Abstract
Viral infections represent a major health problem worldwide. Due to the wide variety of etiological agents and their increasing resistance to anti-virals and antibiotics treatments, new strategies for effective therapies need to be developed. Scientific evidence suggests that probiotics may have prophylactic and therapeutic effects in viral diseases. Indeed, these microorganisms interact harmoniously with the intestinal microbiota and protect the integrity of the intestinal barrier as well as modulate the host immune system. Currently, clinical trials with probiotics have been documented in respiratory tract infections, infections caused by human immunodeficiency viruses, herpes, human papillomavirus and hepatic encephalopathy. However, the benefits documented so far are difficult to extrapolate, due to the strain-dependent effect. In addition, the dose of the microorganism used as well as host characteristics are other parameters that should be consider when advocating the use of probiotics to treat viral infections. This review addresses the scientific evidence of the efficacy of probiotics in clinical strains perspective in viral infectious diseases in the last 10 years.
1. Introduction
Recently, the risk of infections caused by viruses has increased dramatically worldwide [1]. This is mainly due to climate change, global warming and the geographical movement of people and goods [2]. On the other hand, the basis of current therapies to treat these infections are based on antiviral drugs and/or vaccines, which may contribute to the high mutation rates of viruses [3]. In this context, the use of non-pathogenic and beneficial bacteria (i.e., probiotics) represents an attractive alternative to explore new therapies against viral infections [4]. To evaluate the efficacy of probiotics in different diseases, it is necessary to conduct clinical trials, which comprise different stages, such as trial design and registration, enrollment of volunteers, completion of the study and, finally, dissemination of the results [5]. According to the ClinicalTrials.gov database, more than 1178 studies with probiotics were reported in 2020, of which, only few in use for viral infections [6]. Indeed, only about 3.9% of all these clinical trials proposed the use of probiotics as a therapeutic alternative in various viral diseases due to their ability to interact, protect the integrity of the intestinal barrier and modulate the host immune system [7,8,9]. Therefore, the objective of this review is to discuss the scientific evidence on the effect of the use of probiotics in some diseases caused by different viruses in the last 10 years.
For this review, a literature search was conducted in PubMed and Google scholar databases and clinical trials (https://clinicaltrials.gov/ (accessed on 31 January 2021), during the period 2010 to 2020. The terms used were: probiotics, clinical trials, viruses and disease or causative agent: viral gastroenteritis, rhinovirus, enterovirus, adenovirus, influenza, coronavirus, bocavirus, human papillomavirus (HPV), human immunodeficiency virus (HIV), herpes, and liver disease. Reports on probiotics, mixtures of probiotics and synbiotics used in the treatment of diseases caused by viruses were included and reviews and meta-analyses were excluded.
2. Probiotics
The International Scientific Association for Probiotics and Prebiotics (ISAPP) has defined probiotics as: “live microorganisms that, when administered in adequate amounts, confer health benefits” [10]. To exert these benefits, probiotics must remain viable and available in appropriate amounts to survive the stress of the gastrointestinal tract and reach the small intestine and colon with a recommended number of viable cells above 1 × 106 CFU/g [11]. Typically, probiotics are used in the form of single strains; however, some studies suggest that administration of a mixture of probiotics of different strains, or even administering symbiotic, results in additive or even synergistic effects in terms of bioactivity [12,13]. The term synbiotic describe a “mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” [14]. This combination improves the survival of probiotics strains in the gastrointestinal tract, ensuring a superior effect, compared to the activity of the probiotic or prebiotic alone [15]. In the case of a mixture of probiotic strains, this involves a combination of at least two different strains in equal or different proportions [12]. Probiotics include mainly strains of Lactobacillus and Bifidobacterium. Lactobacillus species include L. acidophilus, L. amylovorus, L. brevis, L. bulgaricus, L. casei, L. cellobiosus, L. crispatus, L. curvatus, L. delbrueckii spp. bulgaricus, L. fermentum, L. gallinarum, L. helveticus, L. johnsonii, L. paracasei, L. plantarum, L. reuteri and L. rhamnosus. Bifidobacterium species for instance include B. adolescentis, B. animalis, B. bifidum, B. breve, B. infantis, B. thermophilum, and B. longum. Other species of probiotics include Streptococcus thermophilus, Streptococcus salivarius, Lactococcus lactis, Leuconostoc mesenteroides, Pediococcus pentosaceus, Pediococcus acidilactici, Propionibacterium acidipropionici, Propionibacterium freudenreichii, Propionibacterium jensenii, Propionibacterium thoenii, Enterococcus fecalis, Enterococcus faecium, Bacillus alcolophilus, Bacillus cereus, Bacillus clausii, Bacillus coagulans, Bacillus subtilis, Escherichia coli Nissle 1917, and the yeasts Saccharomyces boulardii and Saccharomyces cerevisiae [16,17,18,19,20]. In addition, thanks to the highlights of studies on the gut microbiota together with the development of new sequencing techniques and bioinformatics tools, it has now been possible to find new “candidate strains” with applications in the food, agricultural, aquaculture and pharmaceutical industries, and which therefore represent a high probiotic potential [21,22,23]. These bacterial strains, less conventional than those mentioned above, are known as next generation probiotics (NGP), and some examples are: Sporolactobacillus inulinus, Akkermansia muciniphila, Feacalibacterium prauznitzii, Roseburia hominis, Eubacterium spp. and Bacteroides spp. [17]. The Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) described guidelines for characterizing microorganisms as probiotics in 2001 [24]. These include: taxonomic identification, functional characterization, and potential health benefits. To determine the beneficial health effects of a probiotic candidate strain, characterization studies and/or assays (such as in vitro cellular models, animal models and human trials) are necessary to determine whether the candidate bacterium provides significant improvement in any of the conditions, symptoms, signs tested, well-being and/or quality of life [24,25]. Although preclinical research provides scientific evidence supporting the use of probiotics and safety, it is essential to establish a proper scientific protocol, such as target population, specific intervention under study, control groups, and safety and efficacy results [23]. When preparing and developing such trials, some aspects have to be considered, such as the dose and strain of the probiotic as well as the type of population and the medical condition [26]. Currently, some clinical trials have successfully determined the use of probiotics as a therapeutic alternative for the management of some viral infections such as: viral gastroenteritis, respiratory tract infections and liver diseases, as well as herpes, HIV and HPV infections [7].
4. Probiotics and Respiratory Tract Infections (RTIs)
Respiratory tract infections (RTIs) represent one of the leading causes of death, ranking third worldwide. The WHO reports that they rank first in the global burden of disease measured each year by the number of disabilities or deaths [44]. Tonsillitis, pharyngitis, laryngitis, sinusitis, otitis media, certain types of influenza, and the common cold represent some of the main RTIs [45]. The main causative agents of RTIs are of viral origin and include rhinoviruses, adenoviruses, influenza viruses, respiratory syncytial virus (RSV), and coronaviruses [46]. In terms of mortality, 20% of deaths occur in the post-neonatal stage, caused by lower respiratory tract infections, with RSV and influenza virus as etiologic agents. In adults, the same pattern exists in upper respiratory tract infections (e.g., viral origin), whereas a predominance of agents of bacterial origin has been described in lower respiratory tract infections [47]. In contrast, adenovirus and rhinovirus have a lower mortality [48]. Finally, the incidence of RTIs caused by coronaviruses has increased exponentially and spread fast. Thus, in December 2019, a case of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (the causative agent of COVID-19, [49]) was reported for the first time; and today this disease is considered by WHO as a pandemic.
