Abstract
Inflammatory processes represent a pivotal element in the development and complications of cardiovascular diseases (CVDs). Targeting these processes can lead to the alleviation of cardiomyocyte (CM) injury and the increase of reparative mechanisms. Loss of CMs from inflammation-associated cardiac diseases often results in heart failure (HF). Evidence of the crosstalk between nuclear factor-kappa B (NF-κB), Hippo, and mechanistic/mammalian target of rapamycin (mTOR) has been reported in manifold immune responses and cardiac pathologies. Since these signaling cascades regulate a broad array of biological tasks in diverse cell types, their misregulation is responsible for the pathogenesis of many cardiac and vascular disorders, including cardiomyopathies and atherosclerosis. In response to a myriad of proinflammatory cytokines, which induce reactive oxygen species (ROS) production, several molecular mechanisms are activated within the heart to inaugurate the structural remodeling of the organ. This review provides a global landscape of intricate protein–protein interaction (PPI) networks between key constituents of NF-κB, Hippo, and mTOR signaling pathways as quintessential targetable candidates for the therapy of cardiovascular and inflammation-related diseases.
1. Introduction
Heart failure (HF) is an elaborate clinical syndrome, which is caused by apoptosis/necrosis/autophagy of cardiomyocytes (CMs) and extracellular matrix (ECM) remodeling, finally leading to ventricular dysfunction and deficient cardiac output [1,2,3]. Despite the current standard therapeutic approaches in HF and the neurohormonal antagonists, which offer improved clinical outcome, novel therapeutic agents are still urgently needed to gain insights into this field [4]. In acute myocardial infarction (MI), an etiology of HF, the subsequent inflammatory feedback plays a key role in rejuvenating the injured cardiac region. Targeting the inflammatory signaling pathways to diminish myocardial and arterial damage in other cardiovascular pathologies that lead to HF, including atherosclerosis and cardiomyopathies, could represent an innovative tool to lessen disease progression and restore CM integrity [5]. The engagement of the immune system is well-recognized as a critical component in the pathological mechanisms of cardiovascular diseases (CVDs) [6,7,8]. Crucially, several inflammatory markers, C-reactive protein (CRP), interleukin-6 (IL-6), d-dimers, lipoprotein-associated phospholipase A2, and long pentraxin 3 (PTX3), are increased in patients with CVDs, connecting cardiovascular incidents with inflammatory status and allowing prognosis [9,10,11,12]. The development of transcriptomic analyses has improved our understanding of signaling pathways in CVD inflammation. Decidedly, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a paramount inflammation-driven mediator, cooperates with Hippo and mechanistic/mammalian target of rapamycin (mTOR) signaling networks to synchronize the immune/inflammatory responses. This review briefly delineates the biological implications of their interchanges to target inflammation-associated CVDs and CM regeneration.
2. Inflammatory Mediators in Cardiovascular Diseases
The concept of inflammation in CVDs has been recognized as the main trigger for the progression and pathogenesis of HF [13]. Importantly, the interactions between inflammatory mediators orchestrate the immune response and tissue remodeling. The persistence of the inflammatory status might be the consequence of a steady activation of proinflammatory cascades and also might be correlated with less likelihood of resolution and repair phase [14,15]. Several mediators, including CRP, PTX3, and tumor necrosis factor α (TNF-α), are enhanced in cardiovascular pathologies (Table 1) [12,16,17]. However, anti-inflammatory approaches used in clinical trials and experimental studies provide information about these mediators’ pathophysiological effects rather than proposing a current treatment for HF and other CVDs [18].
It is primarily understood that atherosclerosis represents a major contributor to the spectrum of coronary heart disease [19]. The atherosclerotic process is caused by endothelial dysfunction and increased permeability of low-density lipoprotein (LDL) and immune cells into the intima [20]. Progressively, subendothelial LDL accumulation generates metabolic distresses, counting hyperlipidemia, hypertension, and diabetes [21,22]. Interestingly, the interconnection between inflammation and atherosclerosis plays a key role in clinical cardiovascular risk, increasing the interest in targeting agents of the inflammatory cascades.
CRP is one of the best-scrutinized acute-phase proteins in CVDs [23,24]. Raised CRP levels are connected with high risks of developing MI, atrial fibrillation, and peripheral artery disease [25,26]. Whether CRP constitutes merely an inflammatory biomarker and contributes to CVD pathogenesis remains an open question. A randomized trial conducted by Chan et al. [27] has shown that rosuvastatin administered in patients with aortic stenosis diminishes CRP levels (Table 1); however, it does not influence the severity of the disease. This trial agrees with the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) and GISSI-HF trials which demonstrated neutral effects of rosuvastatin on the progression of HF [28,29].
Studies from animal models underscored that PTX3 exerts cardioprotective effects, regulating inflammatory conditions and ECM remodeling [30,31]. PTX3 is enhanced by anti-inflammatory agents, including IL-10 and high-density lipoproteins (HDL), substantiating that PTX3, as an essential mediator of innate immunity, regulates the proatherogenic response into the vascular wall [32]. Moreover, using immunohistochemistry, a study has confirmed the expression of PTX3 in patients with acute MI and infectious myocarditis (Table 1) [33]. Specifically, PTX3 was mainly secreted by macrophages and endothelial cells, accumulating in the interstitial medium [33]. Moreover, the Lipid Assessment Trial Italian Network (LATIN) has shown that PTX3 represents an attractive prognostic tool in patients with MI and ST elevation [34]. It was an independent predictor of three-month mortality, compared with other cardiac biomarkers, such as creatine kinase (CK), troponin T, and N-terminal pro-brain natriuretic peptide (NT-proBNP).
TNF-α represents a widely documented proinflammatory cytokine in the development of CVDs and HF [35,36]. TNF-α modulates its different effects by attaching to tumor necrosis factor receptor 1 (TNFR1) and tumor necrosis factor receptor 2 (TNFR2) [37,38]. Essentially, TNFR1 promotes apoptosis in contrast with TNFR2 which is involved in cell survival [39]. In a murine model with TNFR1 loss of function, at the administration of TNF-α in the cardiac tissue, TNFR2 was upregulated, as a feedback-dependent mechanism [40]. Additionally, increased amounts of TNF-α in the myocardium resulted in left ventricular dilation, due to the induction of negative inotropic actions [41]. The treatment of the animals with a TNF-α antagonist (TNFR: Fc) reversed some of the TNF-α-induced effects, emphasizing that neutralization of this cytokine with specific antagonists would show significant benefits in patients with HF and other inflammatory conditions, such as rheumatoid arthritis [41,42]. The effect of etanercept, a human soluble TNF receptor fusion protein, in the RENEWAL (Randomized Etanercept Worldwide Evaluation)—combined data from RECOVER (Research into Etanercept: Cytokine Antagonism in Ventricular Dysfunction) and RENAISSANCE (Randomized Etanercept North American Strategy to Study Antagonism of Cytokines) clinical trials—deserves to be underlined (Table 1). Etanercept failed to improve the clinical status of patients with New York Heart Association (NYHA) class II to IV chronic HF compared with controls [43]. In addition, etanercept injection in induced rheumatoid arthritis rats diminished TNF-α levels and, intriguingly, lowered the nuclear location of NF-κB [44]. A more selective blockade of TNF-α signaling with specific inhibitors remains an open area of research.

Table 1.
Experimental studies/ clinical trials targeting inflammatory mediators in cardiovascular diseases.
Table 1.
Experimental studies/ clinical trials targeting inflammatory mediators in cardiovascular diseases.
| Inflammatory Mediator | Anti-Inflammatory Agent | Experimental Study | Clinical Trial | Outcomes | References |
|---|---|---|---|---|---|
| CRP | Rosuvastatin | - | Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) | ↓ CRP levels ↓ LDL cholesterol | [27] |
| - | GISSI-HF (Gruppo Italiano Per Lo Studio Della Sopravvivenza Nell’Insufficienza Cardiaca-Heart Failure) | [28] | |||
| - | Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) | [29] | |||
| PTX3 | - | ✓ | - | ↑ PTX3 in patients with acute MI and infectious myocarditis | [33] |
| - | - | Lipid Assessment Trial Italian Network (LATIN) | PTX3 prognostic tool: 3 month mortality in patients with MI and ST elevation | [34] | |
| TNF-α | Etanercept | - | RENEWAL (Randomized Etanercept Worldwide Evaluation)—combined data from RECOVER and RENAISSANCE | No improvement on the rate of death or hospitalization in patients with NYHA class II to IV chronic HF | [43] |
| ✓ | - | ↓ TNF-α ↓ NF-κB in induced rheumatoid arthritis rats | [44] |
CRP: C-reactive protein; HF: heart failure; MI: myocardial infarction; NYHA: New York Heart Association; PTX3: long pentraxin 3; and TNF-α: tumor necrosis factor α; ↑: increase; ↓: decrease; ✓: applicable.
3. Reactive Oxygen Species (ROS), NADPH Oxidases (NOXs), and NF-κB: Putative Therapeutic Targets
ROS constitute key mediators in signaling pathways involved in cardiovascular pathophysiology [45]. Upon induction of stressful conditions, such as MI, diabetes mellitus, and hypercholesterolemia, the balance between ROS (oxidants) and antioxidant mechanisms is inclined towards the former, contributing to CMs damage and apoptosis [46]. A growing body of evidence highlighted the pivotal function of NADPH oxidase (NOX) in specific CVDs [47,48]. These enzymes are major sources of ROS, as primary generators of oxidative agents [47]. In essence, there are seven isoforms of NOXs: NOX1–NOX5, dual oxidase 1 (DUOX1), and DUOX2 [49], each presenting a specific subcellular localization that regulates the type of ROS [50]. NOXs promote endothelial dysfunction in atherosclerosis. During the progression of atherosclerotic events, ROS generated by NOXs induction proceed with endothelial nitric oxide synthase (eNOS) uncoupling and mitochondrial dysfunction [51]. Experimental attempts to recouple eNOS include overexpression of GTPCH1 in endothelial cells, resulting in NO bioavailability and reduction in inflammatory conditions in ApoE knockout mice [52]. Interestingly, another recent study emphasized that downregulation of DUOX2 in telocytes, a type of interstitial cell, leads to a decrease in oxidative stress in inflamed lungs of mice with acute respiratory distress syndrome (ARDS) [53]. Targeting telocyte-specific DUOX2 might also be an attractive therapeutic option in CMs regeneration, given that ARDS is connected with cardiac dysfunction and organ failure [54,55]. NF-κB signaling constitutes a crucial mediator of inflammatory processes [56]. NF-κB target genes are of central importance in regulating the production of ROS [57]. Chiefly, NF-κB impedes cellular apoptosis by attenuating ROS expression [57,58]. Along these lines, recombinant serum amyloid A1 (SAA1) protein induces NOX4/ROS inflammatory axis by upregulating the levels of phosphorylated (p)-p38 and p-p65 NF-κB subunit in vascular smooth muscle cells (vSMCs) [59]. Future therapeutic agents mediating SAA1/NOX4/ROS inhibition might improve the clinical status of patients with atherosclerosis and associated-CVDs.
