Obeticholic Acid Improves Aminotransferases Early during Treatment in Patients with Primary Biliary Cholangitis Not Responding to Ursodeoxycholic Acid: A Study in Clinical Practice
Abstract
:1. Introduction
2. Patients and Methods
2.1. Participants and Follow-Up
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Patients Characteristics
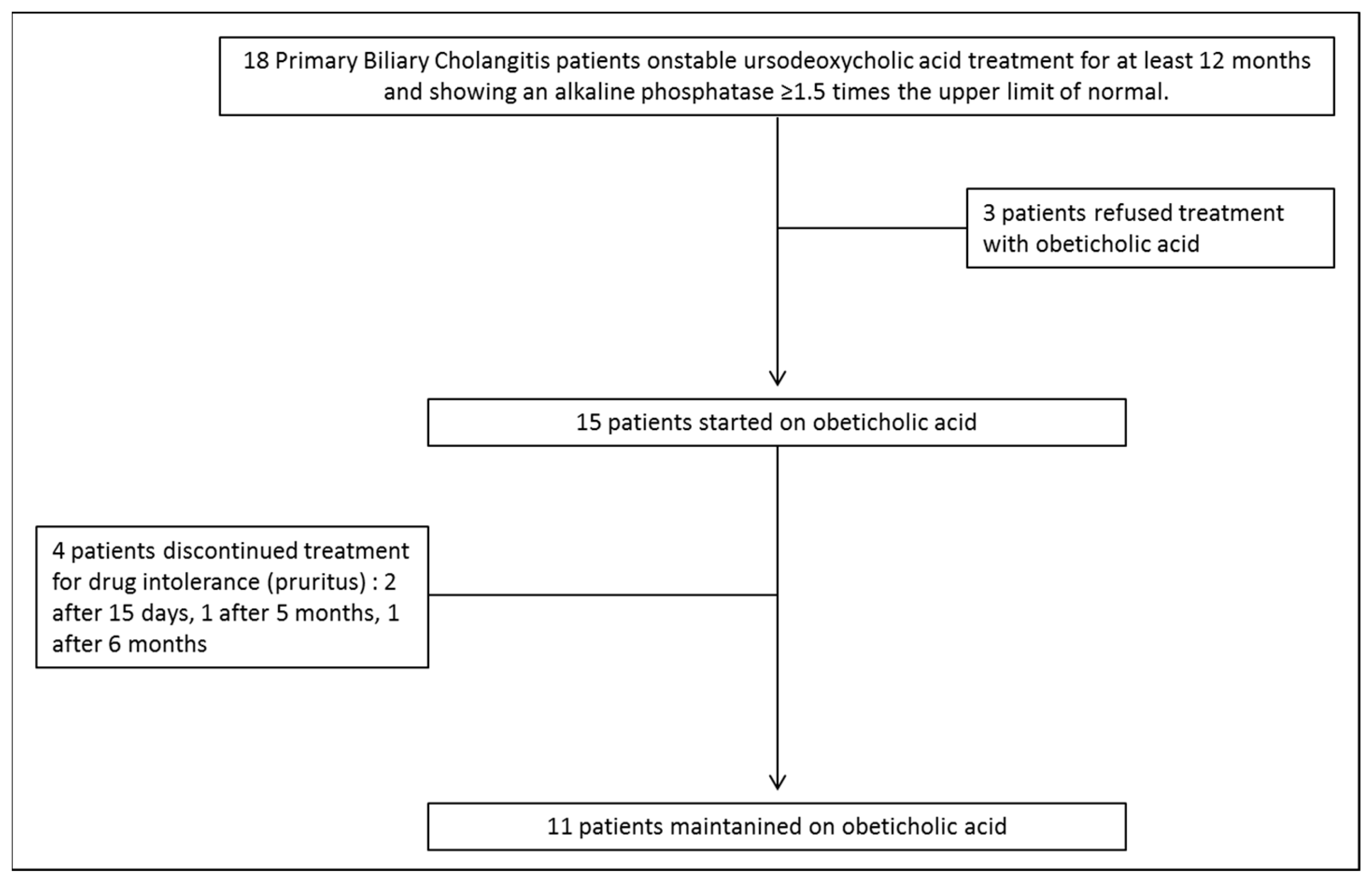
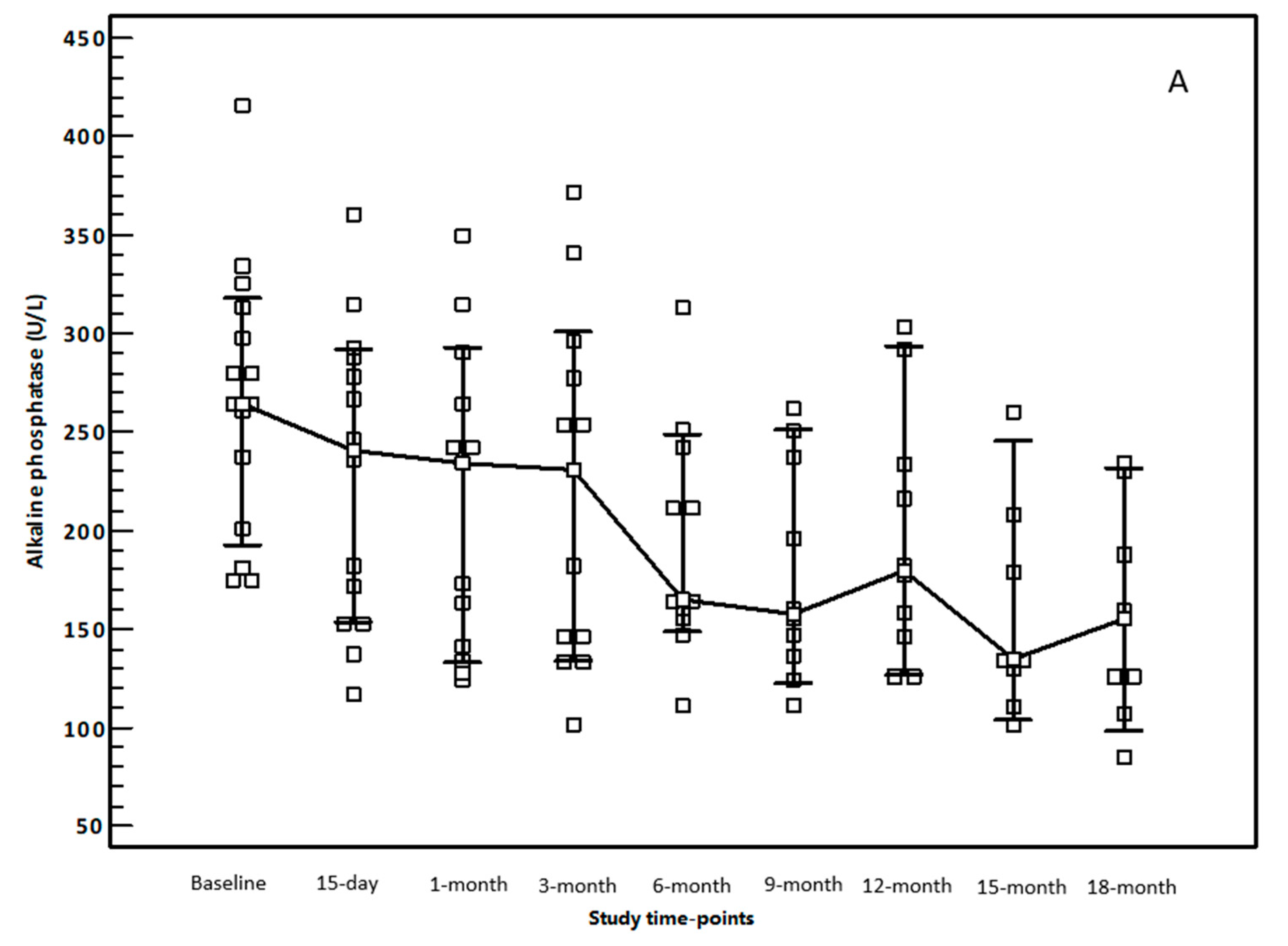
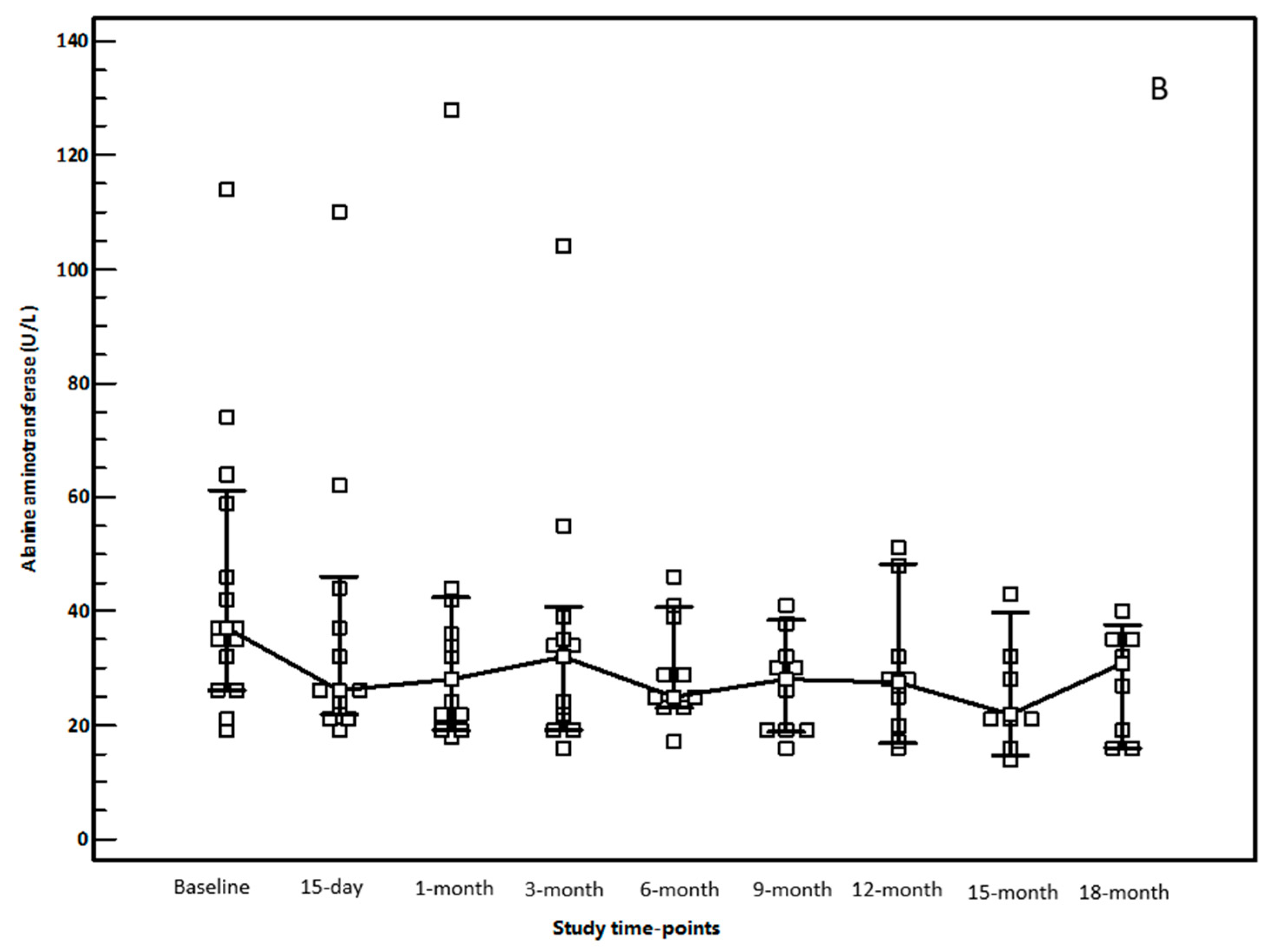
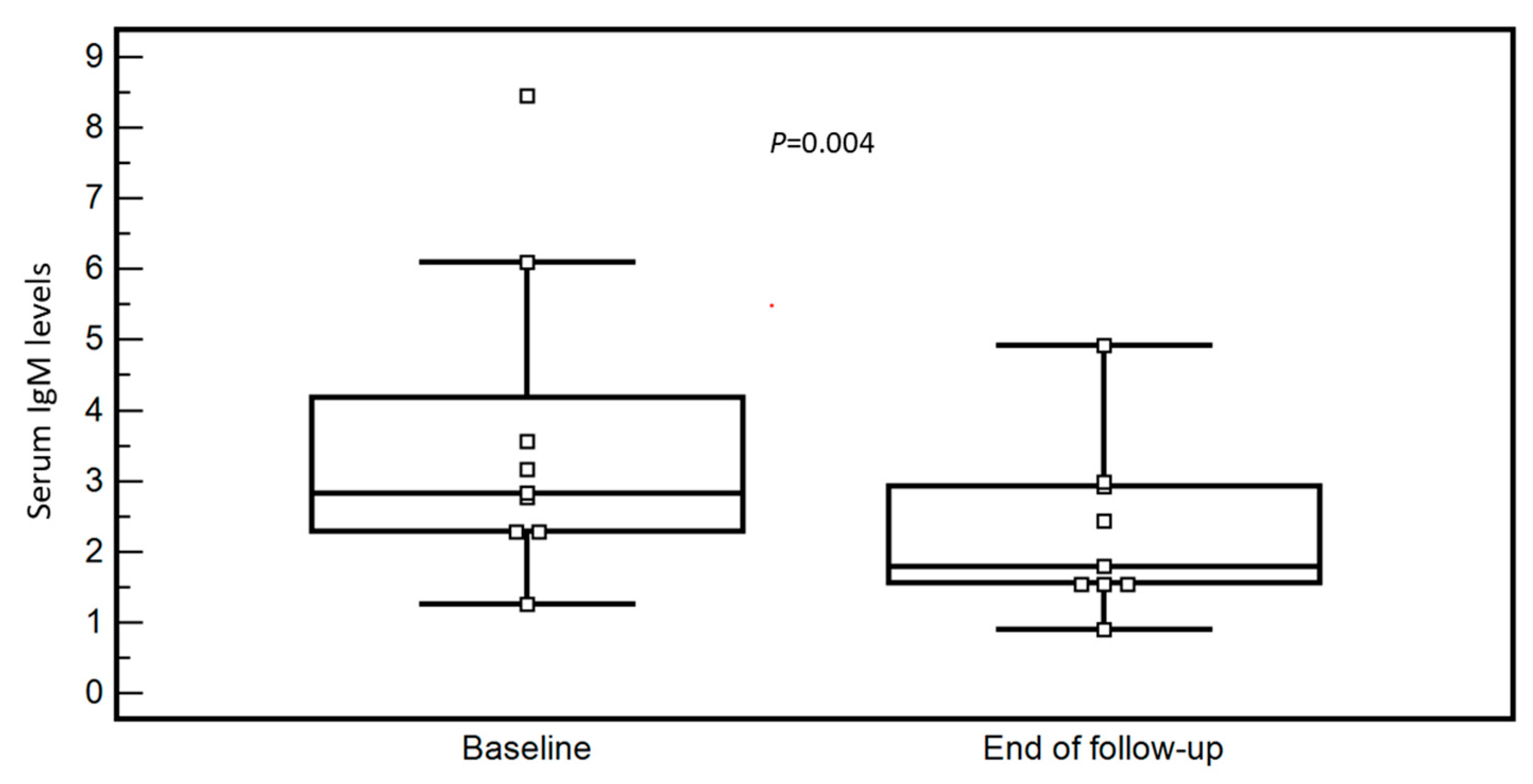
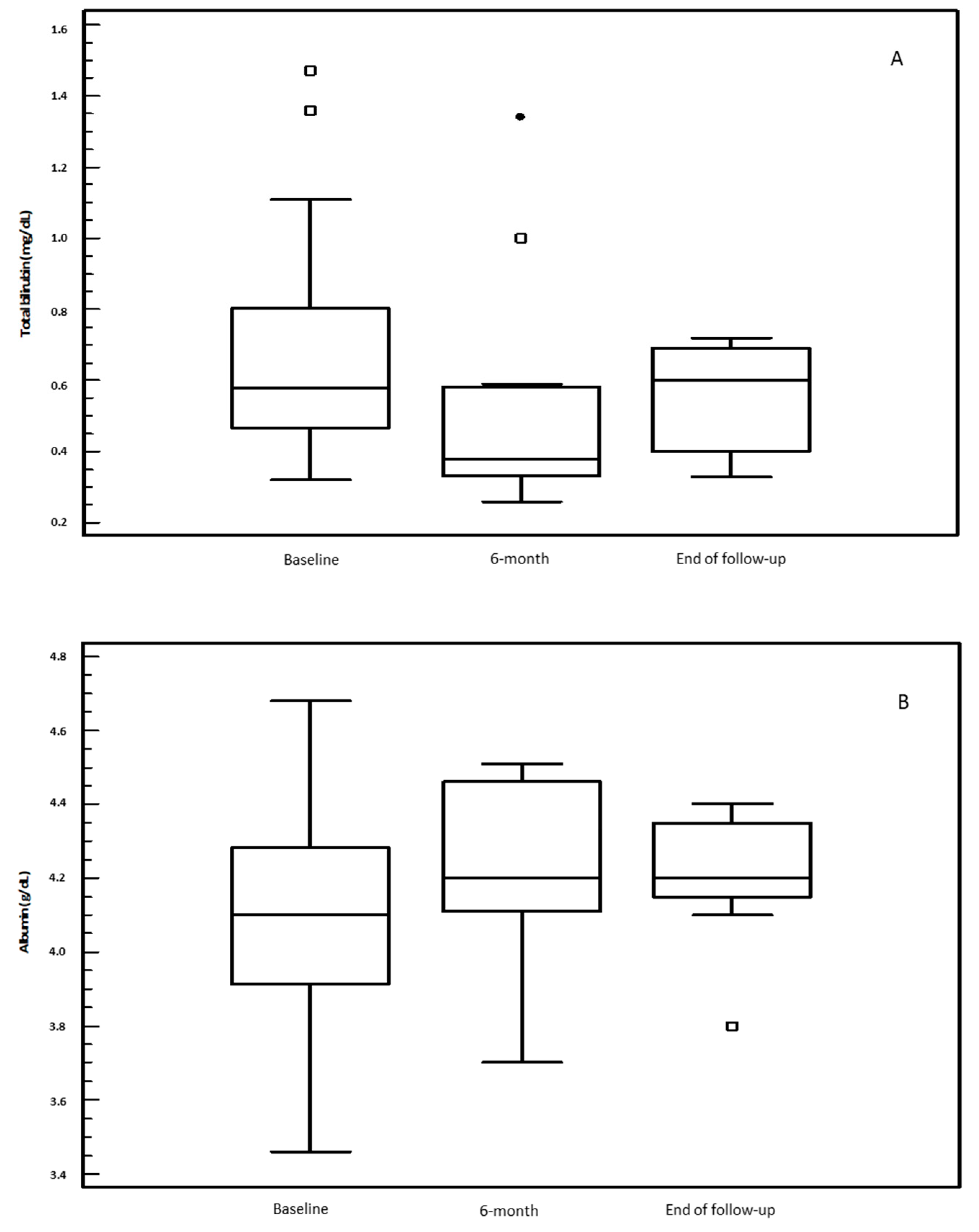
3.2. OCA Treatment and Changes in Biochemical Parameters
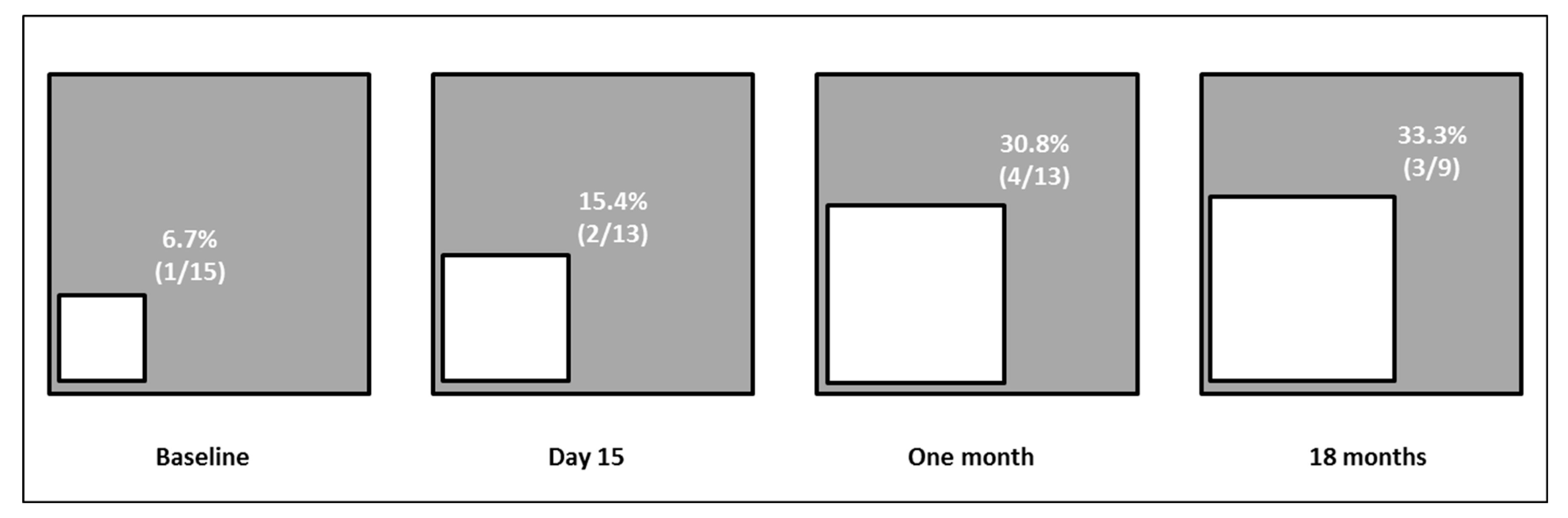
3.3. Evaluation of Alanine Aminotransferases Modification during OCA Treatment According to Up-Dated Upper Limit of Normal
3.4. OCA Tolerability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carey, E.J.; Ali, A.H.; Lindor, K.D. Primary biliary cirrhosis. Lancet 2015, 386, 1565–1575. [Google Scholar] [CrossRef]
