Recent Advances in Understanding the Role of TIGIT+ Follicular Helper T Cells in IgG4-Related Disease
Abstract
:1. Introduction
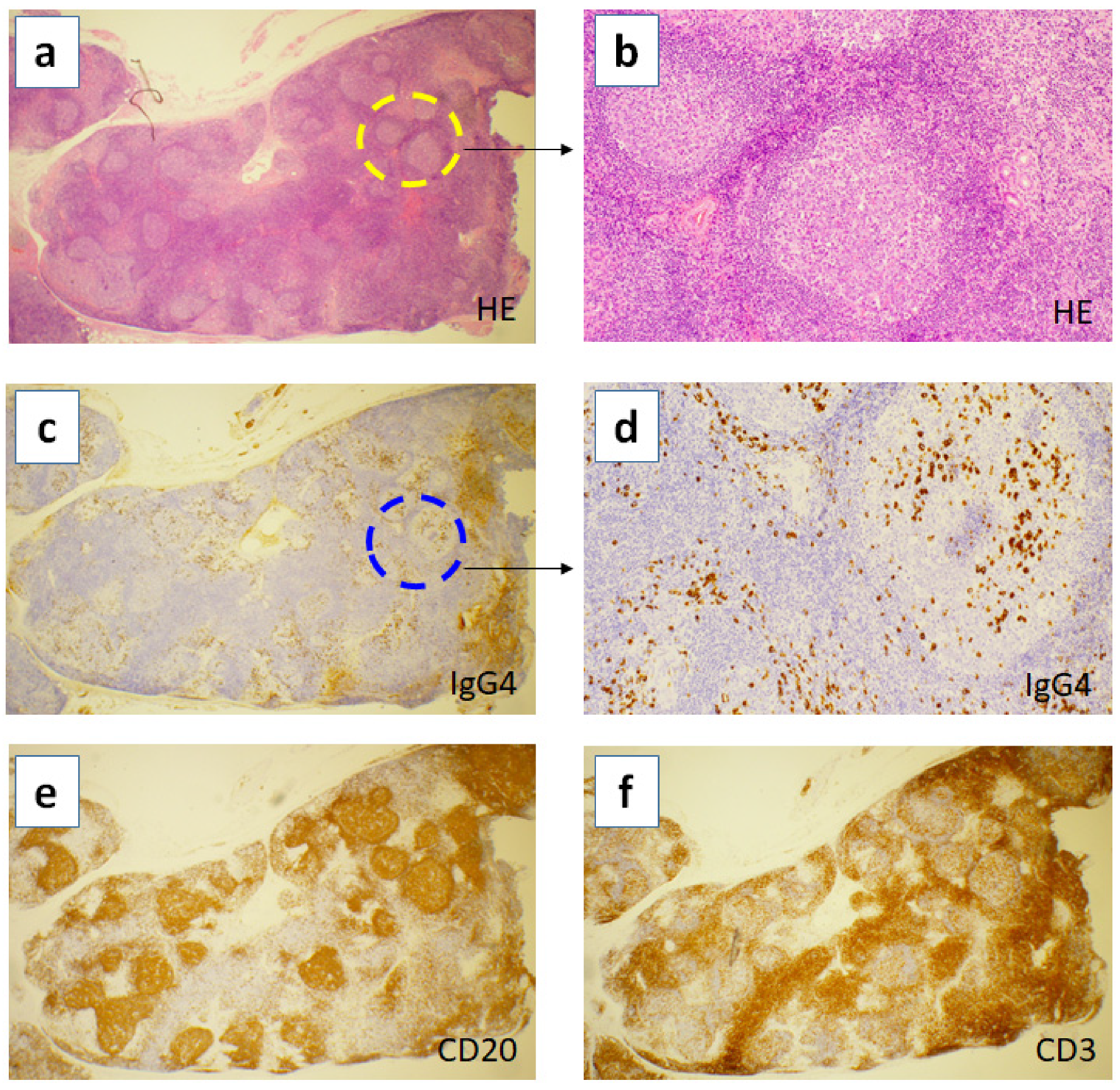
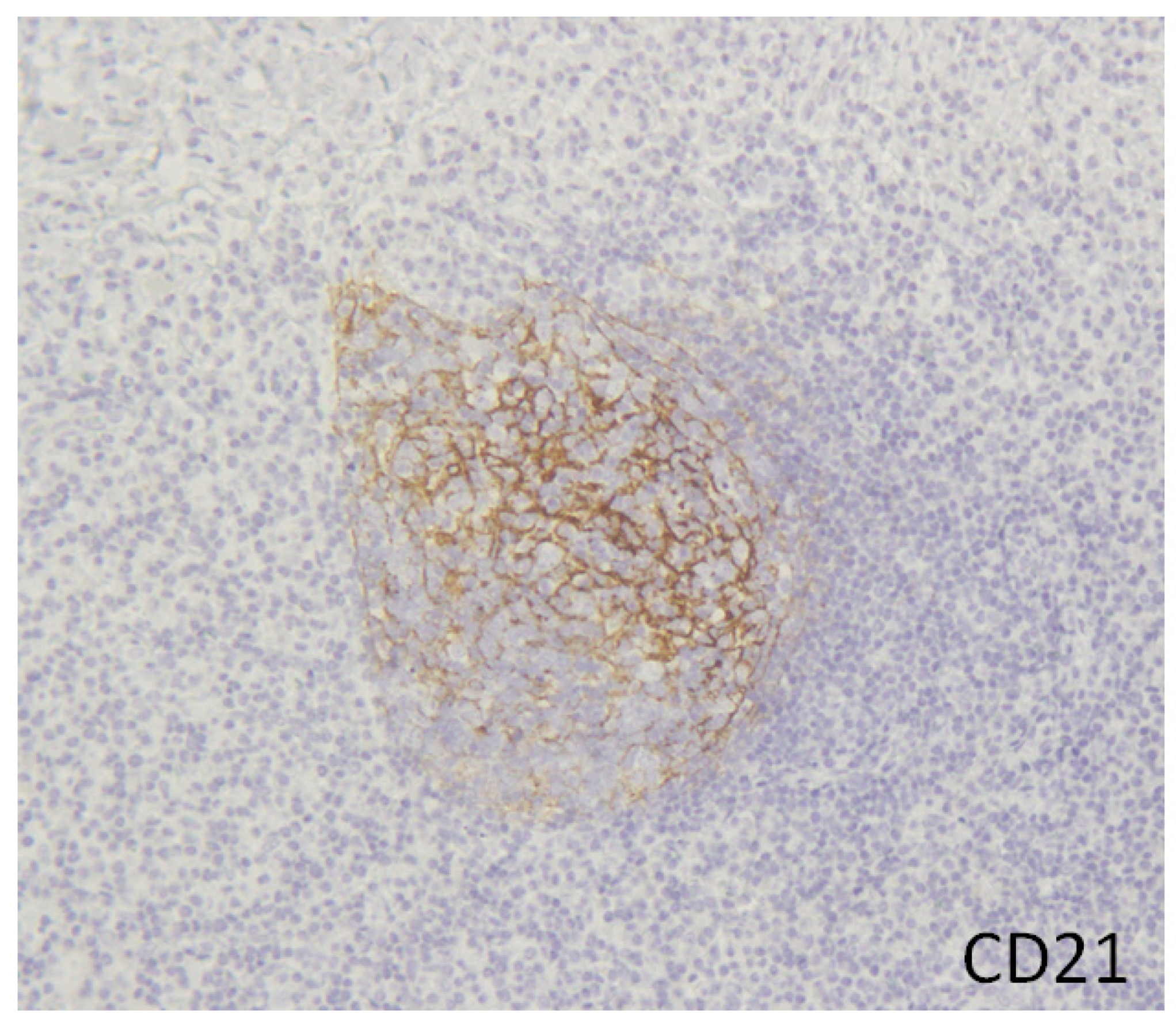
2. Hyper Formation of Tertiary Lymphoid Organs in the Inflamed Tissues of IgG4-RD
3. Tfh Is Essential for TLOs Formation
4. Tfh in IgG4-RD
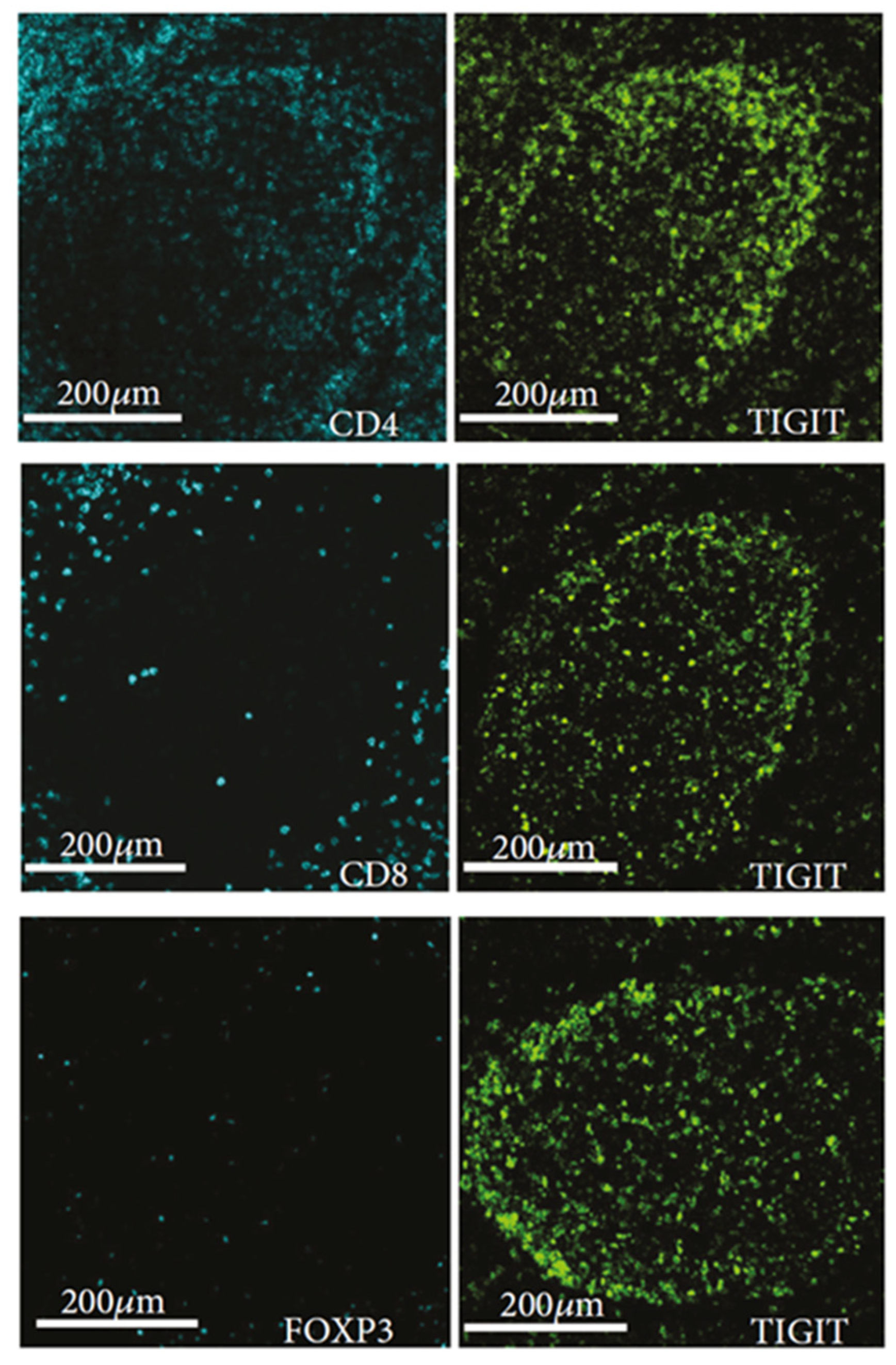
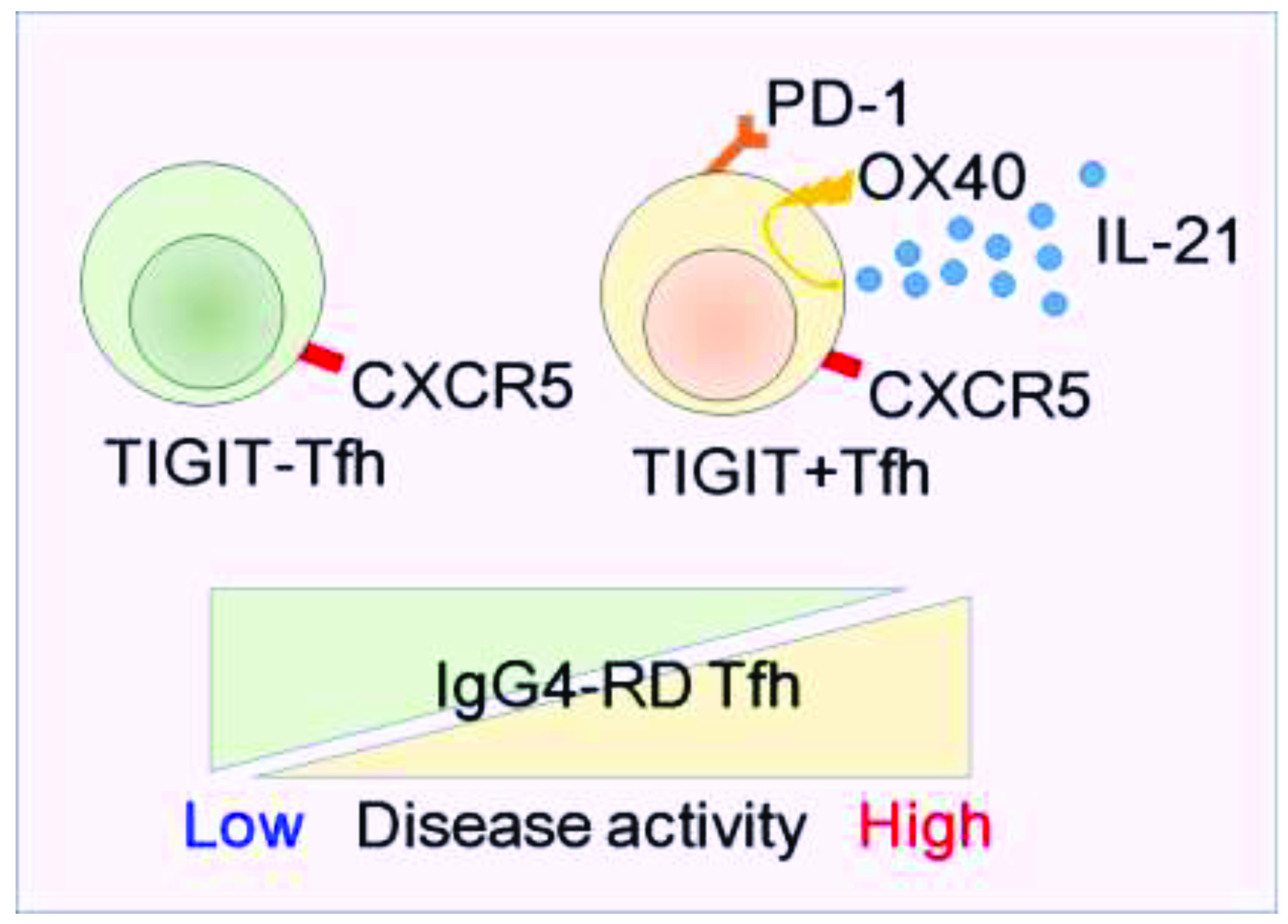
5. Newly Identified Tfh Subset, “TIGIT+ Tfh”
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamano, H.; Kawa, S.; Horiuchi, A.; Unno, H.; Furuya, N.; Akamatsu, T.; Fukushima, M.; Nikaido, T.; Nakayama, K.; Usuda, N.; et al. High Serum IgG4 Concentrations in Patients with Sclerosing Pancreatitis. N. Engl. J. Med. 2001, 344, 732–738. [Google Scholar] [CrossRef]
- Kamisawa, T.; Funata, N.; Hayashi, Y.; Eishi, Y.; Koike, M.; Tsuruta, K.; Okamoto, A.; Egawa, N.; Nakajima, H. A new clinicopathological entity of IgG4-related autoimmune disease. J. Gastroenterol. 2003, 38, 982–984. [Google Scholar] [CrossRef]