4. Targeting Inflammatory Signaling Pathways
4.1. NF-κB Signaling Pathway and Its Role in Cardiovascular Biology
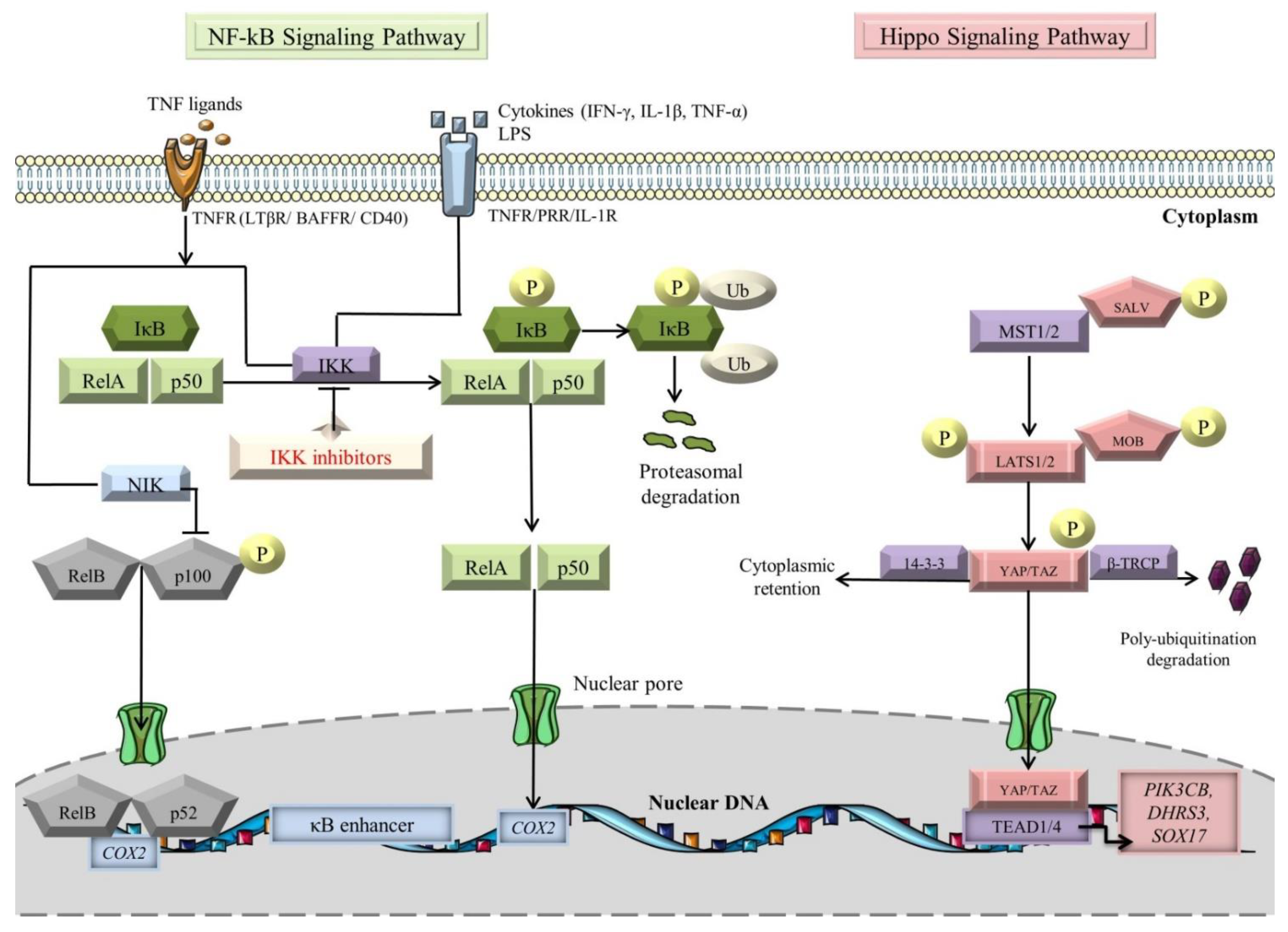
NF-κB signaling represents a well-known regulator of inflammatory and immune responses in a plethora of pathophysiological events [60,61,62,63]. The NF-κB family of transcription factors (TFs) is also specifically involved in the pathogenesis of several CVDs, including MI and HF [64,65,66]. This family comprises five members: NF-κB1 (also known as p50), NF-κB2 (also known as p52), RelA (also known as p65), RelB, and c-Rel, all sharing a common amino-terminal Rel homology domain (RHD), which is pivotal for attaching to cognate DNA promoters, in addition to dimerization [67,68,69]. In terms of their capacity to target promoter/enhancer regions, Rel subfamily members (RelA, RelB, and c-Rel) accommodate transactivation domains at their C-termini, compared with NF-κB1 and NF- κB2, which lack these domains and operate as transcriptional repressors [70,71,72]. Within cells, the members of the NF-κB family assemble as diverse homo- and heterodimers. In the heart, p50/p65 constitutes the most prevalent complex [67,73]. In an inactive state, dimers are bound to inhibitors of NF-κB proteins (IκBs), including IκBα, -β, and -γ, characterized by the presence of ankyrin repeats [69,74].
Activation of NF-κB occurs via the canonical and noncanonical (alternative) signaling pathways, with different operating mechanisms (Figure 1) [75,76]. Molecules such as TNFα, interleukin-1 β (IL-1β), and lipopolysaccharides (LPS) are known to switch on the canonical pathway [77,78,79]. The signal is transduced through tumor necrosis factor receptors (TNFRs), pattern-recognition receptors (PRRs), and interleukin-1 receptors (IL-1Rs) [76,79,80,81]. Under basal conditions, p105 and p100, the precursor proteins of large polypeptides, NF-κB1 and NF-κB2, are cleaved to generate active subunits, p50 and p52 [82]. The mainstay players in the canonical pathway are IκB kinase (IKK) complexes (IKKα (also known as IKK1), IKKβ (also known as IKK2), and IKKγ/NF-κB essential modulator (NEMO)), which induce the phosphorylation of the IκB molecules, followed by their poly-ubiquitination-dependent degradation by the β-transducin repeat containing E3 ubiquitin–protein ligase complex (SCFβ-TRCP) and proteasomal degradation [83,84,85,86]. Notably, transforming growth factor-β-activated kinase 1 (TAK1) is responsible for assimilating the PRR pathways for NF-κB induction [87,88,89]. In essence, TAK1 mediates IκBα phosphorylation by actuating the downstream kinase IKK, thereby promoting NF-κB activation [90]. Moreover, CD40, TNFR2, B-cell activation factor receptor (BAFFR), lymphotoxin β-receptor (LTβR), and receptor activator for nuclear factor kappa B (RANK) constitute decided inducible receptors of the noncanonical NF-κB signaling pathway [75,91]. The composite NF-κB ligand-receptor interplay sets off the NF-κB inducing kinase (NIK) activity, which in turn activates IKKα [92]. Then, the latter enzyme “seizes the opportunity” to phosphorylate p100, bringing about the formation of mature p52, which along with RelB complexes, translocate to the nucleus to induce expression of target genes [75,93,94,95].
Figure 1.
NF-kB and Hippo signaling pathways (see main text for details). The black arrows designate central signaling pathways; the continuous lines denote induction; and the continuous blunt-ended lines signify inhibition. BAFFR: B-cell-activating factor receptor; COX2: cyclooxygenase 2; DHRS3: dehydrogenase/reductase 3; IFN-γ: Interferon γ; IkB: inhibitor of NF-kB; IKK: IκB kinase; LATS1/2: large tumor suppressor 1/2; LPS: lipopolysaccharide; LTβR: lymphotoxin β receptor; MOB: LATS1/2-interacting protein Mps one binder; MST1/2: mammalian Sterile 20-like kinases 1/2; NF-kB: Nuclear factor kappa-light-chain-enhancer of activated B cells; NIK: NF-κB-inducing kinase; P: phosphorylation; PIK3CB: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta; SALV: Salvador; SOX17: SRY-box transcription factor 17; TAZ: transcriptional co-activator with PDZ-binding motif; TEAD1/4: transcription factors with TEA domain; TNF-α: tumor necrosis factor α; TNFR: TNF receptor; Ub: ubiquitination; YAP: Yes-associated protein; and β-TRCP: β-transducin repeat-containing E3 ubiquitin–protein ligase complex. Segments of the figure were portrayed by using artwork from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License, https://creativecommons.org/licenses/by/3.0/ (accessed on 4 August 2022).
NF-κB signaling has been documented in etiologies collateral to cardiometabolic disorders (e.g., diabetes), atherosclerosis, MI, cardiomyopathies, HF, comprising inflammation, thrombosis, endothelial cell (EC) dysfunction, generation of ROS, phenotypic switching of vSMC, and CM apoptosis [58,67,96,97,98,99,100]. Intriguingly, the sophisticated protein–protein interactions (PPIs) between NF-κB components and activators/repressors of its signaling cascade are generated through feedback loops [101]. To date, several pre-clinical studies and clinical trials have been designed to improve the clinical outcome of patients with inflammatory CVDs. Decidedly, the crosstalk between NF-κB and inflammation can be considered a double-edged sword in the pathogenesis of CVDs. On one hand, the cardioprotective role of NF-κB has been ascertained using chromatin immunoprecipitation (ChIP) analysis in a rat model. When postnatal ventricular CMs have gone through hypoxia stress, the transcription of the BCL-2 interacting protein 3 (BNIP3) has been induced by attaching E2F transcription factor 1 (E2F-1) to the BNIP3 promoter and the NF-κB p65 subunit has been displaced, bringing about intrinsic apoptosis [102]. This study provides compelling evidence that NF-κB advocates cell survival, suppressing the apoptotic response induced by transcriptional activation of E2F-1. On the other hand, Hamid and colleagues [103] have demonstrated that NF-κB p65 chronic blockage in CMs impairs cardiac remodeling, apoptosis, fibrosis, counterproductive endoplasmic reticulum stress, and also promotes CM survival in failing hearts. Moreover, in the context of inflammation and stress conditions, vSMCs undergo cytoskeletal rearrangements and build up robust plasticity from the contractile to the synthetic phenotype, opposite to their quiescent physiological state, similar to pericytes, contributing to the generation of atherosclerosis [104,105]. Another study from the Karunakaran lab [106] has shown that receptor-interacting serine/threonine-protein kinase 1 (RIPK1), a downstream signaling mediator of inflammatory receptors, represents a paramount inducer of the NF-κB pathway, which enhances atherosclerosis by promoting the discharge of inflammatory cytokines. Along these same lines, pentraxin family proteins, including CRP and PTX3, mediate some of the inflammatory responses to infectious/proinflammatory origins [107]. In this regard, CRP not only triggers inflammation via the Toll-like receptor 4 (TLR4)/NF-κB/transforming growth factor β (TGF-β) axis but also promotes apoptosis by itself of HL-1 cells (atrial CMs model), and, interestingly, inhibition of NF-κB alone rescues cell proliferation [108]. In comparison, PTX3 could be considered a more “faithful” inflammatory biomarker, as this protein is secreted locally by monocytes/macrophages, ECs, vSMCs, and other cell types, reaching a peak faster than CRP [30]. The NF-κB signaling cascade acts as a central point in PTX3 expression, as IKK2/IκB induces PTX3, leading to EC dysfunction and apoptosis, suggesting that PTX3 might be an atherogenic advocator [11]. Of note, the role of PTX3 has also been assessed in MI. In a model of PTX3 knockout mice, the extent of IL-6 was increased, and mice with IL-1R loss-of-function have not been capable of inducing the expression of PTX3, suggesting that PTX3 could be an attractive target in inflammation. Blocking specific cytokines, cell membrane receptors, the ubiquitin-proteasome system, and the IKK complex could offer an additional advantage over standard therapy [109]. To this end, anti-TNF-α treatment seems to have therapeutic benefits and alleviates the risk of cardiovascular events. The randomized ATTACH (Anti-Tnf alpha Therapy Against Chronic Heart failure) trial testing of the impact of infliximab, a chimeric TNFα-antibody, showed no advantage in HF with reduced ejection fraction (HFrEF) patients (see Table 2). Moreover, high doses of infliximab have been associated with cardiovascular mortality and hospitalization [110]. Furthermore, sulfasalazine, a disease-modifying anti-rheumatic drug (DMARD), exerts its anti-inflammatory and immunosuppressive effects via inhibiting IKKs’ activity, thus this drug abolishes NF-κB induction [111]. The placebo-controlled trial conducted by Tabit et al. [112] in patients with coronary artery disease did not meet its endpoint to reduce endothelial dysfunction as a result of the inflammatory response induced by atherosclerosis. Interestingly, sulfasalazine reduces the expression of TNFα-mediated inflammatory genes in peripheral blood mononuclear cells with no effect on systemic inflammatory biomarkers. A potential explanation for these results implies that the chronic status of coronary disease might be reluctant to manipulation of NF-κB inhibition. Therefore, considering NF-κB signaling a potential target for drugs in the field of inflammatory cardiovascular pathologies will empower researchers to appraise the roles of this pathway in the pathogenesis of various diseases, and further substantiate these findings into clinical translatability.