- Imam, M.H.; Lindor, K.D. The natural history of primary biliary cirrhosis. Semin. Liver Dis. 2014, 34, 329–333. [Google Scholar] [CrossRef]
- Floreani, A.; Franceschet, I.; Cazzagon, N. Primary biliary cirrhosis: Overlaps with other autoimmune disorders. Semin. Liver Dis. 2014, 34, 352–360. [Google Scholar]
- Goel, A.; Kim, W.R. Natural History of Primary Biliary Cholangitis in the Ursodeoxycholic Acid Era: Role of Scoring Systems. Clin. Liver Dis. 2018, 22, 563–578. [Google Scholar] [CrossRef]
- Corpechot, C.; Carrat, F.; Poupon, R.; Poupon, R.E. Primary biliary cirrhosis: Incidence and predictive factors of cirrhosis development in ursodiol-treated patients. Gastroenterology 2002, 122, 652–658. [Google Scholar] [CrossRef]
- Lammers, W.; Hirschfield, G.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Patients with Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015, 149, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Nardi, A.; Flack, S.; Carpino, G.; Varvaropoulou, N.; Gavrila, C.; Spicer, A.; Badrock, J.; Bernuzzi, F.; Cardinale, V.; et al. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: Development and validation of the UDCA Response Score. Lancet Gastroenterol. Hepatol. 2018, 3, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Sharp, S.J.; Flack, S.; Paximadas, D.; Spiess, K.; Adgey, C.; Griffiths, L.; Lim, R.; Trembling, P.; Williamson, K.; et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016, 63, 930–950. [Google Scholar] [CrossRef] [PubMed]
- Momah, N.; Silveira, M.G.; Jorgensen, R.; Sinakos, E.; Lindor, K.D. Optimizing biochemical markers as endpoints for clinical trials in primary biliary cirrhosis. Liver Int. 2012, 32, 790–795. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Roberts, S.B.; Ismail, M.; Kanagalingam, G.; Mason, A.L.; Swain, M.G.; Vincent, C.; Yoshida, E.M.; Tsien, C.; Flemming, J.A.; Janssen, H.L.; et al. Real-World Effectiveness of Obeticholic Acid in Patients with Primary Biliary Cholangitis. Hepatol. Commun. 2020, 4, 1332–1345. [Google Scholar] [CrossRef]
- Gomez, E.; Buey, L.G.; Molina, E.; Casado, M.; Conde, I.; Berenguer, M.; Jorquera, F.; Simón, M.-A.; Olveira, A.; Hernández-Guerra, M.; et al. Effectiveness and safety of obeticholic acid in a Southern European multicenter cohort of patients with primary biliary cholangitis and suboptimal response to ursodeoxycholic acid. Aliment. Pharmacol. Ther. 2020, 53, 519–530. [Google Scholar] [PubMed]
- Hohenester, S.; Oude-Elferink, R.P.; Beuers, U. Primary biliary cirrhosis. Semin. Immunopathol. 2009, 31, 283–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prati, D.; Taioli, E.; Zanella, A.; Della Torre, E.; Butelli, S.; Del Vecchio, E.; Vianello, L.; Zanuso, F.; Mozzi, F.; Milani, S.; et al. Updated Definitions of Healthy Ranges for Serum Alanine Aminotransferase Levels. Ann. Intern. Med. 2002, 137, 1–10. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classificazione del Medicinale per Uso Umano «Ocaliva». Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2017-08-23&atto.codiceRedazionale=17A05919&elenco30giorni=false (accessed on 3 November 2021).
- Ludwig, J.; Dickson, E.R.; McDonald, G.S. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch. A Pathol. Anat. Histol. 1978, 379, 103–112. [Google Scholar] [CrossRef]
- De Franchis, R.; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolondi, L.; Cillo, U.; Colombo, M.; Craxi, A.; Farinati, F.; Giannini, E.G.; Golfieri, R.; Levrero, M.; Pinna, A.D.; Piscaglia, F.; et