New antiviral treatments are being developed worldwide to reduce RTIs, not only caused by influenza virus infections, but also by adenoviruses and, more recently, by SARS-CoV-2 [50]. In this context, probiotics have been proposed as an alternative for the management of viral RTIs, since these microorganisms increase phagocytic activity, increase the expression of CR1, CR3, FccRI, and FcaR receptors (which are associated with phagocytosis), and increase the microbicidal function of neutrophils [51]. In addition, probiotics increase the level of type I interferons, antigen-presenting cells, NKs, and T- and B-lymphocytes in the lung immune system [51]. In this review, we found in the literature that probiotics have a greater beneficial effect on diseases caused by influenza virus compared to rhinovirus, rotavirus and enterovirus [52], while no positive effects have been reported in otitis media infection (Table 1). It should be noted that different strains of probiotic and synbiotic were used, at different doses and duration of treatment in the different clinical trials reviewed (which have shown a promising therapeutic benefit) (Table 1). In particular, the use of probiotics in influenza virus infections, has been shown to result in a reduction of respiratory symptoms and viral load (Table 1). For instance, ingestion of a strain of L. brevis reduces the incidence of influenza, mainly in children not vaccinated against influenza virus (15.7 vs. 23.9 days, p < 0.001) [53]. Also, administration of L. paracasei, L. casei, L. rhamnosus, and L. lactis strains reduces respiratory symptoms (p < 0.0059) and, in particular, the strain of L. lactis induces a transcriptional upregulation of the IFN-α gene and the interferon-stimulated gene 15 (ISG15) (p = 0. 019). In the case of LGG, this strain shows similar protection rates to vaccines against influenza H1N1 and B [54,55,56]. On the other hand, synbiotic administration of B. longum infantis and gluco-oligo saccharides (a type of prebiotic) results in an increase in the number of IgA (p < 0.01) and IgG memory B cells (p < 0.001) and total IgG B cells (p < 0.001), following influenza vaccination [52]. In susceptible populations (such as the elderly), L. rhamnosus decreases the risk of influenza and other viral respiratory infections (35%); however, no significant difference was reported [57].On the other hand, L. coryniformis improves vaccine efficacy (p = 0.036) and protects against respiratory infections (p = 0.007) [58], while L. delbrueckii prevents influenza infection caused by influenza A H3N2 virus and increases IgA (p = 0.04) and H3N2-bound IgA levels (p = 0.001) in saliva of early age subjects [59]. In rotavirus infections, administration of B. animalis spp. lactis decreased rhinovirus replication in nasal secretions, whereas a synbiotic based on LGG and galacto-oligosaccharides (GOS) and polydextrose (PDX) (1:1) reduced the incidence of RTIs in infants receiving prebiotics (rate ratio “RR”, 0.24; 95% CI, 0.12–0.49, p < 0.001) or probiotics RR, 0.50; 95% CI, 0.28–0.90, p = 0.022) compared to placebo, but not rhinovirus viral load [60]. Nowadays, 11 clinical trials on RTIs caused by coronavirus have been reported on the ClinicalTrials.gov platform, but these studies are still in the recruitment phase [61].

Table 1.
Probiotics used in clinical trials of respiratory tract infections caused by viruses.
5. Probiotics and Human Immunodeficiency Virus (HIV)
HIV infects the cells of the immune system, altering or disabling their function [68]. In addition, this virus produces a gradual deterioration of the immune system, which progressively loses CD4+ T lymphocytes, affecting the lymphoid tissue of the intestine, which has a high lymphocyte content, leading to high virus replication [69,70]. HIV infection is also characterized by generating a state of dysbiosis of the gut microbiota, with increased levels of Erysipelotrichaceae, Enterobacteriaceae, Desulfovibrionaceae, Fusobacteria, Pseudomonas aeruginosa and Candida albicans and decreased levels of Bifidobacterium, Lactobacillus, Lachnospiraceae, Ruminococceae, Bacteroides and Rikenellaceae [71,72,73,74,75]. This intestinal dysbiosis severely compromises basic gut functions, such as efficient nutrient absorption and maintenance of intestinal barrier function, and may contribute equally to pathology and disease progression [71]. Antiretroviral therapies (ART) are used to control HIV infection. ART reduces the viral load at the systemic level; however, they may also have side effects, such as diarrhea and other gastrointestinal symptoms leading to treatment interruption [76,77]. The use of probiotics has been proposed as a therapeutic alternative in HIV-infected individuals, as these microorganisms can help restore the host gut microbiota, improving mucosal barrier functions and modulating the immune system [73]. Therefore, it is believed that probiotics could be a cost-effective and clinically efficient strategy to reduce HIV-related morbidity and mortality [76]. In the clinical trials reviewed, probiotics were administered either as a single strain, a mixture of probiotics, or as probiotics supplemented with micronutrients (Table 2). Administration of single strains, such as B. coagulans, increased the percentage of CD4+ T cells (p = 0.018), and showed a decrease in inflammation by correlating D-dimer with CRP and sCD14 with tumor necrosis factor (TNF)-α [70,78]. In addition, Villar-Garcia et al. [79] observed in a study in 2015, that administration of S. boulardii decreased microbial translocation and expression of the inflammation marker IL-6. For their part, Serrano-Villar et al. [80] conducted a similar study in 2019 administering S. boulardii; however, the authors did not find any improvement in the number of circulating T cells neither at the level of inflammation nor immune activation. In the case of probiotic mixture, we found five clinical trials using different probiotics and all studies have beneficial effects on HIV infection (Table 2). Schuther et al. [75] evaluated strains of P. pentosaceus, L. mesenteroides, L. paracasei and L. plantarum strains and observed that supplementation with these bacteria effectively increases the levels of probiotic species (L. plantarum p = 0.001 and P. pentosaceus p = 0.036) in the gut during chronic HIV-1 infection. However, plasma CD14 and C-reactive protein levels were not affected during treatment. In another study, d’Ettorre et al. [81], L. plantarum, S. thermophilus, B. breve, L. paracasei, L. delbrueckii spp. bulgaricus, L. acidophilus, B. longum and B. infantis, can improve immune function by increasing the percentage of Th17 cell subsets (p = 0.059) and reducing the frequency of CD8+ lymphocytes (without reaching significance). Similar results were obtained by Ishizaki et al. [78], indeed, they observed that L. casei Shirota strain increased CD4+ cell count (p < 0.01), especially Th17 (p < 0.05) and decreased CD8+ cells (27.5% to 13.2%, p < 0.001). Other authors evaluated the effect of the administration of L. plantarum, S. thermophilus, B. breve, L. paracasei, L. delbrueckii spp. bulgaricus, L. acidophilus, B. longum, and B. infantis on neuropsychological performance: this clinical trial indicated that patients receiving probiotics showed an improvement in neurological cognitive functions, such as abstract reasoning, as well as short-term (p = 0.0058) and long-term memory (p = 0.0019) [82]. Furthermore, d’Ettorre et al. [83] observed that the administration of S. salivarius, B. breve, B. infantis, B. longum, L. acidophilus, L. plantarum, L. casei, L. delbrueckii, and Streptococcus. faecium provides a specific benefit in HIV-infected patients during anti-retroviral treatment by reducing immune activation on CD4 T-lymphocytes. Probiotic intake also reduces systemic inflammation (CRP plasma levels, p = 0.006) (Table 2). Finally, the administration of probiotics and micronutrients (i.e., vitamin A-1500 IU, vitamin E-5.7 IU, niacinamide-3.8 mg, vitamin B1-0.3 mg, vitamin-B12 0.6 μg, vitamin B6-0.3 mg, vitamin C-21 mg, Fe-3.3 mg, Se-13.8 μg, Zc-2.4 mg DHA (omega-3 fatty acid from fish oil) 13 mg and EPA (omega-3 fatty acid from fish oil) 19 mg), showed an increase in CD 4+ lymphocyte population, while micronutrients (Cu-25 μg, Zn-5 mg, Se-10 μg, I-38μg, vitamin A-1250 IU, vitamin B1 and B2- 0.75 mg, vitamin B6-0.5 mg, vitamin B5-1.25 mg, vitamin B12-0.5 μg, vitamin D-100 IU and vitamin E-2.5 IU), help to significantly delay the progression of advanced stage of the disease, according to WHO clinical staging [84,85]. Further research on the benefits of probiotics is ongoing, but we can conclude that evidence from current clinical trials may have a beneficial effect when administered with ART therapies.