Table 2.
Pre-clinical studies/clinical trials targeting signaling pathways in cardiovascular pathologies.
4.2. Crosstalk between Hippo and NF-κB Signaling Pathways
Originally discovered in the fruit fly (Drosophila melanogaster), the Hippo signaling pathway has emerged as a centerpiece in the regulation of heart development, stemness, CM proliferation, survival, and apoptosis [117,118,119,120,121,122]. This signaling cascade accommodates several serine/threonine kinases, which constantly phosphorylate and sequester the transcriptional coactivators, Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) within the cytosol (Figure 1) [119,123]. Essentially, mammalian Ste20-like kinase 1/2 (MST1/2), in association with its adaptor protein Salvador (SALV), induces the downstream kinase large tumor suppressor 1/2 (LATS1/2) [124]. Sequentially, the latter kinase cooperates with its adaptor Mps one binder 1A and B (MOB1A/B) to phosphorylate YAP/TAZ, warding off their interplay with the TEA domain (TEAD1-4) family of TFs [125,126]. Consequently, the transcriptional coactivators are poly-ubiquitinated by SCFβ-TRCP E3 ligase and are broken down in the proteasome or complexed with 14-3-3 protein to downregulate the target genes (PIK3CB, DHRS3, and SOX17) transcription [127,128,129].
Eminently, Hippo signaling is acknowledged as a critical governor of CM proliferation during embryonic development [130,131], so it can be considered an ideal targetable candidate to promote heart regeneration/repair. Inducing the proliferation of adult CMs is more challenging than that of their embryonic/fetal/neonatal counterparts [132]. YAP/TAZ increases CMs’ resilience to inflammation-induced stresses, capable of reprogramming CMs to a primitive and fetal-like state, which is instrumental to cardiac survival [133,134].
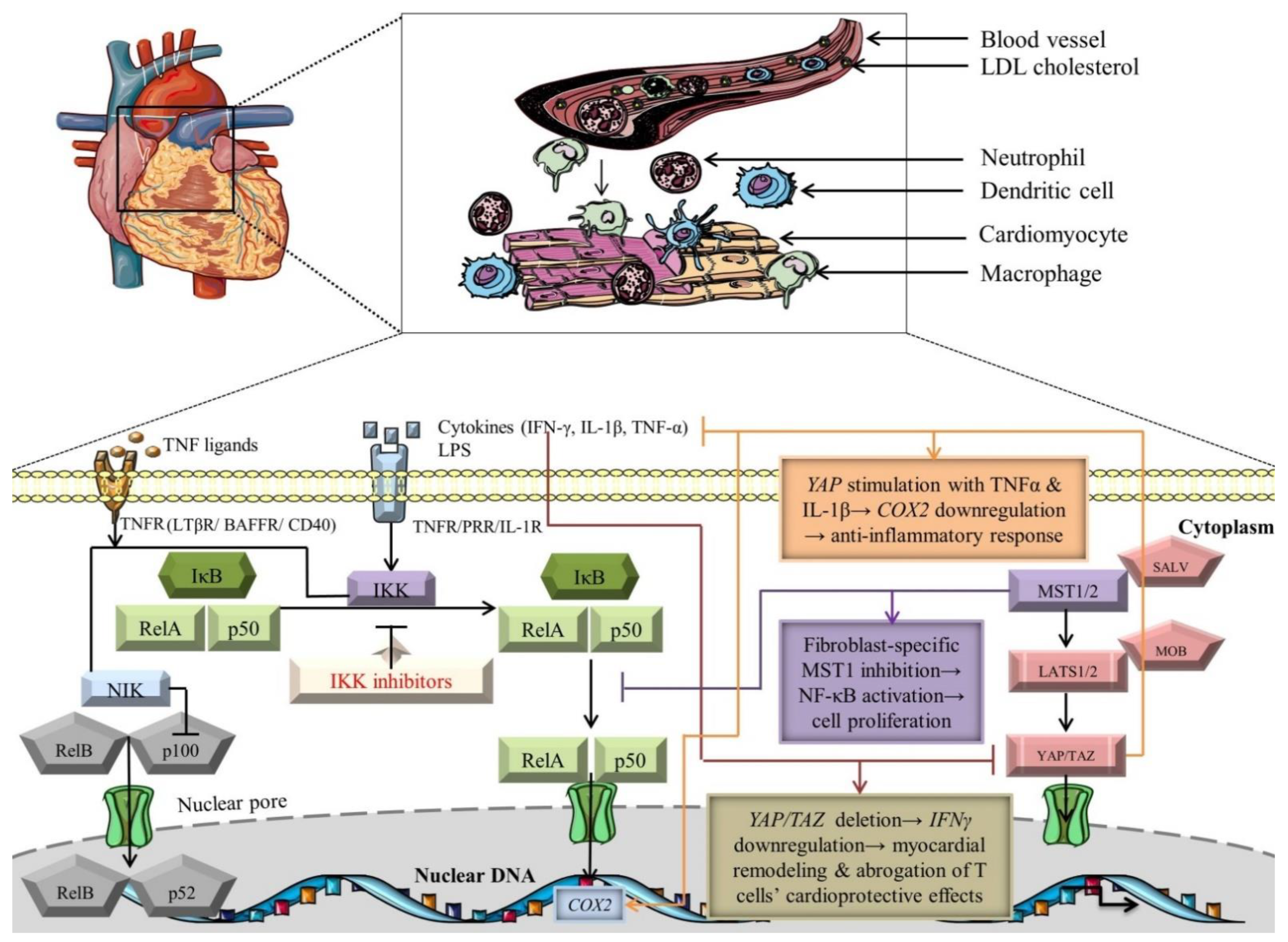
Noteworthy studies have meticulously scrutinized the crosstalk between Hippo and NF-κB signaling pathways (Figure 2) [135,136,137]. Since Hippo constitutes a mainstay regulator of the cardiovascular system homeostasis, it would be reasonable to also represent a modulator of input/output inflammatory and immune signals. In this regard, the deletion of YAP and TAZ in the epicardium is associated with the downregulation of IFN-γ, leading to defective recruitment of T-regulatory cells, which, in fact, possess a cardioprotective function by limiting myocardial remodeling after tissue injury (Figure 2) [114]. Notably, YAP emerges as an elaborate modulator of vascular inflammation, inhibiting NF-κB signaling by collaborating with the E3 ligase to ubiquitinate tumor necrosis factor receptor-associated factor 6 (TRAF6), thus YAP could be an attractive therapeutic target to indirectly alleviate the inflammatory response promoted by NF-κB morphogens [113]. ECs-specific deletion of YAP has negatively synchronized NF-κB activation, TAK1 induction, and cytokine-driven proinflammatory responses (Table 2) [113]. Moreover, another study cast light on the potential interplay loops between these signaling cascades. CMs-specific inhibition of the RAS association domain family 1 isoform A (RASSF1A)/MST1 signaling axis could avoid apoptosis and induce cardioprotective mechanisms, in contrast with cardiac fibroblasts, where suppression of this axis triggers NF-κB expression (inducer of cardiac hypertrophic/fibrotic phenotype) and cell proliferation, highlighting novel perspectives of targeting cell-specific genes/proteins linked to these pathways (Figure 2). Additionally, the cellular microenvironment is not just a simple bystander, but rather has a great impact on restoring tissue homeostasis and adjusting the paracrine/autocrine signals. At low cellular density, cyclooxygenase 2 (COX2), one of the NF-κB target genes, is downregulated in response to TNFα and IL-1β stimulation of a constitutively active form of YAP (YAP5SA)-expressing cells, emphasizing that the Hippo pathway could systematize particular NF-κB-specific genes with high significance and applicability in CVDs to inhibit the pathological settings (Figure 2) [138]. It can be hypothesized that activation of Hippo signaling, using gain-of-function genetic studies targeting YAP/TAZ, in association with inhibition of NF-κB mediators, will better define cardioprotective strategies to minimize cardiac injury and inhibit CMs apoptosis. From a clinical perspective, there have been no anti-inflammatory drugs regarding Hippo signaling targeting inflammation in associated CVDs. Yet, some TEAD inhibitors, such as VT3989, ION537, and IAG933, have been evaluated as therapeutic targets regarding tumor pathology, especially in those presenting mutations of neurofibromin 2 (NF2) [139]. Moreover, an ongoing phase I trial involving another TEAD inhibitor, IK-930, aims to evaluate its safety in subjects with advanced solid tumors with or without NF2 mutations [140]. However, future development of novel therapeutics targeting NF2 in clinical trials involves an exquisite balance regarding benefits in cardiovascular pathologies since NF2 is activated in arrhythmogenic cardiomyopathy, MI, and ischemia/reperfusion (I/R) in response to oxidative stress [141,142]. The molecular mechanisms modulating the activation of these signaling cascades and their cardioprotective/pathological outcome need to be clarified.