- Umehara, H.; Okazaki, K.; Masaki, Y.; Kawano, M.; Yamamoto, M.; Saeki, T.; Matsui, S.; Sumiada, T.; Mimori, T.; Tanaka, Y.; et al. A novel clinical entity, IgG4-related disease (IgG4RD): General concept and details. Mod. Rheumatol. 2012, 22, 1–14. [Google Scholar] [CrossRef]
- Stone, J.H.; Zen, Y.; Deshpande, V. IgG4-related disease. N. Engl. J. Med. 2012, 366, 539–551. [Google Scholar] [CrossRef]
- Akiyama, M.; Kaneko, Y.; Takeuchi, T. Etiology of IgG4-Related Pulmonary Hypertension. Cardiology 2020, 145, 263–266. [Google Scholar] [CrossRef]
- Takanashi, S.; Akiyama, M.; Nishina, N.; Kaneko, Y.; Takeuchi, T. Characteristics and prognosis of IgG4-related skin disease: A case report and systematic literature review. Autoimmun. Rev. 2021, 20, 102805. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kaneko, Y.; Takeuchi, T. Characteristics and prognosis of IgG4-related periaortitis/periarteritis: A systematic literature review. Autoimmun. Rev. 2019, 18, 102354. [Google Scholar] [CrossRef]
