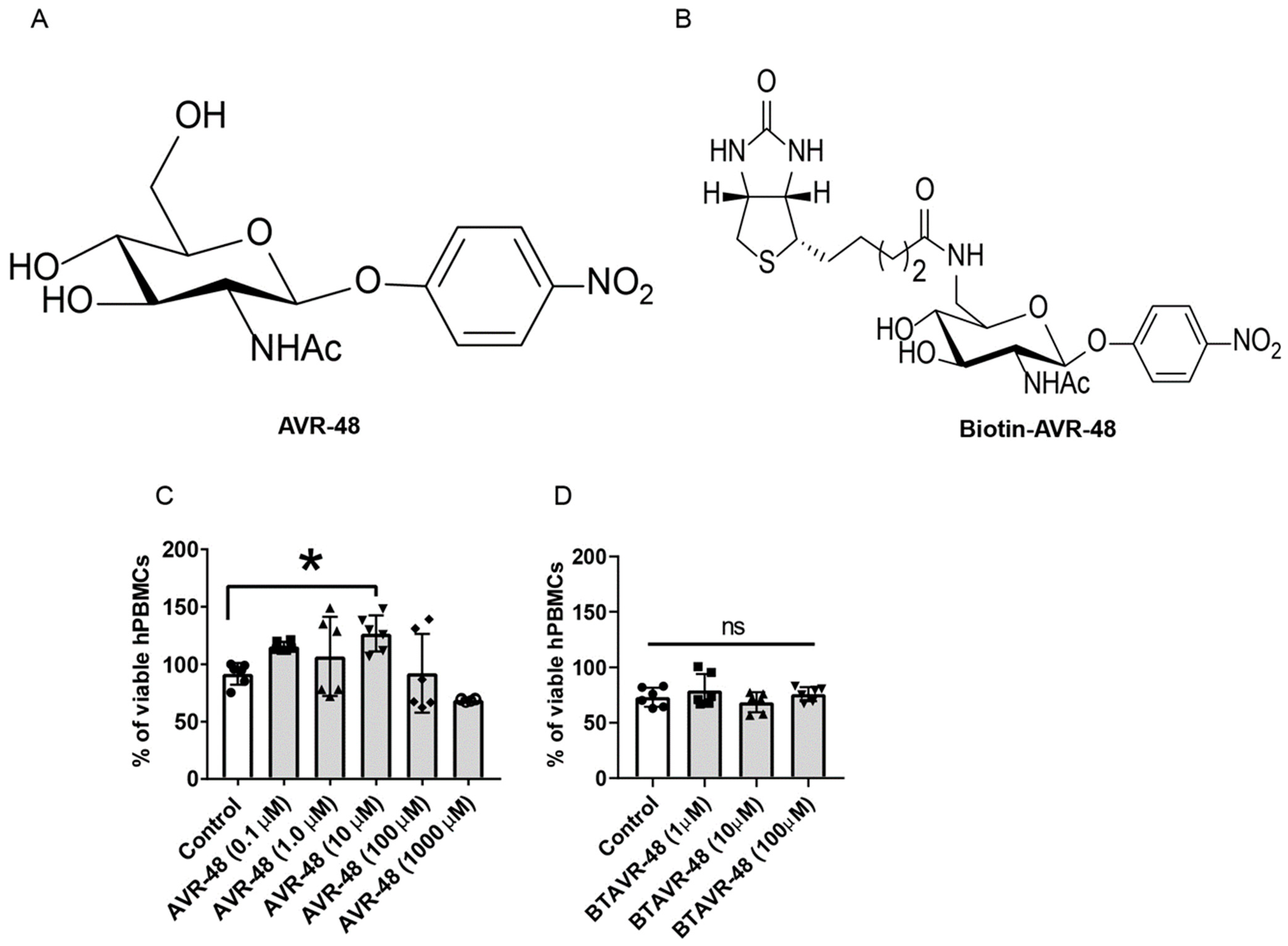
3.1. AVR-48 and Biotin Conjugated AVR-48 Do Not Exhibit Cytotoxicity to hPBMCs In Vitro
3.2. AVR-48 Binds to Both TLR4 and CD163 Receptors in Primary Monocytes
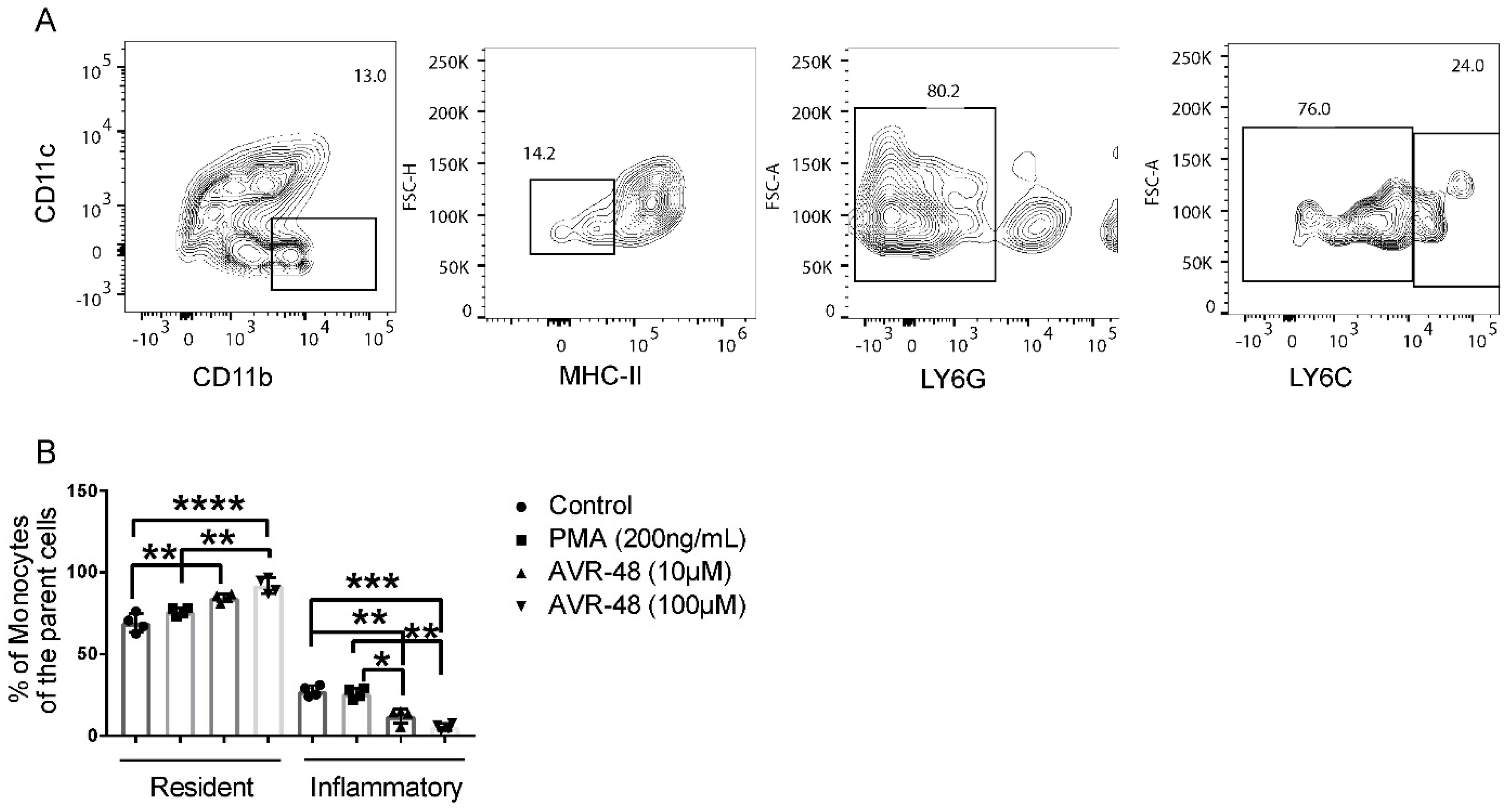
3.3. AVR-48 Treatment Polarizes Mouse Monocytes to Macrophages and Shifts the Monocyte Populations More to a Resident Phenotype
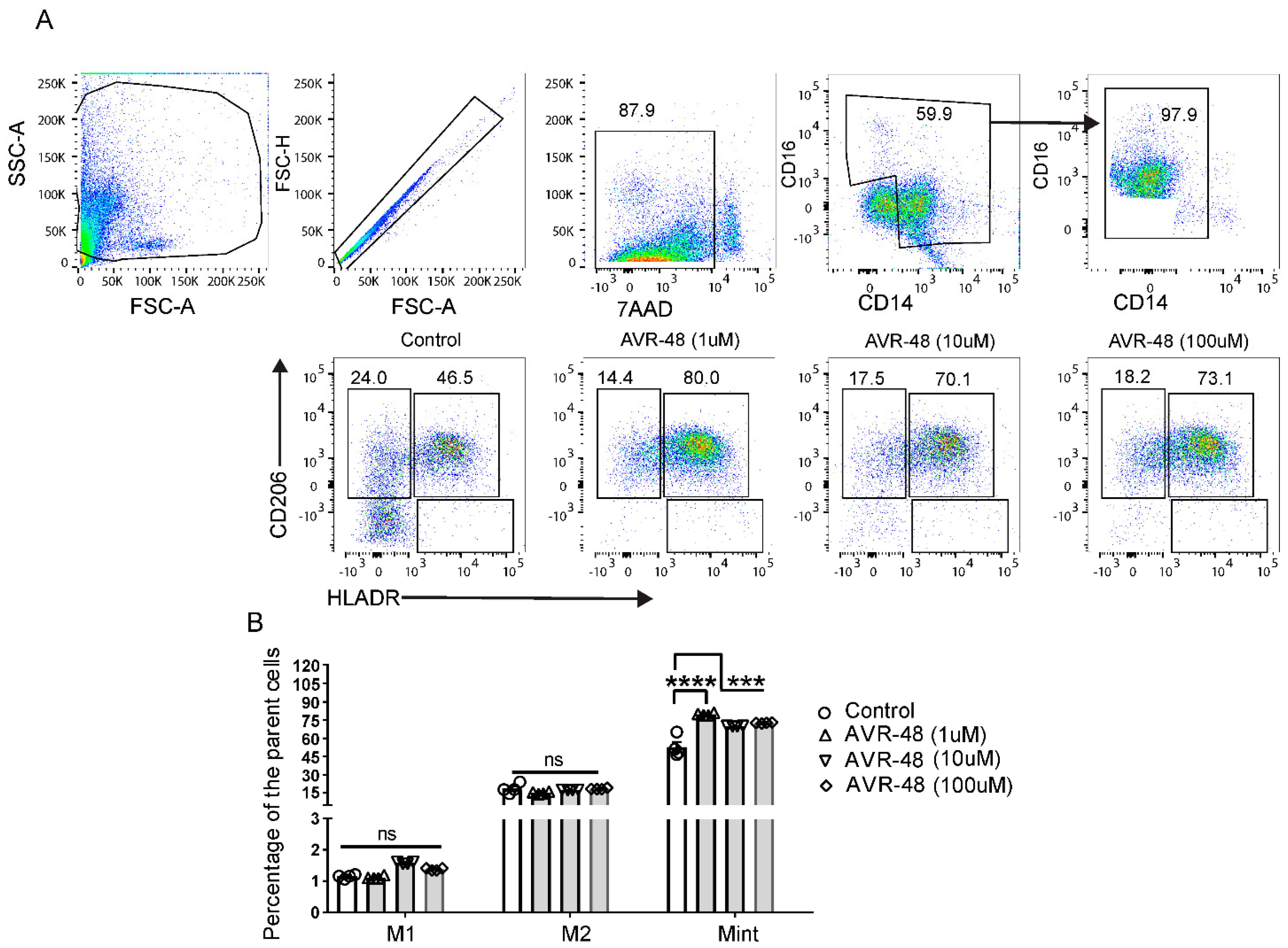
3.4. Treatment of AVR-48 to Human Peripheral Blood Mononuclear Cells Increases the Percentage of Intermediate (Mint) Macrophages
3.5. Effect of LPS and AVR-48 Treatment on Percentage of Macrophages in hPBMCs
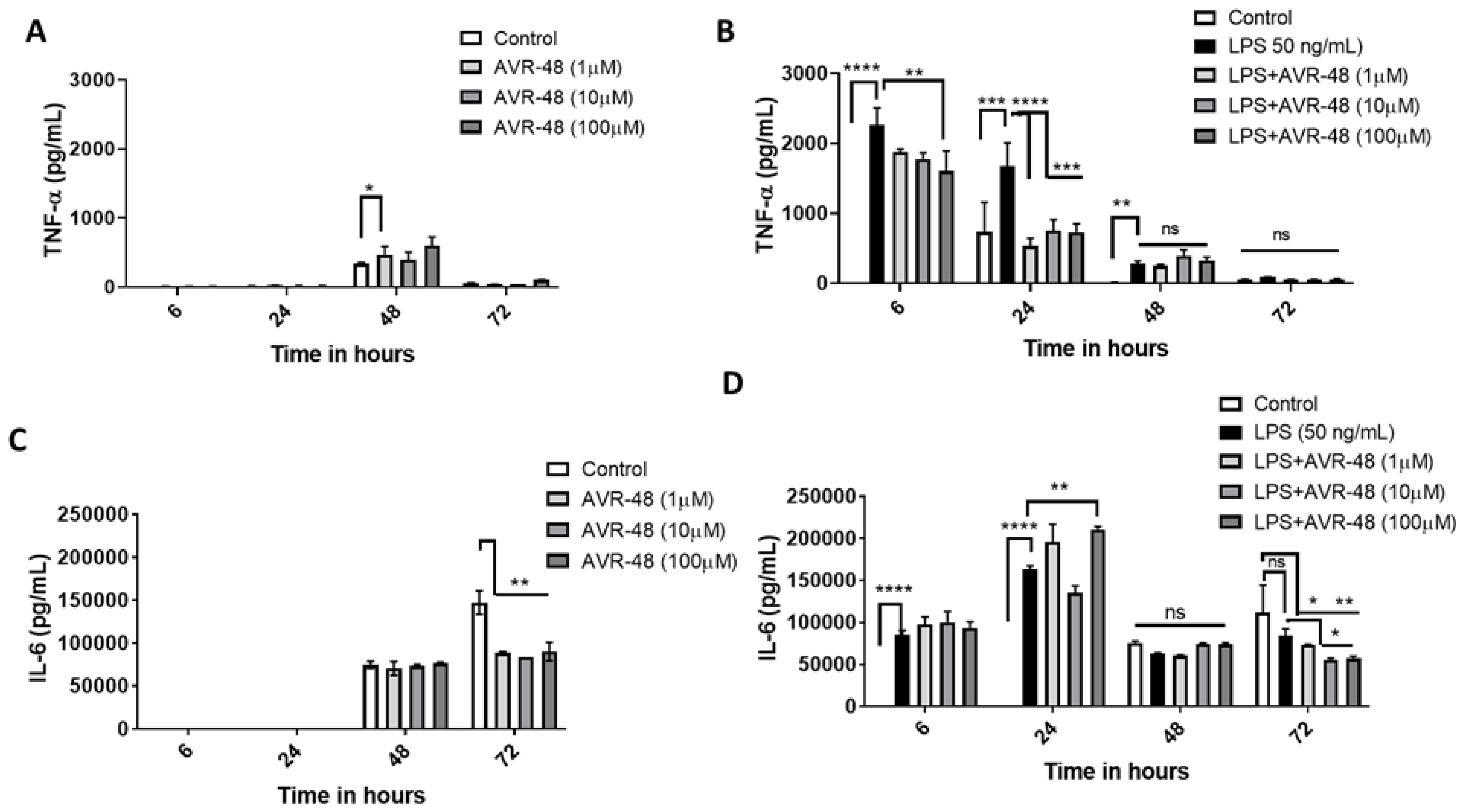
3.6. Effect of AVR-48 and LPS on Concentration of TNF-α and IL-6 in hPBMCs
3.7. Effect of AVR-48 and LPS on Concentration of IL-10 and sCD163 in hPBMCs
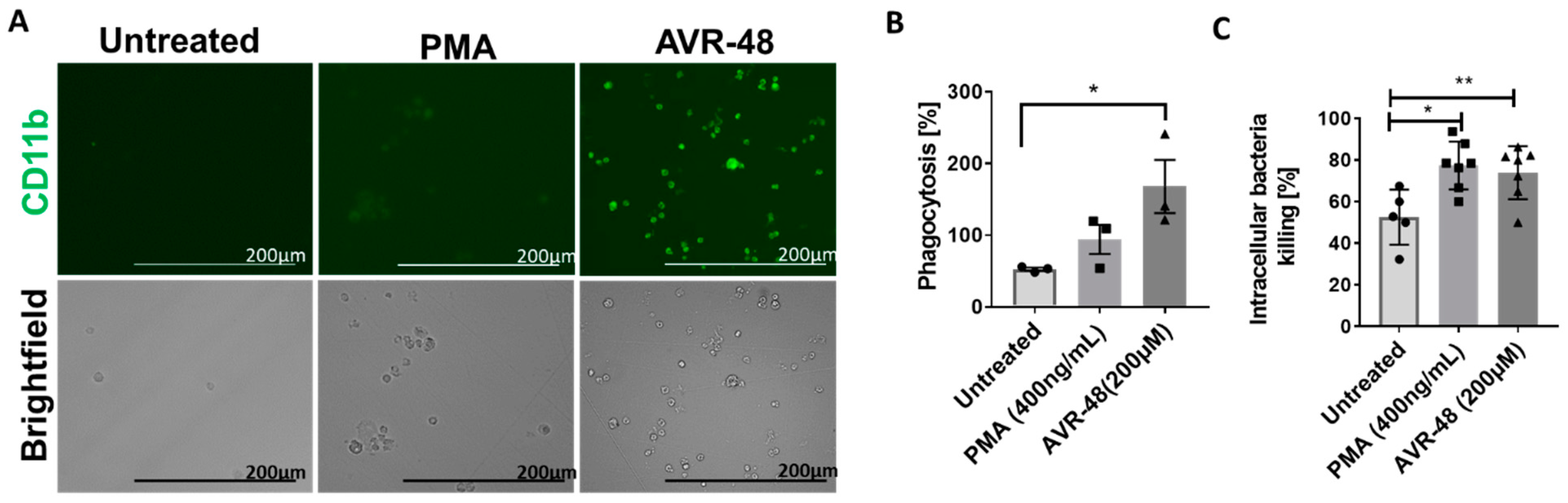
3.8. AVR-48 Induce Differentiation of THP-1 Human Monocytic Cells into Macrophages with Enhanced Phagocytosis of the Bacteria and Promote Intracellular Killing
3.9. AVR-48 Demonstrates Synergy with Standard of Care Antibiotics
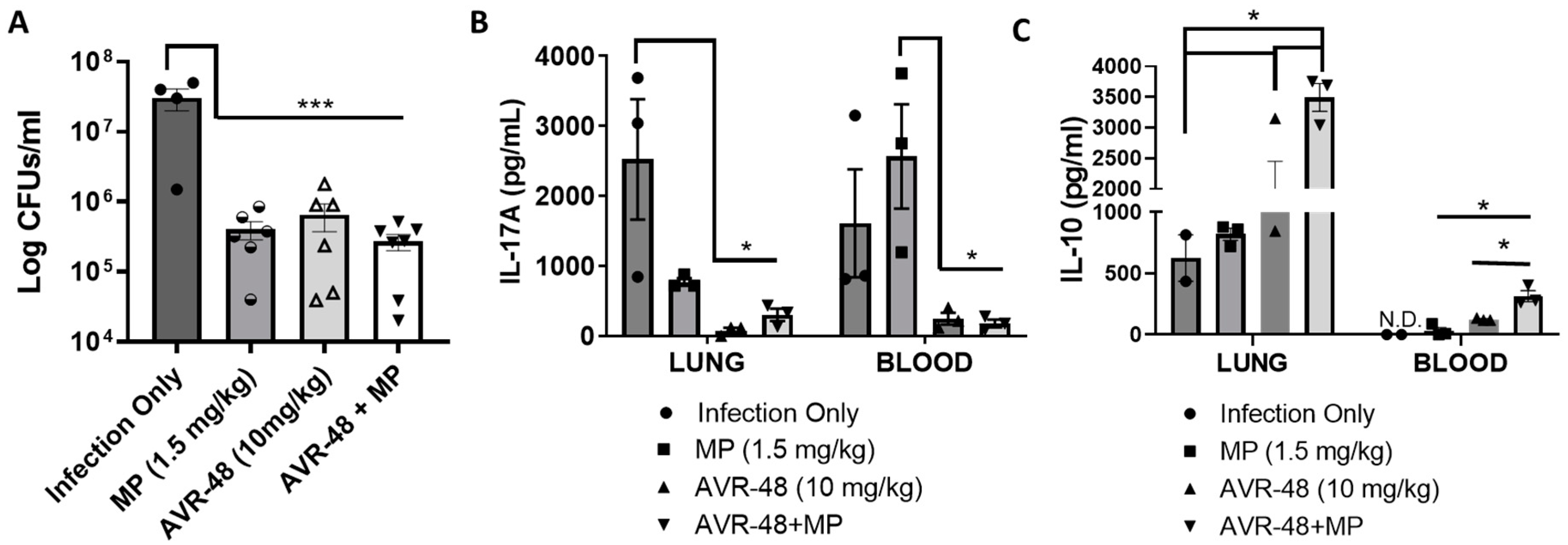
3.10. AVR-48 Decreases Bacteria Load in a Mouse Lung Infection Model and Increases Anti-Inflammatory Cytokine IL-10