Table 2.
Probiotics used in clinical trials to treat human immunodeficiency virus (HIV) infections.
6. Probiotics and Gastrointestinal Infections
Gastroenteritis is a common infectious syndrome which represents the leading cause of hospitalization in children, causing more than 200,000 deaths per year worldwide. Gastrointestinal infection is characterized by nausea, vomiting, diarrhea, anorexia, weight loss, and dehydration [87,88]. The main causative agent of gastroenteritis is rotavirus, followed by norovirus and adenovirus [89,90]. Rotavirus is an RNA virus belonging to the Reoviridae family, which causes more than half a million deaths annually and more than 2 million hospitalizations worldwide [90]. Novovirus belongs to the family Caliciviridae and is a highly infectious RNA virus [91], since only 100 virons are needed to cause an infection, and because of its resistance to various antiseptic agents [92]. Treatment for gastrointestinal diseases consists of controlling hydration and preventing complications. Dehydration is controlled with a course of oral solutions and a return to normal feeding. However, they are not fully effective in shortening the duration of diarrhea or eliminating the causative agent [90]. According to the guidelines of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Pediatric Infectious Diseases (ESPID), the use of probiotic strains of LGG and S. boulardii is recommended for the treatment of diarrheal diseases in children [93]. Indeed, oral administration of LGG in infancy reduces the secretion of anti-inflammatory substances, induces the production of oxygen free radicals and the production of bactericidal substances [94]. While administration of S. boulardii results in increased levels of IgA and IL-10, directly participating in the immunomodulatory response to intestinal infections. In addition, beneficial effects have been attributed in the prevention of Clostridium difficile infections and antibiotic-associated diarrhea [94]. Taken together, these activities could be responsible for their effects in preventing diarrhea caused by rotavirus, so the administration of these probiotics is often recommended [87,95]. The probiotics shown in Table 3 were administered orally, mainly targeting children, and only one study was conducted in older adults. Regarding the beneficial effects of probiotics on viral gastroenteritis, L. plantarum, S. boulardii, B. longum, and L. acidophilus were observed to have effects on rotavirus. Administration of L. plantarum inhibits rotavirus growth, in addition to reducing virus titer (p < 0.001), diarrhea episodes and Vesikari score [87], while administration of S. boulardii produced a decrease in rotavirus viral load [96]. Also, the administration of these microorganisms helps to reduce fever (p < 0.05) and in terms of restoration of gut microbiota, a significant increase in the levels of Bifidobacterium (p < 0.01) and L. casei subgroup (p < 0.01), was obtained when L. casei Shirota strain was administered in older adults [91] (Table 3). Other studies showed that LGG increased IgG serum levels in children with rotavirus diarrhea (456 vs. 2215 EU, p = 0.003) and improved intestinal permeability (p = 0.027) [97], while B. longum and L. acidophilus decreased rotavirus infection in vitro (p < 0.0001) [98] (Table 3). Probiotics were also found to have direct effects on diarrhea symptoms and fever. Thus, administration of L. rhamnosus or probiotic mixtures of C. butyricum, E. faecalis and B. mesentericus [99] or B. longum, B. lactis, L. acidophilus, L. rhamnosus, L. plantarum and P. pentosaceus, leads to a reduction of diarrhea episodes [98] (Table 3). Finally, only one clinical trial was found in which no beneficial effects on rhinovirus clearance were observed when L. rhamnosus and L. helveticus were administered [100]. Therefore, the current evidence suggests that supplementation with probiotics or a probiotic mixture may have a significant effect on reducing symptoms of rotavirus gastroenteritis (Table 3).

Table 3.
Probiotics used in clinical trials in gastroenteritis viral.
7. Probiotics and Human Papillomavirus (HPV)
HPV is the main etiological agent of cervical lesions and is closely associated with the development of benign lesions, intraepithelial neoplasms and cervical cancer (CxCa) [102]. HPV infections usually clear without any intervention within a few months after contact, and approximately 90% clear within two years. However, some HPV infections can persist and progress to CxCa. Worldwide, this type of cancer is the fourth most common cancer in women, with an estimated 570,000 new cases in 2018 and more than 311,000 deaths per year [103]. To date, the main therapies used for precancerous HPV lesions are cryotherapy, ablation, and the electrosurgical excision procedure, which involves the removal of abnormal cells or lesions [104]. Despite the use of these treatments, high rates of HPV recurrence have been observed, as the virus remains in clinically normal skin and/or membranes and mucous membranes [104,105]. According to evidence, probiotic consumption may have a significant effect on HPV clearance [106], by leading to a balanced vaginal microbiota, decreasing rates of mildly abnormal and unsatisfactory cervical smears or increasing clearance of low-grade squamous intraepithelial lesion abnormalities, and reducing genital risk in women with high-risk HPV [106,107,108]; however, the mechanism by which probiotics may exhibit these beneficial effects has not yet been elucidated. Verhoeven et al. [108], administered L. casei Shirota (1 × 1010 CFU/day) orally, in patients with HPV-related precancerous lesions, interestingly, women consuming these probiotics were twice as likely to eliminate cytological abnormalities compared to the control group (60% and 31%, respectively). Furthermore, they observed a 26% clearance of HPV in women who received L. casei Shirota compared to the control group (19%) [108]. In another study by Palma et al. [106], short- and long-term vaginally administration of L. rhamnosus BMX54 (1 × 104 CFU/tablet) was evaluated in women with HPV infections and bacterial vaginosis: BMX54 restored the vaginal microbiota by generating a state of bacterial equilibrium (eubiosis), which reduces bacterial vaginosis characterized by a decrease in Lactobacillus spp. and increase in E. coli, Gardnerella spp., Chlamydia, Ureaplasma spp., and Streptococcus spp. [106,109]. HPV infection was confirmed by polymerase chain reaction (PCR) for subtypes including HPV-16 and -18. Patients who consumed probiotics for a long period had a total HPV decrease of 31.2% (p = 0.044), suggesting that probiotics use reduces HPV-related cytological abnormalities by up to 2-fold (p = 0.041) (Table 4) [106]. Ou et al. [107], investigated the influence of L. rhamnosus GR-1 and L. reuteri RC-14 (5.4 × 109 CFU) on genital risk reduction in women with high-risk HPV; however, no significant difference in HPV clearance rate was found, despite the decrease in cervical smear abnormalities (Table 4) [107].

Table 4.