al. Position paper of the Italian Association for the Study of the Liver (AISF): The multidisciplinary clinical approach to hepatocellular carcinoma. Dig. Liver Dis. 2013, 45, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Cazzagon, N.; Floreani, A. Primary biliary cholangitis: Treatment. Curr. Opin. Gastroenterol. 2021, 37, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.H.; van Buuren, H.R.; Corpechot, C.; Thorburn, D.; Janssen, H.L.; Lindor, K.D.; Hirschfield, G.M.; Parés, A.; Floreani, A.; Mayo, M.J.; et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J. Hepatol. 2019, 71, 357–365. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, D.; De Vincentis, A.; Malinverno, F.; Viganò, M.; Alvaro, D.; Pompili, M.; Picciotto, A.; Palitti, V.P.; Russello, M.; Storato, S.; et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. 2021, 3, 100248. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, L.; Li, B.; Zhan, Y. Response Rate and Impact on Lipid Profiles of Obeticholic Acid Treatment for Patients with Primary Biliary Cholangitis: A Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8829510. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.H.; Hirschfield, G.M.; Floreani, A.; Mayo, M.J.; Parés, A.; Liberman, A.; Malecha, E.S.; Pencek, R.; MacConell, L.; Hansen, B.E. Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis. JHEP Rep. 2021, 3, 100191. [Google Scholar] [CrossRef]
- FDA adds Boxed Warning to Highlight Correct Dosing of Ocaliva (Obeticholic Acid) for Patients with a Rare Chronic Liver Disease. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-highlight-correct-dosing-ocaliva-obeticholic-acid-patients-rare-chronic-liver (accessed on 3 November 2021).