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Panda, S.K.; Kumar, S.; Tupperwar, N.C.; Vaidya, T.; George, A.; Rath, S.; Bal, V.; Ravindran, B. Chitohexaose activates macrophages by alternate pathway through TLR4 and blocks endotoxemia. PLoS Pathog. 2012, 8, e1002717. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Das, P.; Acharya, S.; Agarwal, B.; Christensen, D.J.; Robertson, S.M.; Bhandari, V. Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS). Int. J. Mol. Sci. 2021, 22, 2573. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Acharya, S.; Shah, D.; Agarwal, B.; Prahaladan, V.; Bhandari, V. Chitin Analog AVR-25 Prevents Experimental Bronchopulmonary Dysplasia. J. Pediatr. Intensive Care 2020, 9, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Panda, S.K.; Agarwal, B.; Behera, S.; Ali, S.M.; Pulse, M.E.; Solomkin, J.S.; Opal, S.M.; Bhandari, V.; Acharya, S. Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis. Sci. Rep. 2019, 9, 2904. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Das, P.; Agarwal, B. Novel Immunodulating Small Molecules. U.S. Patent US20200022995A1, 21 June 2022. [Google Scholar]
- Das, P.; Acharya, S.; Prahaladan, V.M.; Kumova, O.K.; Malaeb, S.; Behera, S.; Agarwal, B.; Christensen, D.J.; Carey, A.J.; Bhandari, V. Chitin-Derived AVR-48 Prevents Experimental Bronchopulmonary Dysplasia (BPD) and BPD-Associated Pulmonary Hypertension in Newborn Mice. Int. J. Mol. Sci. 2021, 22, 8547. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Wang, Y.; Liu, X.; Zhang, Y.; Qu, W.; Chen, K.; Francisco, N.M.; Feng, L.; Huang, X.; et al. Beta-Defensin 2 and 3 Promote Bacterial Clearance of Pseudomonas aeruginosa by Inhibiting Macrophage Autophagy through Downregulation of Early Growth Response Gene-1 and c-FOS. Front. Immunol. 2018, 9, 211. [Google Scholar] [CrossRef]
- Lechartier, B.; Hartkoorn, R.C.; Cole, S.T. In vitro combination studies of benzothiazinone lead compound BTZ043 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 5790–5793. [Google Scholar] [CrossRef]
- Rand, K.H.; Houck, H.J.; Brown, P.; Bennett, D. Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob. Agents Chemother. 1993, 37, 613–615. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, W.G.; Hershman, M.J.; Wellhausen, S.R.; Polk, H.C. HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am. J. Surg. 1991, 161, 639–645. [Google Scholar] [CrossRef]