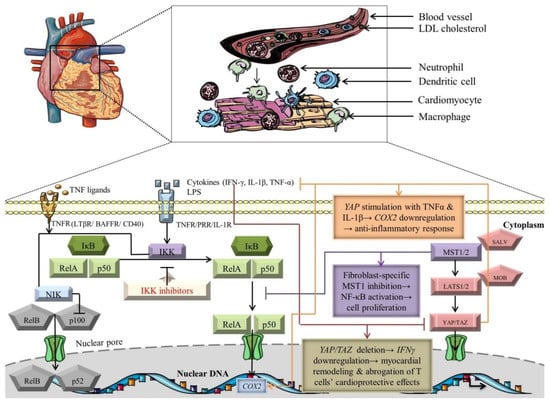
Figure 2.
Crosstalk-dependent outcomes of NF-κB and Hippo signaling pathways. The black arrows designate central signaling pathways; the continuous lines denote induction; and the continuous blunt-ended lines signify inhibition. BAFFR: B cell-activating factor receptor; COX2: cyclooxygenase 2; IFN-γ: Interferon γ; IkB: inhibitor of NF-kB; IKK: IκB kinase; LATS1/2: large tumor suppressor 1/2; LPS: lipopolysaccharide; LTβR: lymphotoxin β receptor; MOB: LATS1/2-interacting protein Mps one binder; MST1/2: mammalian Sterile 20-like kinases 1/2; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; NIK: NF-κB-inducing kinase; SALV: Salvador; TAZ: transcriptional co-activator with PDZ-binding motif; TNF-α: tumor necrosis factor α; TNFR: TNF receptor; and YAP: Yes-associated protein. Segments of the figure were portrayed by using artwork from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License, https://creativecommons.org/licenses/by/3.0/ (accessed on 4 August 2022).
4.3. Crosstalk between Mechanistic/Mammalian Target of Rapamycin (mTOR), NF-κB, and Hippo Signaling Pathways
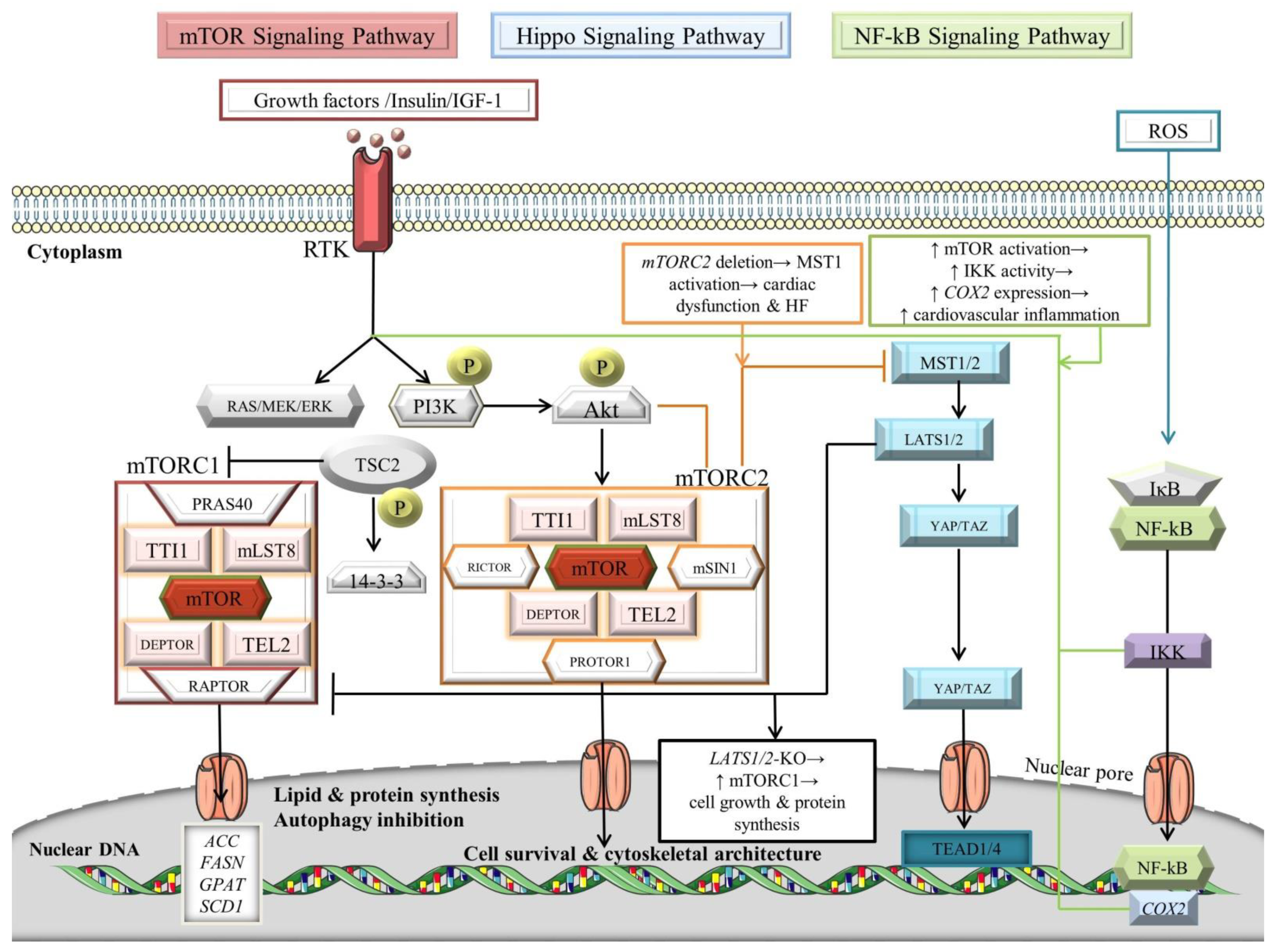
The mTOR complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), govern momentous biological processes, including cell survival, metabolism, myocardial angiogenesis, and stem cells differentiation into CMs [143,144,145,146,147]. The mTOR kinase represents an atypical serine/threonine kinase that belongs to the phosphoinositide kinase-related kinase (PIKK) family [148]. Substantially, both complexes comprise the proteins TELO2 interacting protein 1 (TTI1), mammalian lethal with SEC13 protein 8 (mLST8), DEP domain-containing mTOR-interacting protein (DEPTOR), and telomere length regulation protein TEL2 homolog (TEL2), in addition to mTOR (Figure 3) [149]. Dissimilarly, mTORC1 is composed of Proline-rich Akt substrate of 40 kDa (PRAS40) and regulatory-associated protein of mTOR (RAPTOR), in contrast with mTORC2, which comprises rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR), protein observed with Rictor-1 (PROTOR1), and mammalian ortholog of stress-activated map kinase-interacting protein 1 (mSIN1) [150,151,152]. The signal transduction of mTOR pathways dichotomize into phosphatidylinositol 3-kinase (PI3K)–v-akt murine thymoma viral oncogene homolog (Akt)–mTOR pathway and AMP-activated protein kinase (AMPK)–mTOR pathway (Figure 3) [105,153].
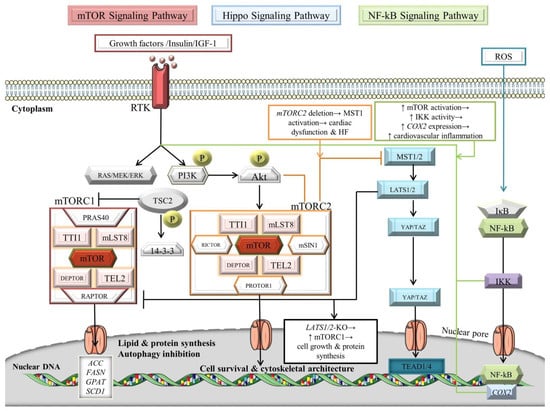
Figure 3.
Signaling crosstalk between NF-κB, Hippo, and mTOR pathways. For details, see main text. The black arrows designate central signaling pathways; the continuous arrows denote activation; and the continuous blunt-ended arrows indicate inhibition. ACC: acetyl-CoA carboxylase; Akt: v-akt murine thymoma viral oncogene homolog/Protein kinase B; COX2: cyclooxygenase 2; DEPTOR: DEP domain-containing mTOR-interacting protein; ERK: extracellular signal-regulated kinase; FASN: fatty acid synthase; GPAT: glycerol-3-phosphate acyltransferase; HF: heart failure; IkB: inhibitor of NF-kB; IGF-1: insulin-like growth factor 1; IKK: IκB kinase; LATS1/2: large tumor suppressor; mLST8: mammalian lethal with SEC13 protein 8; MOB: LATS1/2-interacting protein Mps one binder; mSIN1: mammalian ortholog of stress-activated map kinase-interacting protein 1; MST1/2: mammalian Sterile 20-like kinases 1/2; mTOR: mechanistic/mammalian target of rapamycin; mTORC1/2: mechanistic/mammalian target of rapamycin complex 1/2; NF-κB: nuclear factor-kappa B; P: phosphorylation; PI3K: phosphatidylinositol 3-kinase; PRAS40: Proline-rich Akt substrate of 40 kDa; PROTOR1: protein observed with Rictor-1; RAPTOR: regulatory-associated protein of mTOR; RICTOR: rapamycin-insensitive companion of mammalian target of rapamycin; ROS: reactive oxygen species; RTK: receptor tyrosine kinase; SALV: Salvador; SCD1: stearoyl-CoA desaturase-1; TAZ: transcriptional co-activator with PDZ-binding motif; TEAD1/4: transcription factors with TEA domain; TEL2: telomere length regulation protein TEL2 homolog; TSC2: tuberous sclerosis complex 2/tuberin; TTI1: TELO2 interacting protein 1; and YAP: Yes-associated protein. Parts of the figure were illustrated by using artwork from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License, https://creativecommons.org/licenses/by/3.0/ (accessed on 4 August 2022).
In essence, growth factors and insulin/insulin-like growth factor 1 (IGF-1) act as upstream adjusters by attaching to receptor tyrosine kinases (RTKs), inducing PI3K, which further phosphorylates Akt [154,155,156]. Alternatively, the signal transduction of RTKs could be modulated through RAS/RAF/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) [157]. It is widely recognized that mTORC2 activates Akt and protein kinase C (PKC) by phosphorylating their highly conserved C-terminal tail motifs [158]. In turn, Akt phosphorylates tuberous sclerosis complex 2 (TSC2)/tuberin, which further attaches to the 14-3-3 protein, becomes seized within the cytoplasm, and promotes the induction of mTORC1 by impairing the inhibition activity of the TSC1-TSC2 complex on the small GTPase RHEB [148,159,160,161,162]. The mTORC1 promotes lipogenesis and protein synthesis by processing sterol regulatory element-binding protein-1 (SREBP-1) to induce the transcription of acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN), glycerol-3-phosphate acyltransferase (GPAT), and stearoyl-CoA desaturase-1 (SCD1) lipogenic genes [163] while inhibiting the autophagic program [164]. Moreover, mTORC1 downregulates AMPK, a key activator of autophagy [165]. Interestingly, mTORC1 is responsible for regulating the myocardial ischemia scenario, whereas Beclin1 exerts cardioprotective effects in the reperfusion stage [165,166]. In addition, mTORC2 influences cell survival and cytoskeletal architecture by modulating the cAMP-dependent kinases, cGMP-dependent kinases, PKC, and protein kinase B (PKB)/Akt [154,167].