Probiotics used in clinical trials in HPV infection, hepatic encephalopathy (HE) and Herpes Simplex-2 (HVS-2) Infection.
8. Probiotics and Hepatic Encephalopathy (HE)
HE is a reversible syndrome of brain function defined as: “an alteration in the function of the central nervous system due to liver failure”. This disease includes a wide spectrum of mental and motor disorders observed in patients with hepatic failure [110]. Mental status changes in HE include memory impairment, euphoria or anxiety, inattention, decreased reaction time, sensory abnormalities, poor concentration, inappropriate behavior, confusion and disorientation. In addition, changes in motor function also include rigidity, induced speech, rest and movement disorders such as tremor, asterixis, hyperreflexia or hyporeflexia. However, the prevalence of HE in patients with liver cirrhosis ranges from 30% to 84% [111], and at least 50–70% of patients with cirrhosis will show abnormalities on psychometric tests and many will have significant functional impairment [112]. On the other hand, patients with hepatitis B virus (HBV)-induced cirrhosis harbor a higher amount of E. faecalis, and lower numbers of Enterobacteriaceae and Bifidobacterium, Lactobacillus, Pediococcus, Leuconostoc and Weissella [113]. This structural change in the gut microbiota causes the elevation of ammonia and a disruption of the intestinal barrier; directly affecting the gut-brain axis (and thus behavioral), thus favoring the development of HE [114]. The administration of lactulose and antibiotics is the main treatment for HE. Lactulose is a synthetic, nonabsorbable disaccharide that has multiple effects on the gut microbiota; indeed, it decreases urease enzyme activity and pH, which in turn will decrease ammonia production and absorption in the intestine. Antibiotic use includes neomycin and metronidazole, which are effective in reducing the population of gram-negative and anaerobic urease-producing bacteria. These treatments are effective, but may have toxic side effects, in addition to being expensive [115]. Malaguarnera et al. [116] tested the efficacy of Bifidobacterium spp. in combination with fructo-oligosaccharides (FOS) and lactulose in patients with HE caused by HBV, HCV and cryptogenic cirrhosis. Synbiotic intake significantly (p < 0.001) reduced ammonia levels (50.2 mmol/L) compared to lactulose intake (61.4 mmol/L) and an improvement in traceability tests (p < 0.05), symbol digit modalities (p < 0.001) and block design (p < 0.001). In addition, no adverse effects were observed compared to those who consumed only lactulose (Table 4). Xia et al. [111] investigated the role of C. butyricum (1 × 107 CFU/g) and B. infantis (1 × 106 CFU/g) in the treatment of minimal hepatic encephalopathy (MHE), in patients with HBV-induced liver cirrhosis. The groups receiving the probiotic improved in psychometric, digit symbol, and the number connection tests. In addition, they observed that probiotics modified the diversity of the intestinal microbiota, finding an increase in Clostridium cluster I and Bifidobacterium, while the amount of Enterococcus and Enterobacteriaceae decreased. The increase in Clostridium cluster I and Bifidobacterium was related to an improvement in the integrity and maintenance of the intestinal barrier. Furthermore, this improvement of the intestinal barrier had significant effects on decreasing the blood ammonia concentration of the treated-group compared to the control group (76.4 vs. 152.0 μmol/mL, p = 0.032), which effectively improves the clinical symptoms of MHE (Table 4) [111]. Although improvements in HE symptoms through modulation of the gut microbiota and decreased urease enzymatic activity produced by pathogenic microorganisms in the gut were observed in both clinical trials, no direct effects of probiotics on inhibition of HBV and HCV viruses were observed. For now, and based on the results obtained in clinical trials, probiotics cannot be recommended for the treatment of most liver disorders; indeed, evidence only suggests their use in MHE. Finally, although probiotics have had positive effects over HBV infections in in vitro tests [117], the exact mechanism conferring these effects has not yet been elucidated.
9. Probiotics and Herpes Simplex-2 (HSV-2) Infection
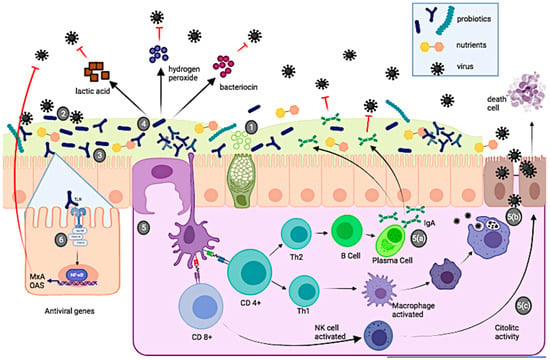
Genital herpes is a sexually transmitted disease caused mainly by herpes simplex virus type 2 (HSV-2) and, to a lesser extent, by herpes simplex virus type 1 (HSV-1), both belonging to the family Herpesviridae (DNA viruses) [118]. HSV-2 infection is a worldwide problem and WHO estimates that 13% of the population aged 15–49 years is infected with HSV-2. Genital herpes infections are often asymptomatic or show mild symptoms that go unnoticed. However, clinical studies show that up to one-third of people with HSV-2 infection may have symptoms, characterized by one or more vesicles, genital and/or anal ulcers, accompanied by other symptoms such as fever, pain, and lymphadenopathy. HSV-2 infection (for which there is currently no cure) is life-threatening and is almost exclusively sexually transmitted [119]. Treatment for this type of infection consists of the use of antivirals drugs; however, although they can reduce the intensity and frequency of symptoms, they cannot reduce HSV-2 transmission [119,120]. A potential therapeutic alternative to combat HSV-2 infections would be the use of probiotics, due to their ability to secrete bacterial metabolites (e.g., lactic acid, hydrogen peroxide, andbacteriocin), modulate the immune system (Figure 1), and restore the vaginal microbiota [121]. Mohseni et al. [122], conducted a clinical trial in which they observed the effect of probiotics on herpes infections. The researchers administered vaginal capsules of L. brevis CD2, L. brevis KB290 and L. brevis SBC8803, in women with HSV-2 infections and compared it with the control group (which was orally administered 400 mg of acyclovir). The results show that both treatments produce similar effects: the probiotic decreases the healing time of the lesion as well as the acyclovir treatment (6.5 vs. 5.2 days, p = 0.06). Furthermore, treatment with all three L. brevis strains shows a significant role in suppressing recurrent HSV-2 infection (p = 0.03). This finding is quite interesting, since a probiotic therapy is cheaper (than the use of a drug) and has no side effects (headache, nausea, diarrhea and abdominal pain) (Table 4) [122]. According to the literature, there are very few clinical trials focused on the use of probiotics to treat HSV-2 infections. Therefore, there is a need for more clinical trials that consider the co-administration of probiotics and retroviral drugs, the duration of probiotic treatment, sample size and route of administration.
10. Discussion
In this review, we found and discussed a total of 40 clinical trials evaluating the effect of probiotics on viral infections. Calculating the percentage of each clinical trial for each viral disease, and considering as 100% the total of selected studies, we found that RTIs showed the highest percentage of studies (40%), followed by HIV infections (25%), and gastrointestinal infections (20%) and finally HE and HSV-2 infections, which showed 5% and 2.5%, respectively. These percentages are probably related to the incidence of viral diseases. RTIs are the diseases in which most studies use probiotics as adjuvant therapy (Figure 1). This is probably because they are the most common infections and have a high impact on health and economy worldwide, as is the case with influenza and SARS-CoV-2.