- Palojärvi, A.; Petäjä, J.; Siitonen, S.; Janér, C.; Andersson, S. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr. Res. 2013, 73, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.; Severin, Y.; Zanotelli, V.R.T.; Bodenmiller, B. In-Depth Characterization of Monocyte-Derived Macrophages using a Mass Cytometry-Based Phagocytosis Assay. Sci. Rep. 2019, 9, 1925. [Google Scholar] [CrossRef] [PubMed]
- Lindner, B.; Burkard, T.; Schuler, M. Phagocytosis assays with different pH-sensitive fluorescent particles and various readouts. BioTechniques 2020, 68, 245–250. [Google Scholar] [CrossRef]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 22. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 2011, 89, 557–563. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Porcheray, F.; Viaud, S.; Rimaniol, A.C.; Léone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yao, X.; Gordon, E.M.; Barochia, A.; Cuento, R.A.; Kaler, M.; Meyer, K.S.; Keeran, K.J.; Nugent, G.Z.; Jeffries, K.R.; et al. A CCL24-dependent pathway augments eosinophilic airway inflammation in house dust mite-challenged Cd163−/−mice. Mucosal Immunol. 2016, 9, 702–717. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, M.; Bae, Y.K.; Kim, G.H.; Choi, S.J.; Oh, W.; Um, S.; Jin, H.J. Decorin Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Induces Macrophage Polarization via CD44 to Repair Hyperoxic Lung Injury. Int. J. Mol. Sci. 2019, 20, 4815. [Google Scholar] [CrossRef]
- Timmermann, M.; Högger, P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic. Biol. Med. 2005, 39, 98–107. [Google Scholar] [CrossRef]
- Zhi, Y.; Gao, P.; Xin, X.; Li, W.; Ji, L.; Zhang, L.; Zhang, X.; Zhang, J. Clinical significance of sCD163 and its possible role in asthma (Review). Mol. Med. Rep. 2017, 15, 2931–2939. [Google Scholar] [CrossRef]
- Weaver, L.K.; Pioli, P.A.; Wardwell, K.; Vogel, S.N.; Guyre, P.M. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J. Leukoc. Biol. 2007, 81, 663–671. [Google Scholar] [CrossRef]
- Kneidl, J.; Löffler, B.; Erat, M.C.; Kalinka, J.; Peters, G.; Roth, J.; Barczyk, K. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell. Microbiol. 2012, 14, 914–936. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J. Neuroinflamm. 2019, 16, 66. [Google Scholar] [CrossRef]