An elegant work from Li et al. [115] has revealed that upon stimulation with hesperidin, myocardial I/R injury has been alleviated by the downregulation of Beclin1 and upregulation of phosphorylated (p)-mTOR, p-Akt, and p-PI3K. It is well-described that reperfusion promotes extensive autophagy that produces detrimental consequences within the heart via ROS-mediated robust upregulation of Beclin 1, intriguingly, through NF-κB (Figure 3) [168,169,170,171,172,173]. Another recent work has disclosed that cardiac mTORC2 has negatively adjusted the MST1 kinase scheme, evocative of the crosstalk with the Hippo signaling pathway [174]. Interestingly, mTORC2 promotes survival and growth of CMs, and acts as a “guardian” of MST1 induction, to support the physiological state and preserve cardiac function. Besides, LATS1 and LATS2 phosphorylate RAPTOR to attenuate mTORC1 activation, suggesting again a direct interconnection between the Hippo and mTOR pathways [175]. This study highlights that LATS1/2 impairs mTORC1 signaling, leading to the inhibition of cellular proliferation, protein synthesis, and metabolism. It would be interesting to assess the regulatory capacity of other Hippo signaling molecules on the mTOR cascade in CMs to determine the putative application of the crosstalk-dependent mediators in future cardiac regenerative therapies. Additionally, a recent paper has provided evidence that mTOR signaling is involved in the pathogenesis of the atherosclerotic process. The transcriptome analyses of CD68+ myeloid cells attested that prosaposin (PSAP), a hub gene, is downregulated upon mTOR/ribosomal protein S6 kinase 1 (S6K1)-dependent inhibition [176]. Prosaposin, a member of the saposin family of proteins, exerts its roles in the regulation of lysosomal lipid metabolism and is mainly expressed in monocytes and macrophages [176,177]. This protein possesses anti-inflammatory properties, so it could serve as an excellent future targeted therapy for atherosclerosis. Moreover, dihydrotanshinone I (DHT) might support the significant role of the mTOR/transcription factor EB (TFEB)/NF-κB signaling axis in the inhibition of inflammation induced by cardiotoxicity upon administration of doxorubicin [178]. DHT possesses anti-inflammation roles by inhibiting the proinflammatory cytokines produced by M1 macrophages. To unravel the molecular mechanisms of the mTOR signaling pathway in CMs, Wang et al. [178] performed a co-incubation of doxorubicin-stimulated cells with an mTOR agonist, resulting in increased expression of TNF-α and COX2, and abolished levels of nuclear TFEB, along with an upregulation of p-IKKα/β. DHT has assumed the role of a therapeutic adjuvant, diminishing the levels of p-IKKα/β and p-NF-κB, and exerting its anti-apoptotic effects via the mTOR signaling pathway, thus DHT could be considered a cardioprotective agent with future implications in cardiotoxicity studies.
Clinical data have sustained proof of concept that mTOR inhibitors confer therapeutic benefits in CVDs [179,180]. The Controlled Level EVERolimus in Acute Coronary Syndromes (CLEVER-ACS) clinical trial [116] has employed patients going through acute ST-elevation MI (STEMI) to receive everolimus, an mTOR inhibitor, for five days (Table 2). The results warrant attention regarding the efficacy of everolimus, as neither primary endpoints (no reduction in MI size) nor secondary ones (no great improvement of microvascular obstruction) have been accomplished at 30 days, attested by cardiac magnetic resonance imaging (CMRI) [181].
Consequently, a better comprehension of the mechanisms underlying the pathological induction of mTOR pathways will provide a basis for designing state-of-the-art therapeutic strategies.
5. An Update of Targeted Therapy to Subdue Inflammatory Pathways
Given their implications in inflammatory processes, NF-κB, Hippo, and mTOR signaling pathways serve as key therapeutic targets [61,137,182].
NF-κB signaling cascade is essentially inaugurated at the cell membrane through the activation of receptors. Remarkably, the therapeutic monoclonal antibodies (mAbs), along with fusion proteins, constitute a novel technology to inhibit the proinflammatory cytokines in clinical trials. Several drugs, including TNF inhibitors (infliximab, etanercept, adalimumab, certolizumab pegol, and golimumab), Bruton’s Tyrosine Kinase (BTK) inhibitors (zanubrutinib), proteasome inhibitors (carfilzomib and ixazomib), and nuclear export inhibitors (selinexor) have been approved by Food and Drug Administration (FDA) [183,184,185,186,187]. Moreover, TRAF-STOP inhibitor 6877002 constitutes a selective inhibitor of CD40-TRAF6 interplay, which impedes the activation of the NF-κB pathway, and has been acknowledged as a repressor of the atherosclerotic process in Apoe-deficient mice [188]. Atorvastatin possesses immunomodulatory, anti-inflammatory, and lipid-lowering effects by inhibiting NF-κB, TNF-α, and IL-6 after zymosan-induced vascular inflammation [189].
Accumulating evidence has proved that the Hippo pathway represents a promising therapeutic target for CVD therapy [122,190]. XMU-MP-1, an MST1/2 inhibitor, diminishes CMs hypertrophy and enhances cell survival by inducing the activity of YAP in response to pressure overload [191]. IRF3 constitutes an agonist of YAP, promoting its activation and nuclear translocation [192]. Knockdown of IRF3 has been shown to suppress gastric tumor progression in a YAP-dependent manner [193]. Prospective therapeutic advances will be able to confirm IRF3-mediated responses to the induction of YAP/TAZ signaling regarding the modulation of inflammatory processes in cardiovascular pathologies and cardiac regeneration.
The effectiveness of mTOR inhibitors, including rapamycin and rapamycin analogs (rapalogs), has been substantiated in manifold pathological states, including hypertrophy, autophagy, and immunosuppression [194,195,196,197]. Rapamycin exerts cardioprotective effects on mice with transverse aortic constriction (TAC) [198]. As a potential contributor to hypertrophy, mTOR has been considered an ideal target to regulate metabolic processes by decreasing the activity of S6K1, eventually leading to a reduction in protein translation. Furthermore, rapamycin avoids CM apoptosis and induces CM autophagy by inhibiting mTOR and endoplasmic reticulum stress pathways in chronic HF [199]. Of interest, some rapalogs, including everolimus, temsirolimus, and deforolimus, possess antineoplastic roles and harmonize the immunomodulation of adaptive immunity [200,201,202,203,204]. Although everolimus has not shown great improvement in reversing the condition of STEMI patients in the CLEVER-ACS trial, still, it deserves further investigation, due to its potential benefits. For instance, everolimus-treated rats after induction of MI exhibited an improvement in ventricular function and an increase in the autophagy process [180].
Undoubtedly, targeting NF-κB, Hippo, and mTOR signaling components represents a concept that has gained momentum in the cardiovascular area. The regulation of CVDs’ pathogenesis plays a crucial role in the determination of targeted therapeutic strategies. Thereby, CM/immune cell-specific modulation of signaling pathways might be a promising modus operandi to prevent and treat inflammation in cardiovascular pathologies and to induce myocardial regeneration.
6. Conclusions and Perspectives
Inflammation constitutes an essential mechanism in the development and advancement of CVDs. Cardiac remodeling with persistent inflammation results in myocardial fibrosis and ECM adjustments, which cause arrhythmias, exacerbate cardiac function, and bring about HF. In the crosstalk between inflammation and CVDs, the NF-κB, Hippo, and mTOR signaling pathways intermingle to synchronize cell fate plasticity, shaping diverse biological functions within CMs and immune cells. Computational approaches and cell-specific nanobiologics in conjunction with accurate genetical analyses will provide ingenious answers to the long-lasting question of how signaling networks mediate diverse transcriptional programs to promote CM regeneration and reverse cardiac dysfunction in the associated inflammatory phenotypes. Since these signaling cascades play a key role in the inflammation and regeneration processes, further research is required to more comprehensively characterize the proliferative orchestration of CMs and to develop therapeutic blueprints without potential side effects.