Scientific evidence suggests that the use of probiotics in viral infections may enhance the immune system response, leading to health benefits [15,16,123,124,125]. Although the antiviral mechanisms produced by probiotics are not fully understood [123], a potential antiviral effect of these microorganisms is usually associated with improvement of the barrier function of the intestinal mucosa, production of antimicrobial substances (hydrogen peroxide or organic acids) and modulation of the immune system [32,123].
Moreover, the effects of probiotics may also include enhanced phagocytic activity, increased secretion of immunoglobulins (IgA, IgG, and IgM) and increased cytokine production (interleukins, TNF-α, and interferon-α) [126]. In the case of SARS-CoV-2 infection, disease progression, generates an increase in free radicals, causing cell damage and triggering a storm of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-2, IL-6, IL-7, and granulocyte colony stimulating factor (G-CSF), affecting the balance of the gut microbiota (i.e., reduction of Bifidobacterium spp. and Lactobacillus spp. counts) [127]. In this sense, probiotics could restore the altered intestinal microbiota and modulate the immune system, so they could be useful to generate health benefits in this disease [15,16,123,124,125]. Furthermore, probiotics co-administered with retroviral drugs could enhance the beneficial health effects, as together they could restore the structure, function and integrity of the gastrointestinal mucosa. These beneficial effects are offered by probiotics together with modulation of the immune system (by increasing the CD4+ lymphocyte population, especially Th17, and decreasing the number of CD8+ lymphocytes), inhibition of epithelial invasion and prevention of microbial translocation of pathogens and production of metabolites of health concern [32,70,78,79]. In clinical trials, the rationale for the use of probiotics for HPV elimination is probably based on the interaction of such microorganisms (e.g., Lactobacillus spp. mainly) with the vaginal microbiota, resulting in an increased innate and adaptive immune response and a probable direct antiviral effect. On the other hand, clinical trials demonstrating the effect of probiotics in liver diseases were only observed in HE, since, probiotics used in HBV infections were performed in in vitro tests. In such assays, probiotic strains that inhibit HBV do so by an antiviral mechanism associated with the Mx GTPase pathway. However, it is very likely that it will not be possible to perform a similar evaluation in clinical trials, due to the site of infection, which does not allow adequate interaction with probiotics. Finally, several probiotics have been used in viral diseases, as they are low cost and non-invasive. Yet, available probiotics are still limited and research with different probiotic strains and NGP should continue.
Although probiotics are an interesting alternative and represent an emerging multi-billion-dollar industry, regulatory authorities must implement adequate legislation to establish standardization, good quality manufacturing practices, evaluation of efficacy, and studies to document any potential adverse effects [128,129]. Currently, probiotics are regulated as dietary supplements, so proof of efficacy is not mandatory [128,130]; however, this could change in the short time, as countries are starting to discuss the legal framework for probiotic [130]. A study by Phavichitr et al. [131] showed that probiotics shortened the duration of hospitalization of children, but without a significant impact on total expenses. Probiotics may be an economically attractive intervention for disease prevention, however, information on cost-effectiveness is still very scarce and only future clinical studies will be able to provide such an answer in terms of cost [132]. For instance, European Food Safety Authority (EFSA) and the US Food and Drug Administration(FDA) have not yet approved any probiotic formulation as a therapy [128,130]. Regarding the side effects of probiotics (which by definition seems to be somewhat contradictory), the risk of infection by any microorganism considered as a probiotic is very low, but their administration in particular cases must be extremely careful, for example in people with long-term hospitalizations, suppressed immune systems or in post-surgery patients [133,134,135].
11. Conclusions
Recently published studies have shown that probiotics have beneficial effects against various viral infections (i.e., hepatic encephalopathy and respiratory, gastrointestinal, HIV, HPV and HVS-2 infections). However, the probiotic effect attributed to one strain cannot be extrapolated to other strains of the same species. The potential antiviral effect associated with probiotics includes: (1) interaction and modification of the host microbiota, (2) adhesion of probiotics to the epithelial surface, which may block viral attachment and compete for specific carbohydrate receptors, (3) production of antimicrobial compounds such as lactic acid, hydrogen peroxide, and bacteriocins, and (4) modulation of the immune system. On the other hand, clinical trials are not harmonized in terms of dosage, sample size and control groups, route of administration and duration of probiotic treatment. Therefore, standardization of protocols will allow better selection of strains, and data recorded, as well as their outcomes, will be very helpful for outgoing and future studies.
Finally, probiotics represent an interesting and promising strategy for health promotion and could be used as adjuvants in therapies against viral infections in order to improve the effect of vaccines. Since probiotics are not considered drugs, it is necessary to maintain strict control in legal regulation, sufficient scientific evidence on efficacy and safety, and post-marketing documentation of possible undesirable effects for consumers. In conclusion, we believe that the application of probiotics and NGP in COVID-19 and other diseases requires further investigation, as the evidence suggests a promising effect.
Author Contributions
All authors have equally contributed to the conceptualization, investigation, formal analysis, discussion, writing-original draft and revision and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parvez, M.K.; Parveen, S. Evolution and Emergence of Pathogenic Viruses: Past, Present, and Future. Intervirology 2017, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Monto, A.S. Vaccines and antiviral drugs in pandemic preparedness. Emerg. Infect. Dis. 2006, 12, 55–60. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Lamas, A.; Del Carmen Mondragon, A.; Cardelle-Cobas, A.; Regal, P.; Rodriguez-Avila, J.A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Probiotic Effects against Virus Infections: New Weapons for an Old War. Foods 2021, 10, 130. [Google Scholar] [CrossRef]
- Committee on Strategies for Responsible Sharing of Clinical Trial Data; Board on Health Sciences Policy; Institute of Medicine. The clinical trial life cycle and when to share data. In Sharing Clinical Trial Data: Maximizing Benefits; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- WHO. Clinical Trial. Available online: https://www.who.int/health-topics/clinical-trials/-tab=tab_1 (accessed on 31 January 2021).
- Clinicaltrials.gov. Clinical Trials. Available online: https://clinicaltrials.gov/ct2/about-studies (accessed on 2 December 2020).
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.H.; Heinemann, R.J.B.; Pedroso, D.L. Developments in probiotic encapsulation. CAB Rev. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Flesch, A.G.T.; Poziomyck, A.K.; Damin, D.C. The therapeutic use of symbiotics. Arq. Bras. De Cir. Dig. ABCD 2014, 27, 206–209. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-L.; Shu, C.-C.; Lai, W.-F.; Tzeng, C.-M.; Lai, H.-C.; Lu, C.-C. Investiture of next generation probiotics on amelioration of diseases–Strains do matter. Med. Microecol. 2019, 1–2, 100002. [Google Scholar] [CrossRef]
- WGO. Global Guidelines Probiotics and Prebiotics. Available online: http://www.spg.pt/wp-content/uploads/2015/07/2017-Probiotics-and-Prebiotics.pdf (accessed on 13 December 2020).
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT -Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Bron, P.A.; van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.-D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Dronkers, T.M.G.; Ouwehand, A.C.; Rijkers, G.T. Global analysis of clinical trials with probiotics. Heliyon 2020, 6, e04467. [Google Scholar] [CrossRef]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 15 December 2020).
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Brüssow, H. Probiotics and prebiotics in clinical tests: An update [version 1; peer review: 2 approved]. F1000Research 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cuestas, M.L.; Minassian, M.L. Virus emergentes y reemergentes: Un nuevo reto para la salud mundial del milenio. Rev. Argent. De Microbiol. 2020, 52, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Ahmed, A.B.F.; Bhattacharjee, S.; Slama, P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411. [Google Scholar] [CrossRef]
- Ginglen, J.G.; Doyle, M.Q. Immunization. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- WHO. Vaccines and Immunization. Available online: https://www.who.int/health-topics/vaccines-and-immunization-tab=tab_1 (accessed on 30 January 2021).