- Garingo, A.; Tesoriero, L.; Cayabyab, R.; Durand, M.; Blahnik, M.; Sardesai, S.; Ramanathan, R.; Jones, C.; Kwong, K.; Li, C.; et al. Constitutive IL-10 expression by lung inflammatory cells and risk for bronchopulmonary dysplasia. Pediatr. Res. 2007, 61, 197–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McColm, J.R.; Stenson, B.J.; Biermasz, N.; McIntosh, N. Measurement of interleukin 10 in bronchoalveolar lavage from preterm ventilated infants. Arch. Dis. Childhood. Fetal Neonatal Ed. 2000, 82, F156–F159. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.; Lui, K.; Wang, H.; Henry, R. Decreased interleukin-10 in tracheal aspirates from preterm infants developing chronic lung disease. Acta Paediatr. 2002, 91, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Vento, G.; Capoluongo, E.; Matassa, P.G.; Concolino, P.; Vendettuoli, V.; Vaccarella, C.; Frezza, S.; Zuppi, C.; Romagnoli, C.; Ameglio, F. Serum levels of seven cytokines in premature ventilated newborns: Correlations with old and new forms of bronchopulmonary dysplasia. Intensive Care Med. 2006, 32, 723–730. [Google Scholar] [CrossRef]
- Thompson, A.; Bhandari, V. Pulmonary Biomarkers of Bronchopulmonary Dysplasia. Biomark. Insights 2008, 3, 361–373. [Google Scholar] [CrossRef]
- McGowan, E.C.; Kostadinov, S.; McLean, K.; Gotsch, F.; Venturini, D.; Romero, R.; Laptook, A.R.; Sharma, S. Placental IL-10 dysregulation and association with bronchopulmonary dysplasia risk. Pediatr. Res. 2009, 66, 455–460. [Google Scholar] [CrossRef]
- Rossato, M.; Curtale, G.; Tamassia, N.; Castellucci, M.; Mori, L.; Gasperini, S.; Mariotti, B.; Luca, M.D.; Mirolo, M.; Cassatella, M.A.; et al. IL-10 induced microRNA-187 negatively regulates TNF, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, E3101–E3110. [Google Scholar] [CrossRef]



| MIC90 (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Bacteria | Mero | Cipro | Colistin | AVR-48 | Meropenem (AVR-48) | Ciprofloxacin (AVR-48) | Colistin (AVR-48) |
| P. aeruginosa (10145) | 4.0 | 2.0–4.0 | 8.0 | >200 | 1.5 ± 0.3 (4.6 ± 3.0) | 2.0 (3.6 ± 2.3) | 2.0 (3.6 ± 2.3) |
| A. baumannii (19606) | 0.5–1.0 | 2.0–4.0 | ND | >200 | ND | 1.0 (4.6 ± 3.0) | ND |
| MRSA* (BAA 1760) | ND | ND | >200 | >200 | ND | ND | 14.5 ± 9.5 (4.6 ± 3.