Funding
This work received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S. Cardiomyocyte and Heart Failure; Chapter 8; Muramatsu, T., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; Hill, J.A.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Vishram-Nielsen, J.K.K.; Pagnesi, M.; Adamo, M.; Lombardi, C.M.; Gustafsson, F.; Metra, M. Advanced heart failure: Guideline-directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. 2022, 9, 1507–1523. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A.J.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, II2–II10. [Google Scholar] [CrossRef]
- Riehle, C.; Bauersachs, J. Key inflammatory mechanisms underlying heart failure. Herz 2019, 44, 96–106. [Google Scholar] [CrossRef]
- Vos, A.G.; Idris, N.S.; Barth, R.E.; Klipstein-Grobusch, K.; Grobbee, D.E. Pro-Inflammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PLoS ONE 2016, 11, e0147484. [Google Scholar] [CrossRef]
- Tousoulis, D.; Antoniades, C.; Stefanadis, C. Assessing inflammatory status in cardiovascular disease. Heart 2007, 93, 1001–1007. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, G.; Wang, Y.; Yue, Y.; Zhao, W. A key mediator, PTX3, of IKK/IκB/NF-κB exacerbates human umbilical vein endothelial cell injury and dysfunction. Int. J. Clin. Exp. Pathol. 2014, 7, 7699–7707. [Google Scholar]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in cardiovascular disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L.J. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Reina-Couto, M.; Vale, L.; Carvalho, J.; Bettencourt, P.; Albino-Teixeira, A.; Sousa, T. Resolving Inflammation in Heart Failure: Novel Protective Lipid Mediators. Curr. Drug Targets 2016, 17, 1206–1223. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Reina-Couto, M.; Pereira-Terra, P.; Quelhas-Santos, J.; Silva-Pereira, C.; Albino-Teixeira, A.; Sousa, T. Inflammation in Human Heart Failure: Major Mediators and Therapeutic Targets. Front. Physiol. 2021, 12, 746494. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and Cardiovascular Disease: From Pathogenesis to Therapeutic Target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Karadag, F.; Kirdar, S.; Karul, A.B.; Ceylan, E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur. J. Intern. Med. 2008, 19, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wu, Y.; Liu, E. C-reactive protein and cardiovascular disease: From animal studies to the clinic (Review). Exp. Med. 2020, 20, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J. Cell. Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Latini, R.; Florea, V.G.; Kuskowski, M.A.; Rector, T.; Masson, S.; Signorini, S.; Mocarelli, P.; Hester, A.; Glazer, R.; et al. C-reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005, 112, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Dumesnil, J.G.; Tam, J.; Ni, A.; Teo, K. Effect of rosuvastatin on C-reactive protein and progression of aortic stenosis. Am. Heart J. 2011, 161, 1133–1139. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Kjekshus, J.; Apetrei, E.; Barrios, V.; Böhm, M.; Cleland, J.G.F.; Cornel, J.H.; Dunselman, P.; Fonseca, C.; Goudev, A.; Grande, P.; et al. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 2007, 357, 2248–2261. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef]
- Fornai, F.; Carrizzo, A.; Forte, M.; Ambrosio, M.; Damato, A.; Ferrucci, M.; Biagioni, F.; Busceti, C.; Puca, A.A.; Vecchione, C. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immun. Ageing 2016, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Marchesi, P.; Pirillo, A.; Uboldi, P.; Chiesa, G.; Maina, V.; Garlanda, C.; Mantovani, A.; Catapano, A.L. Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 925–931. [Google Scholar] [CrossRef]
- Nebuloni, M.; Pasqualini, F.; Zerbi, P.; Lauri, E.; Mantovani, A.; Vago, L.; Garlanda, C. PTX3 expression in the heart tissues of patients with myocardial infarction and infectious myocarditis. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2011, 20, e27–e35. [Google Scholar] [CrossRef]
- Latini, R.; Maggioni, A.P.; Peri, G.; Gonzini, L.; Lucci, D.; Mocarelli, P.; Vago, L.; Pasqualini, F.; Signorini, S.; Soldateschi, D.; et al. Prognostic Significance of the Long Pentraxin PTX3 in Acute Myocardial Infarction. Circulation 2004, 110, 2349–2354. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Naga Prasad, S.V. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 117. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory Cytokines in Heart Failure: Mediators and Markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Urschel, K.; Cicha, I. TNF-α in the cardiovascular system: From physiology to therapy. Int. J. Interf. Cytokine Mediat. Res. 2015, 7, 9–25. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kribbs, S.B.; Clubb, F.J.; Michael, L.H.; Didenko, V.V.; Hornsby, P.J.; Seta, Y.; Oral, H.; Spinale, F.G.; Mann, D.L. Pathophysiologically Relevant Concentrations of Tumor Necrosis Factor-α Promote Progressive Left Ventricular Dysfunction and Remodeling in Rats. Circulation 1998, 97, 1382–1391. [Google Scholar] [CrossRef]
- Hussain, A.; Tarahomi, T.; Singh, L.; Bollampally, M.; Heydari-Kamjani, M.; Kesselman, M.M. Cardiovascular Risk Associated With TNF Alpha Inhibitor Use in Patients With Rheumatoid Arthritis. Cureus 2021, 13, e17938. [Google Scholar] [CrossRef]
- Mann, D.L.; McMurray, J.J.V.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.S.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef]
- Liu, C.-L.; Wang, Y.-Y. Effects of TNF-alpha/NF-kappa B signaling pathway on etanercept alleviating rheumatoid arthritis. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2017, 33, 373–376. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Karachaliou, G.-S.; Triantafyllis, A.S.; Karaolanis, G.I.; Tsilimigras, D.I.; Theocharis, S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann. Transl. Med. 2017, 5, 324. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Sirker, A.; Zhang, M.; Shah, A.M. NADPH oxidases in cardiovascular disease: Insights from in vivo models and clinical studies. Basic Res. Cardiol. 2011, 106, 735–747. [Google Scholar] [CrossRef]
- Augsburger, F.; Filippova, A.; Rasti, D.; Seredenina, T.; Lam, M.; Maghzal, G.; Mahiout, Z.; Jansen-Dürr, P.; Knaus, U.G.; Doroshow, J.; et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019, 26, 101272. [Google Scholar] [CrossRef]
- Lassègue, B.; San Martín, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Alp, N.J.; McAteer, M.A.; Khoo, J.; Choudhury, R.P.; Channon, K.M. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liang, T.; Zhou, Y.; Ju, H.; Song, D.; Fang, H. Telocytes reduce oxidative stress by downregulating DUOX2 expression in inflamed lungs of mice. Acta Biochim. Biophys. Sin. 2022, 54, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.M.; Mohr, N.M.; Graetz, T.J.; Lynch, I.P.; Dettmer, M.; Cullison, K.; Coney, T.; Gogineni, S.; Gregory, R. The impact of cardiac dysfunction on acute respiratory distress syndrome and mortality in mechanically ventilated patients with severe sepsis and septic shock: An observational study. J. Crit. Care 2015, 30, 65–70. [Google Scholar] [CrossRef]
- Dimai, S.; Semmler, L.; Prabhu, A.; Stachelscheid, H.; Huettemeister, J.; Klaucke, S.C.; Lacour, P.; Blaschke, F.; Kruse, J.; Parwani, A.; et al. COVID19-associated cardiomyocyte dysfunction, arrhythmias and the effect of Canakinumab. PLoS ONE 2021, 16, e0255976. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Yu, M.-H.; Li, X.; Li, Q.; Mo, S.-J.; Ni, Y.; Han, F.; Wang, Y.-B.; Tu, Y.-X. SAA1 increases NOX4/ROS production to promote LPS-induced inflammation in vascular smooth muscle cells through activating p38MAPK/NF-κB pathway. BMC Mol. Cell Biol. 2019, 20, 15. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Haudek, S.B.; Knuefermann, P.; Vallejo, J.G.; Chen, Z.J.; Michael, L.H.; Sivasubramanian, N.; Olson, E.N.; Entman, M.L.; Mann, D.L. Nuclear Factor-κB Protects the Adult Cardiac Myocyte Against Ischemia-Induced Apoptosis in a Murine Model of Acute Myocardial Infarction. Circulation 2003, 108, 3075–3078. [Google Scholar] [CrossRef]
- Santos, D.G.B.; Resende, M.F.; Mill, J.G.; Mansur, A.J.; Krieger, J.E.; Pereira, A.C. Nuclear Factor (NF) κB polymorphism is associated with heart function in patients with heart failure. BMC Med. Genet. 2010, 11, 89. [Google Scholar] [CrossRef]
- Chen, F.; Castranova, V.; Shi, X.; Demers, L.M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999, 45, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell type specific roles of nf-kb linking inflamation and thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Saccani, S.; Pantano, S.; Natoli, G. Modulation of NF-kappaB activity by exchange of dimers. Mol. Cell 2003, 11, 1563–1574. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Marienfeld, R.; May, M.J.; Berberich, I.; Serfling, E.; Ghosh, S.; Neumann, M. RelB forms transcriptionally inactive complexes with RelA/p65. J. Biol. Chem. 2003, 278, 19852–19860. [Google Scholar] [CrossRef]
- Cartwright, T.; Perkins, N.D.; Wilson, C.L. NFKB1: A suppressor of inflammation, ageing and cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-κB in the heart: To be or not to NF-κB. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef]
- Huguet, C.; Crepieux, P.; Laudet, V. Rel/NF-κB transcription factors and IκB inhibitors: Evolution from a unique common ancestor. Oncogene 1997, 15, 2965–2974. [Google Scholar] [CrossRef][Green Version]
- Sun, S.-C. Non-canonical NF-κB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and Function of NF-κB Transcription Factors in the Immune System. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Greten, F.R.; Arkan, M.C.; Bollrath, J.; Hsu, L.C.; Goode, J.; Miething, C.; Göktuna, S.I.; Neuenhahn, M.; Fierer, J.; Paxian, S.; et al. NF-κB Is a Negative Regulator of IL-1β Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKβ. Cell 2007, 130, 918–931. [Google Scholar] [CrossRef]
- Rex, J.; Lutz, A.; Faletti, L.E.; Albrecht, U.; Thomas, M.; Bode, J.G.; Borner, C.; Sawodny, O.; Merfort, I. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes During LPS-Induced Inflammation. Front. Physiol. 2019, 10, 117. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Chen, L.-F.; Greene, W.C. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 2004, 5, 392–401. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Caamaño, J.; Hunter, C.A. NF-kappaB family of transcription factors: Central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 2002, 15, 414–429. [Google Scholar] [CrossRef]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Heissmeyer, V.; Krappmann, D.; Hatada, E.N.; Scheidereit, C. Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol. Cell. Biol. 2001, 21, 1024–1035. [Google Scholar] [CrossRef]
- Orian, A.; Gonen, H.; Bercovich, B.; Fajerman, I.; Eytan, E.; Israël, A.; Mercurio, F.; Iwai, K.; Schwartz, A.L.; Ciechanover, A. SCF(beta)(-TrCP) ubiquitin ligase-mediated processing of NF-kappaB p105 requires phosphorylation of its C-terminus by IkappaB kinase. EMBO J. 2000, 19, 2580–2591. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Gohda, J.; Matsumura, T.; Inoue, J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J. Immunol. 2004, 173, 2913–2917. [Google Scholar] [CrossRef]