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef]
- Al Kassaa, I. Antiviral Probiotics: A New Concept in Medical Sciences. In New Insights on Antiviral Probiotics: From Research to Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1–46. [Google Scholar]
- Tiwari, S.K.; Dicks, L.M.T.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at War Against Viruses: What Is Missing From the Picture? Front. Microbiol. 2020, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Ismail, N.; Matar, C. Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules 2020, 25, 4891. [Google Scholar] [CrossRef]
- Llewellyn, A.; Foey, A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef]
- Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef]
- Mahooti, M.; Miri, S.M.; Abdolalipour, E.; Ghaemi, A. The immunomodulatory effects of probiotics on respiratory viral infections: A hint for COVID-19 treatment? Microb. Pathog. 2020, 148, 104452. [Google Scholar] [CrossRef]
- Lee, Y.N.; Youn, H.N.; Kwon, J.H.; Lee, D.H.; Park, J.K.; Yuk, S.S.; Erdene-Ochir, T.O.; Kim, K.T.; Lee, J.B.; Park, S.Y.; et al. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against influenza virus infection in mice. Antivir. Res. 2013, 98, 284–290. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune System Stimulation by Probiotic Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- WHO. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 23 January 2021).
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- van Doorn, H.R.; Yu, H. 33—Viral Respiratory Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: London, UK, 2020; pp. 284–288. [Google Scholar]
- Perk, Y.; Özdil, M. Respiratory syncytial virüs infections in neonates and infants. Turk. Pediatri Ars. 2018, 53, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, J.K.; Virgin, H.W. Transkingdom control of viral infection and immunity in the mammalian intestine. Science 2016, 351, aad5872. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Lee, T.; Ahn, J.-H.; Park, S.Y.; Kim, G.-H.; Kim, J.; Kim, T.-H.; Nam, I.; Park, C.; Lee, M.-H. Recent Advances in AIV Biosensors Composed of Nanobio Hybrid Material. Micromachines 2018, 9, 651. [Google Scholar] [CrossRef]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef]
- Enani, S.; Przemska-Kosicka, A.; Childs, C.E.; Maidens, C.; Dong, H.; Conterno, L.; Tuohy, K.; Todd, S.; Gosney, M.; Yaqoob, P. Impact of ageing and a synbiotic on the immune response to seasonal influenza vaccination; A randomised controlled trial. Clin. Nutr. 2018, 37, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Waki, N.; Matsumoto, M.; Fukui, Y.; Suganuma, H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study. Lett. Appl. Microbiol. 2014, 59, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.E.; Fiorino, A.M.; Snydman, D.R.; Hibberd, P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: A randomized double-blind placebo-controlled trial. Eur. J. Clin. Nutr. 2011, 65, 501–507. [Google Scholar] [CrossRef]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; Tanihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Tarnow, I.; Eskesen, D.; Morberg, C.M.; Michelsen, B.; Bügel, S.; Dragsted, L.O.; Rijkers, G.T.; Calder, P.C. Effect of Lactobacillus paracasei subsp. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. Am. J. Clin. Nutr. 2015, 101, 1188–1196. [Google Scholar] [CrossRef]
- Wang, B.; Hylwka, T.; Smieja, M.; Surrette, M.; Bowdish, D.M.E.; Loeb, M. Probiotics to Prevent Respiratory Infections in Nursing Homes: A Pilot Randomized Controlled Trial. J. Am. Geriatr. Soc. 2018, 66, 1346–1352. [Google Scholar] [CrossRef]
- Fonollá, J.; Gracián, C.; Maldonado-Lobón, J.A.; Romero, C.; Bédmar, A.; Carrillo, J.C.; Martín-Castro, C.; Cabrera, A.L.; García-Curiel, J.M.; Rodríguez, C.; et al. Effects of Lactobacillus coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 83–90. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: A randomized controlled trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef]
- Luoto, R.; Ruuskanen, O.; Waris, M.; Kalliomäki, M.; Salminen, S.; Isolauri, E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2014, 133, 405–413. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. COVID 19 and Procbiotics in Clinical Trials. Available online: https://clinicaltrials.gov/ct2/results?recrs=&cond=Covid19&term=Probiotics&cntry=&state=&city=&dist= (accessed on 20 June 2021).
- Kumpu, M.; Lehtoranta, L.; Roivainen, M.; Rönkkö, E.; Ziegler, T.; Söderlund-Venermo, M.; Kautiainen, H.; Järvenpää, S.; Kekkonen, R.; Hatakka, K.; et al. The use of the probiotic Lactobacillus rhamnosus GG and viral findings in the nasopharynx of children attending day care. J. Med. Virol. 2013, 85, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Kalima, K.; He, L.; Lappalainen, M.; Roivainen, M.; Närkiö, M.; Mäkelä, M.; Siitonen, S.; Korpela, R.; Pitkäranta, A. Specific probiotics and virological findings in symptomatic conscripts attending military service in Finland. J. Clin. Virol. 2014, 60, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Tapiovaara, L.; Lehtoranta, L.; Swanljung, E.; Mäkivuokko, H.; Laakso, S.; Roivainen, M.; Korpela, R.; Pitkäranta, A. Lactobacillus rhamnosus GG in the middle ear after randomized, double-blind, placebo-controlled oral administration. Int. J. Pediatric Otorhinolaryngol. 2014, 78, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Tapiovaara, L.; Kumpu, M.; Mäkivuokko, H.; Waris, M.; Korpela, R.; Pitkäranta, A.; Winther, B. Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study. Int. Forum Allergy Rhinol. 2016, 6, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Woodfolk, J.A.; Borish, L.; Steinke, J.W.; Patrie, J.T.; Muehling, L.M.; Lahtinen, S.; Lehtinen, M.J. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—A randomised controlled trial. Benef. Microbes 2017, 8, 207–215. [Google Scholar] [CrossRef]
- Kinoshita, T.; Maruyama, K.; Suyama, K.; Nishijima, M.; Akamatsu, K.; Jogamoto, A.; Katakami, K.; Saito, I. The effects of OLL1073R-1 yogurt intake on influenza incidence and immunological markers among women healthcare workers: A randomized controlled trial. Food Funct. 2019, 10, 8129–8136. [Google Scholar] [CrossRef]
- WHO. VIH/Sida. Available online: https://www.who.int/es/news-room/fact-sheets/detail/hiv-aids (accessed on 30 January 2021).