- John, B.V.; Schwartz, K.; Levy, C.; Dahman, B.; Deng, Y.; Martin, P.; Taddei, T.H.; Kaplan, D.E. Impact of Obeticholic acid Exposure on Decompensation and Mortality in Primary Biliary Cholangitis and Cirrhosis. Hepatol. Commun. 2021, 5, 1426–1436. [Google Scholar] [CrossRef]
| Data | Unit | Reference Range | Value |
|---|---|---|---|
| Age | years | 64 (56–73) | |
| Gender | female | 13 (86.7) | |
| Alanine aminotransferase | IU/L | <40 IU/L | 37 (26–61) |
| Alkaline phosphatase | IU/L | <116 IU/L | 264 (193–318) |
| Total bilirubin | mg/dL | <1.2 mg/dL | 0.58 (0.44–0.98) |
| Albumin | g/dL | >3.5 g/dL | 4.1 (3.9–4.3) |
| INR | ratio | 0.8–1.2 | 0.99 (0.95–1.07) |
| Platelet count | x109/L | 145–450 × 109/L | 231 (174–277) |
| Total cholesterol | mg/dL | <200 mg/dL | 205 (179–217) |
| HDL cholesterol | mg/dL | >45 mg/dL | 64 (48–87) |
| LDL cholesterol | mg/dL | <130 mg/dL | 122 (100–143) |
| Triglycerides | mg/dL | <170 mg/dL | 103 (67–162) |
| Immunoglobulin M | g/L | 0.4–2.5 g/L | 2.51 (2.13–5.80) |
| Comorbidities, n (%) | present | ||
| Histological stage, n (%) * | I, II, III, IV | 0, 0, 5 (35.7), 7 (50.0), 2 (14.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labanca, S.; Cacciato, V.; Borro, P.; Marenco, S.; Pieri, G.; Picciotto, A.; Plaz Torres, M.C.; Giannini, E.G. Obeticholic Acid Improves Aminotransferases Early during Treatment in Patients with Primary Biliary Cholangitis Not Responding to Ursodeoxycholic Acid: A Study in Clinical Practice. Immuno 2021, 1, 457-467. https://doi.org/10.3390/immuno1040033
Labanca S, Cacciato V, Borro P, Marenco S, Pieri G, Picciotto A, Plaz Torres MC, Giannini EG. Obeticholic Acid Improves Aminotransferases Early during Treatment in Patients with Primary Biliary Cholangitis Not Responding to Ursodeoxycholic Acid: A Study in Clinical Practice. Immuno. 2021; 1(4):457-467. https://doi.org/10.3390/immuno1040033
Chicago/Turabian StyleLabanca, Sara, Valentina Cacciato, Paolo Borro, Simona Marenco, Giulia Pieri, Antonino Picciotto, Maria Corina Plaz Torres, and Edoardo G. Giannini. 2021. "Obeticholic Acid Improves Aminotransferases Early during Treatment in Patients with Primary Biliary Cholangitis Not Responding to Ursodeoxycholic Acid: A Study in Clinical Practice" Immuno 1, no. 4: 457-467. https://doi.org/10.3390/immuno1040033
APA StyleLabanca, S., Cacciato, V., Borro, P., Marenco, S., Pieri, G., Picciotto, A., Plaz Torres, M. C., & Giannini, E. G. (2021). Obeticholic Acid Improves Aminotransferases Early during Treatment in Patients with Primary Biliary Cholangitis Not Responding to Ursodeoxycholic Acid: A Study in Clinical Practice. Immuno, 1(4), 457-467. https://doi.org/10.3390/immuno1040033