- Hu, H.; Sun, S.-C. Ubiquitin signaling in immune responses. Cell Res. 2016, 26, 457–483. [Google Scholar] [CrossRef]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.-C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Sun, S.-C. The noncanonical NF-κB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef]
- Tian, B.; Brasier, A.R. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog. Horm. Res. 2003, 58, 95–130. [Google Scholar] [CrossRef]
- Maier, H.J.; Schips, T.G.; Wietelmann, A.; Krüger, M.; Brunner, C.; Sauter, M.; Klingel, K.; Böttger, T.; Braun, T.; Wirth, T. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. USA 2012, 109, 11794–11799. [Google Scholar] [CrossRef]
- Bonomini, F. NF-Κb—A Key Factor in Atherogenesis and Atheroprogression; Chapter 2; Favero, G., Ed.; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar]
- Gaspar-Pereira, S.; Fullard, N.; Townsend, P.A.; Banks, P.S.; Ellis, E.L.; Fox, C.; Maxwell, A.G.; Murphy, L.B.; Kirk, A.; Bauer, R.; et al. The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am. J. Pathol. 2012, 180, 929–939. [Google Scholar] [CrossRef]
- Dai, X.; Thiagarajan, D.; Fang, J.; Shen, J.; Annam, N.P.; Yang, Z.; Jiang, H.; Ju, D.; Xie, Y.; Zhang, K.; et al. SM22α suppresses cytokine-induced inflammation and the transcription of NF-κB inducing kinase (Nik) by modulating SRF transcriptional activity in vascular smooth muscle cells. PLoS ONE 2017, 12, e0190191. [Google Scholar] [CrossRef]
- Siednienko, J.; Jankowska, E.A.; Banasiak, W.; Gorczyca, W.A.; Ponikowski, P. Nuclear factor-kappaB activity in peripheral blood mononuclear cells in cachectic and non-cachectic patients with chronic heart failure. Int. J. Cardiol. 2007, 122, 111–116. [Google Scholar] [CrossRef]
- Tieri, P.; Termanini, A.; Bellavista, E.; Salvioli, S.; Capri, M.; Franceschi, C. Charting the NF-κB pathway interactome map. PLoS ONE 2012, 7, e32678. [Google Scholar] [CrossRef]
- Shaw, J.; Yurkova, N.; Zhang, T.; Gang, H.; Aguilar, F.; Weidman, D.; Scramstad, C.; Weisman, H.; Kirshenbaum, L.A. Antagonism of E2F-1 regulated Bnip3 transcription by NF-kappaB is essential for basal cell survival. Proc. Natl. Acad. Sci. USA 2008, 105, 20734–20739. [Google Scholar] [CrossRef]
- Hamid, T.; Guo, S.Z.; Kingery, J.R.; Xiang, X.; Dawn, B.; Prabhu, S.D. Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc. Res. 2011, 89, 129–138. [Google Scholar] [CrossRef]
- Finsterwalder, R.; Ganesan, M.K.; Leb, H.; Habertheuer, A.; Basílio, J.; Lang, I.; Krunic, M.; Wiedemann, D.; Petzelbauer, P. Hypoxia/reperfusion predisposes to atherosclerosis. PLoS ONE 2018, 13, e0205067. [Google Scholar] [CrossRef] [PubMed]
- Cucu, I.; Nicolescu, M.I. A Synopsis of Signaling Crosstalk of Pericytes and Endothelial Cells in Salivary Gland. Dent. J. 2021, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Nguyen, M.-A.; Geoffrion, M.; Vreeken, D.; Lister, Z.; Cheng, H.S.; Otte, N.; Essebier, P.; Wyatt, H.; Kandiah, J.W.; et al. RIPK1 Expression Associates With Inflammation in Early Atherosclerosis in Humans and Can Be Therapeutically Silenced to Reduce NF-κB Activation and Atherogenesis in Mice. Circulation 2021, 143, 163–177. [Google Scholar] [CrossRef]
- Peri, G.; Introna, M.; Corradi, D.; Iacuitti, G.; Signorini, S.; Avanzini, F.; Pizzetti, F.; Maggioni, A.P.; Moccetti, T.; Metra, M.; et al. PTX3, A Prototypical Long Pentraxin, Is an Early Indicator of Acute Myocardial Infarction in Humans. Circulation 2000, 102, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, Y.; Gao, M.; Tian, Y.; Qi, P.; Shen, Y.; Huang, L.; Shi, L.; Wang, Y.; Liu, X. C-reactive protein promotes inflammation through TLR4/NF-κB/TGF-β pathway in HL-1 cells. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-κB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-α, in Patients with Moderate-to-Severe Heart Failure. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef]
- Weber, C.K.; Liptay, S.; Wirth, T.; Adler, G.; Schmid, R.M. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 2000, 119, 1209–1218. [Google Scholar] [CrossRef]
- Tabit, C.E.; Holbrook, M.; Shenouda, S.M.; Dohadwala, M.M.; Widlansky, M.E.; Frame, A.A.; Kim, B.H.; Duess, M.-A.; Kluge, M.A.; Levit, A.; et al. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc. Med. 2012, 17, 101–107. [Google Scholar] [CrossRef]
- Lv, Y.; Kim, K.; Sheng, Y.; Cho, J.; Qian, Z.; Zhao, Y.-Y.; Hu, G.; Pan, D.; Malik, A.B.; Hu, G. YAP Controls Endothelial Activation and Vascular Inflammation Through TRAF6. Circ. Res. 2018, 123, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ramjee, V.; Li, D.; Manderfield, L.J.; Liu, F.; Engleka, K.A.; Aghajanian, H.; Rodell, C.B.; Lu, W.; Ho, V.; Wang, T.; et al. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J. Clin. Investig. 2017, 127, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, X.; Wang, J.; Xu, W.; Yi, C.; Ma, R.; Jiang, H. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. Int. J. Mol. Med. 2018, 42, 1917–1924. [Google Scholar] [CrossRef]
- Klingenberg, R.; Stähli, B.E.; Heg, D.; Denegri, A.; Manka, R.; Kapos, I.; von Eckardstein, A.; Carballo, D.; Hamm, C.W.; Vietheer, J.; et al. Controlled-Level EVERolimus in Acute Coronary Syndrome (CLEVER-ACS)—A phase II, randomized, double-blind, multi-center, placebo-controlled trial. Am. Heart J. 2022, 247, 33–41. [Google Scholar] [CrossRef]
- Harvey, K.F.; Pfleger, C.M.; Hariharan, I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 2003, 114, 457–467. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Dong, J.; Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003, 114, 445–456. [Google Scholar] [CrossRef]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tumaneng, K.; Guan, K.-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- Flinn, M.A.; Link, B.A.; O’Meara, C.C. Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration. Semin. Cell Dev. Biol. 2020, 100, 11–19. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Zhao, B.; Guan, K.-L. The Hippo Pathway in Heart Development, Regeneration, and Diseases. Circ. Res. 2015, 116, 1431–1447. [Google Scholar] [CrossRef]
- Mo, J.-S.; Park, H.W.; Guan, K.-L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014, 15, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zheng, Y.; Hara, M.; Pan, D.; Luo, X. Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes Dev. 2015, 29, 1416–1431. [Google Scholar] [CrossRef] [PubMed]
- Praskova, M.; Xia, F.; Avruch, J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 2008, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.H.Y.; Nousiainen, M.; Chalamalasetty, R.B.; Schäfer, A.; Nigg, E.A.; Silljé, H.H.W. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 2005, 24, 2076–2086. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zha, Z.-Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J. Biol. Chem. 2010, 285, 37159–37169. [Google Scholar] [CrossRef]
- Freeman, A.K.; Morrison, D.K. 14-3-3 Proteins: Diverse functions in cell proliferation and cancer progression. Semin. Cell Dev. Biol. 2011, 22, 681–687. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Luo, J.; Hou, N. Molecular Mechanism of Hippo-YAP1/TAZ Pathway in Heart Development, Disease, and Regeneration. Front. Physiol. 2020, 11, 389. [Google Scholar] [CrossRef]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Qi, X.; McAnally, J.; Schwartz, R.J.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 2011, 4, ra70. [Google Scholar] [CrossRef]
- Cucu, I.; Nicolescu, M.I.; Busnatu, Ș.-S.; Manole, C.G. Dynamic Involvement of Telocytes in Modulating Multiple Signaling Pathways in Cardiac Cytoarchitecture. Int. J. Mol. Sci. 2022, 23, 5769. [Google Scholar] [CrossRef]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P. Beyond the Cardiomyocyte: Consideration of HIPPO Pathway Cell-Type Specificity. Circ. Res. 2018, 123, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, J.; Li, W.; Wu, A.; Zhang, X.; Tong, W.; Ho, K.K.; Qin, L.; Song, H.; Mak, K.K. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 2018, 9, 4564. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Chen, W.; Tang, Z.; Yu, X.; Zhu, W.; Zhang, S.; Qiu, J. Role of the Hippo-YAP/NF-κB signaling pathway crosstalk in regulating biological behaviors of macrophages under titanium ion exposure. J. Appl. Toxicol. 2021, 41, 561–571. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Ling, L.; Meng, X.; Chu, F.; Zhang, S.; Zhou, F. The Crosstalk Between Hippo-YAP Pathway and Innate Immunity. Front. Immunol. 2020, 11, 323. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, X.; Chen, J.; Xie, X.; Xu, J.; Zhao, Y.; Shen, J.; Hu, L.; Xu, P.; Song, H.; et al. Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) mediate cell density-dependent proinflammatory responses. J. Biol. Chem. 2018, 293, 18071–18085. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.R.; Simov, V.; Valtingojer, I.; Venier, O. Recent Therapeutic Approaches to Modulate the Hippo Pathway in Oncology and Regenerative Medicine. Cells 2021, 10, 2715. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Lakhani, N.J.; McKean, M.; Lingaraj, T.; Victor, L.; Sanchez-Martin, M.; Kacena, K.; Malek, K.S.; Santillana, S. A phase 1, first-in-human study of IK-930, an oral TEAD inhibitor targeting the Hippo pathway in subjects with advanced solid tumors. J. Clin. Oncol. 2022, 40, TPS3168. [Google Scholar] [CrossRef]
- Matsuda, T.; Zhai, P.; Sciarretta, S.; Zhang, Y.; Jeong, J.I.; Ikeda, S.; Park, J.; Hsu, C.-P.; Tian, B.; Pan, D.; et al. NF2 Activates Hippo Signaling and Promotes Ischemia/Reperfusion Injury in the Heart. Circ. Res. 2016, 119, 596–606. [Google Scholar] [CrossRef]
- Mia, M.M.; Chelakkot-Govindalayathil, A.L.; Singh, M.K. Targeting NF2-Hippo/Yap signaling pathway for cardioprotection after ischemia/reperfusion injury. Ann. Transl. Med. 2016, 4, 545. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, J.; Tang, L.; Shi, J.; Zhu, D. mTORC1 and mTORC2 play different roles in regulating cardiomyocyte differentiation from embryonic stem cells. Int. J. Dev. Biol. 2017, 61, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Liu, L.; Wang, S.; Dai, Z. ILK regulates MSCs survival and angiogenesis partially through AKT and mTOR signaling pathways. Acta Histochem. 2017, 119, 400–406. [Google Scholar] [CrossRef]
- Aoyagi, T.; Kusakari, Y.; Xiao, C.-Y.; Inouye, B.T.; Takahashi, M.; Scherrer-Crosbie, M.; Rosenzweig, A.; Hara, K.; Matsui, T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H75–H85. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 2010, 40, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.-L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Limon, J.J.; Fruman, D.A. Akt and mTOR in B Cell Activation and Differentiation. Front. Immunol. 2012, 3, 228. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Hall, M.N. Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014, 15, 155–162. [Google Scholar] [CrossRef]