- Reikvam, D.H.; Meyer-Myklestad, M.H.; Trøseid, M.; Stiksrud, B. Probiotics to manage inflammation in HIV infection. Curr. Opin. Infect. Dis. 2020, 33, 34–43. [Google Scholar] [CrossRef]
- Yang, O.O.; Kelesidis, T.; Cordova, R.; Khanlou, H. Immunomodulation of Antiretroviral Drug-Suppressed Chronic HIV-1 Infection in an Oral Probiotic Double-Blind Placebo-Controlled Trial. AIDS Res. Hum. Retrovir. 2014, 30, 988–995. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Somsouk, M. HIV and the Gut Microbiota: Composition, Consequences, and Avenues for Amelioration. Curr. HIV/AIDS Rep. 2019, 16, 204–213. [Google Scholar] [CrossRef]
- Lu, W.; Feng, Y.; Jing, F.; Han, Y.; Lyu, N.; Liu, F.; Li, J.; Song, X.; Xie, J.; Qiu, Z.; et al. Association Between Gut Microbiota and CD4 Recovery in HIV-1 Infected Patients. Front. Microbiol. 2018, 9, 1451. [Google Scholar] [CrossRef]
- Dillon, S.M.; Lee, E.J.; Kotter, C.V.; Austin, G.L.; Dong, Z.; Hecht, D.K.; Gianella, S.; Siewe, B.; Smith, D.M.; Landay, A.L.; et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014, 7, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Tincati, C.; Rizzardini, G.; Torti, C.; Quirino, T.; Haarman, M.; Amor, K.B.; van Schaik, J.; Vriesema, A.; Knol, J.; et al. Early Impairment of Gut Function and Gut Flora Supporting a Role for Alteration of Gastrointestinal Mucosa in Human Immunodeficiency Virus Pathogenesis. J. Clin. Microbiol. 2008, 46, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Schunter, M.; Chu, H.; Hayes, T.L.; McConnell, D.; Crawford, S.S.; Luciw, P.A.; Bengmark, S.; Asmuth, D.M.; Brown, J.; Bevins, C.L.; et al. Randomized pilot trial of a synbiotic dietary supplement in chronic HIV-1 infection. BMC Complement. Altern. Med. 2012, 12, 84. [Google Scholar] [CrossRef]
- D’Angelo, C.; Reale, M.; Costantini, E. Microbiota and Probiotics in Health and HIV Infection. Nutrients 2017, 9, 615. [Google Scholar] [CrossRef]
- Rajasuriar, R.; Wright, E.; Lewin, S.R. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr. Opin. HIV AIDS 2015, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Bi, X.; Nguyen, L.V.; Matsuda, K.; Pham, H.V.; Phan, C.T.T.; Khu, D.T.K.; Ichimura, H. Effects of Short-Term Probiotic Ingestion on Immune Profiles and Microbial Translocation among HIV-1-Infected Vietnamese Children. Int. J. Mol. Sci. 2017, 18, 2185. [Google Scholar] [CrossRef] [PubMed]
- Villar-García, J.; Hernández, J.J.; Güerri-Fernández, R.; González, A.; Lerma, E.; Guelar, A.; Saenz, D.; Sorlí, L.; Montero, M.; Horcajada, J.P.; et al. Effect of Probiotics (Saccharomyces boulardii) on Microbial Translocation and Inflammation in HIV-Treated Patients: A Double-Blind, Randomized, Placebo-Controlled Trial. JAIDS J. Acquir. Immune Defic. Syndr. 2015, 68, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; de Lagarde, M.; Vázquez-Castellanos, J.; Vallejo, A.; Bernadino, J.I.; Madrid, N.; Matarranz, M.; Díaz-Santiago, A.; Gutiérrez, C.; Cabello, A.; et al. Effects of Immunonutrition in Advanced Human Immunodeficiency Virus Disease: A Randomized Placebo-controlled Clinical Trial (Promaltia Study). Clin. Infect. Dis. 2018, 68, 120–130. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Rossi, G.; Scagnolari, C.; Andreotti, M.; Giustini, N.; Serafino, S.; Schietroma, I.; Scheri, G.C.; Fard, S.N.; Trinchieri, V.; et al. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun. Inflamm. Dis. 2017, 5, 244–260. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Fratino, M.; Selvaggi, C.; Giustini, N.; Serafino, S.; Schietroma, I.; Corano Scheri, G.; Pavone, P.; Passavanti, G.; Alunni Fegatelli, D.; et al. A pilot study on the effects of probiotic supplementation on neuropsychological performance and microRNA-29a-c levels in antiretroviral-treated HIV-1-infected patients. Brain Behav. 2017, 7, e00756. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Ceccarelli, G.; Giustini, N.; Serafino, S.; Calantone, N.; De Girolamo, G.; Bianchi, L.; Bellelli, V.; Ascoli-Bartoli, T.; Marcellini, S.; et al. Probiotics Reduce Inflammation in Antiretroviral Treated, HIV-Infected Individuals: Results of the “Probio-HIV” Clinical Trial. PLoS ONE 2015, 10, e0137200. [Google Scholar] [CrossRef]
- Hemsworth, J.C.; Hekmat, S.; Reid, G. Micronutrient supplemented probiotic yogurt for HIV-infected adults taking HAART in London, Canada. Gut Microbes 2012, 3, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Dayal, R.; Agarwal, D.; Kumar, R.; Singh, T.P.; Hussain, T.; Singh, S.P. Role of Multivitamins, Micronutrients and Probiotics Supplementation in Management of HIV Infected Children. Indian J. Pediatrics 2014, 81, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Walmsley, S.L.; Raboud, J.M.; Kovacs, C.; Coburn, B.; Rousseau, R.; Reinhard, R.; Rosenes, R.; Kaul, R. Can Probiotics Reduce Inflammation and Enhance Gut Immune Health in People Living with HIV: Study Designs for the Probiotic Visbiome for Inflammation and Translocation (PROOV IT) Pilot Trials. HIV Clin. Trials 2016, 17, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Yi, D.Y.; Jo, S.; Lee, Y.M.; Kim, J.-H.; Kim, W.; Park, M.r.; Yoon, S.m.; Kim, Y.; Yang, S.; et al. Effect of a new Lactobacillus plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine 2020, 99, e22192. [Google Scholar] [CrossRef]
- Graves, N.S. Acute Gastroenteritis. Prim. Care: Clin. Off. Pract. 2013, 40, 727–741. [Google Scholar] [CrossRef]
- Stuempfig, N.D.; Seroy, J. Viral Gastroenteritis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Nagata, S.; Asahara, T.; Ohta, T.; Yamada, T.; Kondo, S.; Bian, L.; Wang, C.; Yamashiro, Y.; Nomoto, K. Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br. J. Nutr. 2011, 106, 549–556. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky Benjamin, A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Pieścik-Lech, M.; Shamir, R.; Guarino, A.; Szajewska, H. Review article: The management of acute gastroenteritis in children. Aliment. Pharmacol. Ther. 2013, 37, 289–303. [Google Scholar] [CrossRef]
- Machado, K. Uso de probióticos en el tratamiento y la prevención de diarrea aguda en niños. Arch. De Pediatría Del Urug. 2020, 91, 35–45. [Google Scholar]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef]
- Grandy, G.; Medina, M.; Soria, R.; Terán, C.G.; Araya, M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect. Dis. 2010, 10, 253. [Google Scholar] [CrossRef]
- Sindhu, K.N.C.; Sowmyanarayanan, T.V.; Paul, A.; Babji, S.; Ajjampur, S.S.R.; Priyadarshini, S.; Sarkar, R.; Balasubramanian, K.A.; Wanke, C.A.; Ward, H.D.; et al. Immune Response and Intestinal Permeability in Children With Acute Gastroenteritis Treated With Lactobacillus rhamnosus GG: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2014, 58, 1107–1115. [Google Scholar] [CrossRef]
- Lee, D.K.; Park, J.E.; Kim, M.J.; Seo, J.G.; Lee, J.H.; Ha, N.J. Probiotic bacteria, B. longum and L. acidophilus inhibit infection by rotavirus in vitro and decrease the duration of diarrhea in pediatric patients. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 237–244. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Liu, P.-Y.; Chen, Y.-Y.; Nong, B.-R.; Huang, I.F.; Hsieh, K.-S.; Chen, K.-T. Three-Combination Probiotics Therapy in Children With Salmonella and Rotavirus Gastroenteritis. J. Clin. Gastroenterol. 2014, 48, 37–42. [Google Scholar] [CrossRef]
- Freedman, S.B.; Xie, J.; Nettel-Aguirre, A.; Pang, X.-L.; Chui, L.; Williamson-Urquhart, S.; Schnadower, D.; Schuh, S.; Sherman, P.M.; Lee, B.E.; et al. A randomized trial evaluating virus-specific effects of a combination probiotic in children with acute gastroenteritis. Nat. Commun. 2020, 11, 2533. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gupta, P.K.; Das, R.R. Efficacy and Safety of Saccharomyces boulardii in Acute Rotavirus Diarrhea: Double Blind Randomized Controlled Trial from a Developing Country. J. Trop. Pediatrics 2016, 62, 464–470. [Google Scholar] [CrossRef][Green Version]
- Martins, A.E.S.; Lucena-Silva, N.; Garcia, R.G.; Welkovic, S.; Barboza, A.; Menezes, M.L.B.; Maruza, M.; Tenório, T.; Ximenes, R.A.A. Prevalence of human papillomavirus infection, distribution of viral types and risk factors in cervical samples from human immunodeficiency virus-positive women attending three human immunodeficiency virus-acquired immune deficiency syndrome reference centres in northeastern Brazil. Mem. Do Inst. Oswaldo Cruz 2014, 109, 738–747. [Google Scholar] [CrossRef][Green Version]
- WHO. Human Papillomavirus (HPV) and Cervical Cancer. Available online: https://www.who.int/es/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed on 10 January 2021).