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, S.; Panda, S.K.; Donkor, M.; Acharya, E.; Jones, H.; Acharya, S. Chitin Derived Small Molecule AVR-48 Reprograms the Resting Macrophages to an Intermediate Phenotype and Decrease Pseudomonas aeruginosa Mouse Lung Infection. Immuno 2022, 2, 651-670. https://doi.org/10.3390/immuno2040040
Behera S, Panda SK, Donkor M, Acharya E, Jones H, Acharya S. Chitin Derived Small Molecule AVR-48 Reprograms the Resting Macrophages to an Intermediate Phenotype and Decrease Pseudomonas aeruginosa Mouse Lung Infection. Immuno. 2022; 2(4):651-670. https://doi.org/10.3390/immuno2040040
Chicago/Turabian StyleBehera, Sumita, Santosh K. Panda, Michael Donkor, Eesha Acharya, Harlan Jones, and Suchismita Acharya. 2022. "Chitin Derived Small Molecule AVR-48 Reprograms the Resting Macrophages to an Intermediate Phenotype and Decrease Pseudomonas aeruginosa Mouse Lung Infection" Immuno 2, no. 4: 651-670. https://doi.org/10.3390/immuno2040040
APA StyleBehera, S., Panda, S. K., Donkor, M., Acharya, E., Jones, H., & Acharya, S. (2022). Chitin Derived Small Molecule AVR-48 Reprograms the Resting Macrophages to an Intermediate Phenotype and Decrease Pseudomonas aeruginosa Mouse Lung Infection. Immuno, 2(4), 651-670. https://doi.org/10.3390/immuno2040040