- Pearce, L.R.; Huang, X.; Boudeau, J.; Pawłowski, R.; Wullschleger, S.; Deak, M.; Ibrahim, A.F.M.; Gourlay, R.; Magnuson, M.A.; Alessi, D.R. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 2007, 405, 513–522. [Google Scholar] [CrossRef]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Dilly, A.K.; Rajala, R.V.S. Insulin growth factor 1 receptor/PI3K/AKT survival pathway in outer segment membranes of rod photoreceptors. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4765–4773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Moerkens, M.; Ramaiahgari, S.; de Bont, H.; Price, L.; Meerman, J.; van de Water, B. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011, 13, R52. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.M.; Morrison, D.K. Integrating signals from RTKs to ERK/MAPK. Oncogene 2007, 26, 3113–3121. [Google Scholar] [CrossRef]
- Baffi, T.R.; Lordén, G.; Wozniak, J.M.; Feichtner, A.; Yeung, W.; Kornev, A.P.; King, C.C.; Del Rio, J.C.; Limaye, A.J.; Bogomolovas, J.; et al. mTORC2 controls the activity of PKC and Akt by phosphorylating a conserved TOR interaction motif. Sci. Signal. 2021, 14, eabe4509. [Google Scholar] [CrossRef]
- Potter, C.J.; Pedraza, L.G.; Xu, T. Akt regulates growth by directly phosphorylating Tsc2. Nat. Cell Biol. 2002, 4, 658–665. [Google Scholar] [CrossRef]
- Li, Y.; Inoki, K.; Vacratsis, P.; Guan, K.-L. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J. Biol. Chem. 2003, 278, 13663–13671. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Huang, J.; Manning, B.D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 2009, 37, 217–222. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 2009, 19, R1046–R1052. [Google Scholar] [CrossRef] [PubMed]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR–Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 1519. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.X.Y.; Kaczmarek, A.; Hoque, A.; Davie, E.; Ngoei, K.R.W.; Morrison, K.R.; Smiles, W.J.; Forte, G.M.; Wang, T.; Lie, S.; et al. mTORC1 directly inhibits AMPK to promote cell proliferation under nutrient stress. Nat. Metab. 2020, 2, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Ma, M.; Zheng, Y.; Pan, Y.; Lin, X. mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J. Cell. Physiol. 2019, 234, 12562–12568. [Google Scholar] [CrossRef]
- Kazyken, D.; Magnuson, B.; Bodur, C.; Acosta-Jaquez, H.A.; Zhang, D.; Tong, X.; Barnes, T.M.; Steinl, G.K.; Patterson, N.E.; Altheim, C.H.; et al. AMPK directly activates mTORC2 to promote cell survival during acute energetic stress. Sci. Signal. 2019, 12, eaav3249. [Google Scholar] [CrossRef]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef]
- Valentim, L.; Laurence, K.M.; Townsend, P.A.; Carroll, C.J.; Soond, S.; Scarabelli, T.M.; Knight, R.A.; Latchman, D.S.; Stephanou, A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J. Mol. Cell. Cardiol. 2006, 40, 846–852. [Google Scholar] [CrossRef]
- Hariharan, N.; Zhai, P.; Sadoshima, J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid. Redox Signal. 2011, 14, 2179–2190. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Hill, J.A.; Diwan, A. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 2012, 125, 3170–3181. [Google Scholar] [CrossRef]
- Haar, L.; Ren, X.; Liu, Y.; Koch, S.E.; Goines, J.; Tranter, M.; Engevik, M.A.; Nieman, M.; Rubinstein, J.; Jones, W.K. Acute consumption of a high-fat diet prior to ischemia-reperfusion results in cardioprotection through NF-κB-dependent regulation of autophagic pathways. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1705–H1713. [Google Scholar] [CrossRef]
- Zeng, M.; Wei, X.; Wu, Z.; Li, W.; Li, B.; Zhen, Y.; Chen, J.; Wang, P.; Fei, Y. NF-κB-mediated induction of autophagy in cardiac ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2013, 436, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Zhai, P.; Maejima, Y.; Del Re, D.P.; Nagarajan, N.; Yee, D.; Liu, T.; Magnuson, M.A.; Volpe, M.; Frati, G.; et al. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. 2015, 11, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.; Dai, X.; Dai, X.; Xie, J.; Yin, S.; Zhu, J.; Wang, C.; Liu, Y.; Guo, J.; Wang, M.; et al. LATS suppresses mTORC1 activity to directly coordinate Hippo and mTORC1 pathways in growth control. Nat. Cell Biol. 2020, 22, 246–256. [Google Scholar] [CrossRef] [PubMed]
- van Leent, M.M.T.; Beldman, T.J.; Toner, Y.C.; Lameijer, M.A.; Rother, N.; Bekkering, S.; Teunissen, A.J.P.; Zhou, X.; van der Meel, R.; Malkus, J.; et al. Prosaposin mediates inflammation in atherosclerosis. Sci. Transl. Med. 2021, 13, eabe1433. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.M.; Rodriguez-Navas, C.; Tesla, R.J.; Prange-Kiel, J.; Wasser, C.R.; Yoo, K.S.; McDonald, J.; Cenik, B.; Ravenscroft, T.A.; Plattner, F.; et al. Lipidomic and Transcriptomic Basis of Lysosomal Dysfunction in Progranulin Deficiency. Cell Rep. 2017, 20, 2565–2574. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Li, W.; Zhang, Q.; Jiang, Y.; Guo, D.; Sun, X.; Lu, W.; Li, C.; Wang, Y. TFEB-NF-κB inflammatory signaling axis: A novel therapeutic pathway of Dihydrotanshinone I in doxorubicin-induced cardiotoxicity. J. Exp. Clin. Cancer Res. 2020, 39, 93. [Google Scholar] [CrossRef]
- Palmerini, T.; Kirtane, A.J.; Serruys, P.W.; Smits, P.C.; Kedhi, E.; Kereiakes, D.; Sangiorgi, D.; Reggiani, L.B.; Kaiser, C.; Kim, H.-S.; et al. Stent Thrombosis With Everolimus-Eluting Stents. Circ. Cardiovasc. Interv. 2012, 5, 357–364. [Google Scholar] [CrossRef][Green Version]
- Buss, S.J.; Muenz, S.; Riffel, J.H.; Malekar, P.; Hagenmueller, M.; Weiss, C.S.; Bea, F.; Bekeredjian, R.; Schinke-Braun, M.; Izumo, S.; et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2435–2446. [Google Scholar] [CrossRef]
- Stähli, B.E.; Roland, K.; Dik, H.; Mattia, B.; Robert, M.; Ioannis, K.; Oliver, M.; Andrea, D.; Rahel, K.; Florence, B.; et al. Mammalian Target of Rapamycin Inhibition in Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2022, 80, 1802–1814. [Google Scholar] [CrossRef]
- Kaldirim, M.; Lang, A.; Pfeiler, S.; Fiegenbaum, P.; Kelm, M.; Bönner, F.; Gerdes, N. Modulation of mTOR Signaling in Cardiovascular Disease to Target Acute and Chronic Inflammation. Front. Cardiovasc. Med. 2022, 9, 907348. [Google Scholar] [CrossRef]
- Lis, K.; Kuzawińska, O.; Bałkowiec-Iskra, E. Tumor necrosis factor inhibitors—State of knowledge. Arch. Med. Sci. 2014, 10, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Wang, Y.; Jain, P.; Wang, M. Zanubrutinib in lymphoproliferative disorders: A comprehensive review. Ther. Adv. Hematol. 2022, 13, 20406207221093980. [Google Scholar] [CrossRef] [PubMed]
- Perel, G.; Bliss, J.; Thomas, C.M. Carfilzomib (Kyprolis): A Novel Proteasome Inhibitor for Relapsed And/or Refractory Multiple Myeloma. Pharm. Ther. 2016, 41, 303–307. [Google Scholar]
- Gupta, N.; Hanley, M.J.; Xia, C.; Labotka, R.; Harvey, R.D.; Venkatakrishnan, K. Clinical Pharmacology of Ixazomib: The First Oral Proteasome Inhibitor. Clin. Pharmacokinet. 2019, 58, 431–449. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Savona, M.; Baz, R.; Mau-Sorensen, P.M.; Gabrail, N.; Garzon, R.; Stone, R.; Wang, M.; Savoie, L.; Martin, P.; et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood 2017, 129, 3175–3183. [Google Scholar] [CrossRef]
- Seijkens, T.T.P.; van Tiel, C.M.; Kusters, P.J.H.; Atzler, D.; Soehnlein, O.; Zarzycka, B.; Aarts, S.A.B.M.; Lameijer, M.; Gijbels, M.J.; Beckers, L.; et al. Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 527–542. [Google Scholar] [CrossRef]
- Arya, P.; Nabi, S.; Bhandari, U. Modulatory role of atorvastatin against high-fat diet and zymosan-induced activation of TLR2/NF-κB signaling pathway in C57BL/6 mice. Iran. J. Basic Med. Sci. 2021, 24, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Neininger, A.C.; Dai, X.; Liu, Q.; Burnette, D.T. The Hippo pathway regulates density-dependent proliferation of iPSC-derived cardiac myocytes. Sci. Rep. 2021, 11, 17759. [Google Scholar] [CrossRef]
- Triastuti, E.; Nugroho, A.B.; Zi, M.; Prehar, S.; Kohar, Y.S.; Bui, T.A.; Stafford, N.; Cartwright, E.J.; Abraham, S.; Oceandy, D. Pharmacological inhibition of Hippo pathway, with the novel kinase inhibitor XMU-MP-1, protects the heart against adverse effects during pressure overload. Br. J. Pharmacol. 2019, 176, 3956–3971. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, K.; Ji, K.; Zhang, C.; Jiang, Y.; Zhang, Q.; Tian, Z.; Wang, X.; Zhang, M.; Li, X. microRNA-365 inhibits YAP through TLR4-mediated IRF3 phosphorylation and thereby alleviates gastric precancerous lesions. Cancer Cell Int. 2020, 20, 549. [Google Scholar] [CrossRef]
- Jiao, S.; Guan, J.; Chen, M.; Wang, W.; Li, C.; Wang, Y.; Cheng, Y.; Zhou, Z. Targeting IRF3 as a YAP agonist therapy against gastric cancer. J. Exp. Med. 2018, 215, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Marin, T.M.; Keith, K.; Davies, B.; Conner, D.A.; Guha, P.; Kalaitzidis, D.; Wu, X.; Lauriol, J.; Wang, B.; Bauer, M.; et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J. Clin. Investig. 2011, 121, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Kuzman, J.A.; O’Connell, T.D.; Gerdes, A.M. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology 2007, 148, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, D.; Neagoe, P.-E.; Sirois, M.G.; White, M. Effect of everolimus on the immunomodulation of the human neutrophil inflammatory response and activation. Cell. Mol. Immunol. 2015, 12, 40–52. [Google Scholar] [CrossRef]
- Arriola Apelo, S.I.; Neuman, J.C.; Baar, E.L.; Syed, F.A.; Cummings, N.E.; Brar, H.K.; Pumper, C.P.; Kimple, M.E.; Lamming, D.W. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell 2016, 15, 28–38. [Google Scholar] [CrossRef]
- Shioi, T.; McMullen, J.R.; Tarnavski, O.; Converso, K.; Sherwood, M.C.; Manning, W.J.; Izumo, S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 2003, 107, 1664–1670. [Google Scholar] [CrossRef]
- Gao, G.; Chen, W.; Yan, M.; Liu, J.; Luo, H.; Wang, C.; Yang, P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int. J. Mol. Med. 2020, 45, 195–209. [Google Scholar] [CrossRef]
- Shi, G.; Chiramel, A.I.; Majdoul, S.; Lai, K.K.; Dempsey, T.; Kenney, A.; Zani, A.; Eddy, A.; Zhang, L.; Beare, P.A.; et al. Rapalogs downmodulate intrinsic immunity and promote cell entry of SARS-CoV-2. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hoda, M.A.; Mohamed, A.; Ghanim, B.; Filipits, M.; Hegedus, B.; Tamura, M.; Berta, J.; Kubista, B.; Dome, B.; Grusch, M.; et al. Temsirolimus inhibits malignant pleural mesothelioma growth in vitro and in vivo: Synergism with chemotherapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 852–863. [Google Scholar] [CrossRef]
- Ohara, T.; Takaoka, M.; Toyooka, S.; Tomono, Y.; Nishikawa, T.; Shirakawa, Y.; Yamatsuji, T.; Tanaka, N.; Fujiwara, T.; Naomoto, Y. Inhibition of mTOR by temsirolimus contributes to prolonged survival of mice with pleural dissemination of non-small-cell lung cancer cells. Cancer Sci. 2011, 102, 1344–1349. [Google Scholar] [CrossRef]
- Chiong, E.; Lee, I.-L.; Dadbin, A.; Sabichi, A.L.; Harris, L.; Urbauer, D.; McConkey, D.J.; Dickstein, R.J.; Cheng, T.; Grossman, H.B. Effects of mTOR inhibitor everolimus (RAD001) on bladder cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.; Sankhala, K.; Abdel-Karim, I.; Mita, A.; Giles, F. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin. Investig. Drugs 2008, 17, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).