- D’Alessandro, P.; Arduino, B.; Borgo, M.; Saccone, G.; Venturella, R.; Di Cello, A.; Zullo, F. Loop Electrosurgical Excision Procedure versus Cryotherapy in the Treatment of Cervical Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gynecol Minim Invasive Ther. 2018, 7, 145–151. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomaviruses. Available online: https://www.ncbi.nlm.nih.gov/books/NBK321760/ (accessed on 10 February 2021).
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-C.; Fu, H.-C.; Tseng, C.-W.; Wu, C.-H.; Tsai, C.-C.; Lin, H. The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: A randomized placebo-controlled trial. BMC Women’s Health 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, V.; Renard, N.; Makar, A.; Royen, P.V.; Bogers, J.-P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: A prospective controlled pilot study. Eur. J. Cancer Prev. 2013, 22, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Recine, N.; Palma, E.; Domenici, L.; Giorgini, M.; Imperiale, L.; Sassu, C.; Musella, A.; Marchetti, C.; Muzii, L.; Benedetti Panici, P. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case–control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch. Gynecol. Obstet. 2016, 293, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Hepatic encephalopathy. Gastroenterol. Rep. 2017, 5, 138–147. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Xia, J.; Wang, B.; Liu, H.; Yang, L.; Wang, Y.; Ling, Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J. Int. Med. Res. 2018, 46, 3596–3604. [Google Scholar] [CrossRef]
- Bleibel, W.; Al-Osaimi, A. Hepatic encephalopathy. Saudi J. Gastroenterol. 2012, 18, 301–309. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Gut microbiota and hepatitis-B-virus-induced chronic liver disease: Implications for faecal microbiota transplantation therapy. J. Hosp. Infect. 2017, 96, 342–348. [Google Scholar] [CrossRef]
- Mancini, A.; Campagna, F.; Amodio, P.; Tuohy, K.M. Gut:Liver:Brain axis: The microbial challenge in the hepatic encephalopathy. Food Funct. 2018, 9, 1373–1388. [Google Scholar] [CrossRef] [PubMed]
- Patidar, K.R.; Bajaj, J.S. Antibiotics for the treatment of hepatic encephalopathy. Metab. Brain Dis. 2013, 28, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Gargante, M.P.; Malaguarnera, G.; Salmeri, M.; Mastrojeni, S.; Rampello, L.; Pennisi, G.; Volti, G.L.; Galvano, F. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur. J. Gastroenterol. Hepatol. 2010, 22, 199–206. [Google Scholar] [CrossRef]
- Lee, D.K.; Kang, J.Y.; Shin, H.S.; Park, I.H.; Ha, N.J. Antiviral activity of Bifidobacterium adolescentis SPM0212 against Hepatitis B virus. Arch. Pharmacal Res. 2013, 36, 1525–1532. [Google Scholar] [CrossRef]
- Parra-Sánchez, M. Úlceras genitales por virus herpes simplex. Enferm. Infecc. Y Microbiol. Clínica 2019, 37, 260–264. [Google Scholar] [CrossRef] [PubMed]
- WHO. Herpes simplex virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 20 January 2021).
- Bodsworth, N.; Fife, K.; Koltun, W.; Tyring, S.; Abudalu, M.; Prichard, M.; Hamed, K. Single-day famciclovir for the treatment of genital herpes: Follow-up results of time to next recurrence and assessment of antiviral resistance. Curr. Med. Res. Opin. 2009, 25, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, E.; Makvandi, M.; Teimoori, A.; Ataei, A.; Ghafari, S.; Samarbaf-Zadeh, A. Antiviral effects of Lactobacillus crispatus against HSV-2 in mammalian cell lines. J. Chin. Med. Assoc. 2018, 81, 262–267. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Taghinezhad-S, S.; Keyvani, H.; Ghobadi, N. Comparison of Acyclovir and Multistrain Lactobacillus brevis in Women with Recurrent Genital Herpes Infections: A Double-Blind, Randomized, Controlled Study. Probiotics Antimicrob. Proteins 2018, 10, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; Ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front. Cell. Infect. Microbiol. 2021, 10, 925. [Google Scholar] [CrossRef]
- Kurian, S.J.; Unnikrishnan, M.K.; Miraj, S.S.; Bagchi, D.; Banerjee, M.; Reddy, B.S.; Rodrigues, G.S.; Manu, M.K.; Saravu, K.; Mukhopadhyay, C.; et al. Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. Arch. Med. Res. 2021, 52, 582–594. [Google Scholar] [CrossRef]
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential beneficial role of probiotics on the outcome of COVID-19 patients: An evolving perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 295–301. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015, 2, Cd006895. [Google Scholar] [CrossRef]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of corona virus disease-19 (COVID-19): The Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Koirala, S.; Anal, A.K. Probiotics-based foods and beverages as future foods and their overall safety and regulatory claims. Future Foods 2021, 3, 100013. [Google Scholar] [CrossRef]
- de Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Phavichitr, N.; Puwdee, P.; Tantibhaedhyangkul, R. Cost-benefit analysis of the probiotic treatment of children hospitalized for acute diarrhea in Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 2013, 44, 1065–1071. [Google Scholar]
- Lau, V.I.; Rochwerg, B.; Xie, F.; Johnstone, J.; Basmaji, J.; Balakumaran, J.; Iansavichene, A.; Cook, D.J. Probiotics in hospitalized adult patients: A systematic review of economic evaluations. Can. J. Anaesth. 2020, 67, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Coria, D.; Canto-Losa, J.; Carrillo-Vázquez, D.; Carbajal-Morelos, L.; Estrada-León, R.; Corona-Rodarte, E. Lactobacillus gasseri liver abscess and bacteremia: A case report. BMC Infect. Dis. 2021, 21, 518. [Google Scholar] [CrossRef]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).