- Takanashi, S.; Akiyama, M.; Suzuki, K.; Otomo, K.; Takeuchi, T. IgG4-related fibrosing mediastinitis diagnosed with computed tomography-guided percutaneous needle biopsy: Two case reports and a review of the literature. Medicine 2018, 97, e10935. [Google Scholar] [CrossRef]
- Akiyama, M.; Kaneko, Y.; Hayashi, Y.; Takeuchi, T. IgG4-related disease involving vital organs diagnosed with lip biopsy: A case report and literature review. Medicine 2016, 95, e3970. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kaneko, Y.; Takeuchi, T. Eosinophilic granulomatosis with polyangiitis can manifest lacrimal and salivary glands swelling by granulomatous inflammation: A potential mimicker of IgG4-related disease. Ann. Rheum. Dis. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kaneko, Y.; Takeuchi, T. Characteristics and prognosis of ANCA-positive retroperitoneal fibrosis: A systematic literature review. Autoimmun. Rev. 2020, 19, 102642. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Takanashi, S.; Takeuchi, T.; Kaneko, Y. Salivary gland involvement in ANCA-associated vasculitis: A systematic literature review. Autoimmun. Rev. 2021, 20, 102940. [Google Scholar] [CrossRef]
- Akiyama, M.; Zeisbrich, M.; Ibrahim, N.; Ohtsuki, S.; Berry, G.J.; Hwang, P.H.; Goronzy, J.J.; Weyand, C.M. Neutrophil Extracellular Traps Induce Tissue-Invasive Monocytes in Granulomatosis with Polyangiitis. Front. Immunol. 2019, 10, 2617. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akiyama, M.; Kajio, N.; Otomo, K.; Suzuki, K.; Nishina, N.; Kasuya, K.; Oishi, N.; Kameyama, K.; Takeuchi, T. Adolescent PR3-ANCA-positive hypertrophic pachymeningitis: A case report and review of the literature. Medicine 2018, 97, e0521. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Akiyama, M.; Kaneko, Y.; Takeuchi, T. IgG4-related disease and idiopathic multicentric Castleman’s disease: Confusable immune-mediated disorders. Rheumatology 2021, keab634, Online ahead of print. [Google Scholar] [CrossRef]
- Sasaki, T.; Akiyama, M.; Kaneko, Y.; Mori, T.; Yasuoka, H.; Suzuki, K.; Yamaoka, K.; Okamoto, S.; Takeuchi, T. Distinct features distinguishing IgG4-related disease from multicentric Castleman’s disease. RMD Open 2017, 3, e000432. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Yasuoka, H.; Takeuchi, T. Interleukin-6 in idiopathic multicentric Castleman’s disease after long-term tocilizumab. Ann. Hematol. 2017, 96, 2117–2119. [Google Scholar] [CrossRef] [PubMed]
- Anan, R.; Akiyama, M.; Kaneko, Y.; Kikuchi, J.; Suzuki, K.; Matsubara, S.; Takeuchi, T. Polymyositis with elevated serum IgG4 levels and abundant IgG4+plasma cell infiltration: A case report and literature review. Medicine 2017, 96, e8710. [Google Scholar] [CrossRef]
- Akiyama, M.; Takeuchi, T. IgG4-Related Disease: Beyond Glucocorticoids. Drugs Aging 2018, 35, 275–287. [Google Scholar] [CrossRef]
- Khosroshahi, A.; Wallace, Z.S.; Crowe, J.L.; Akamizu, T.; Azumi, A.; Carruthers, M.N.; Chari, S.T.; Della-Torre, E.; Frulloni, L.; Goto, H.; et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015, 67, 1688–1699. [Google Scholar] [CrossRef]
- Sasaki, T.; Akiyama, M.; Kaneko, Y.; Yasuoka, H.; Suzuki, K.; Yamaoka, K.; Takeuchi, T. Risk factors of relapse following glucocorticoid tapering in IgG4-related disease. Clin. Exp. Rheumatol. 2018, 36, 186–189. [Google Scholar]
- Masamune, A.; Nishimori, I.; Kikuta, K.; Tsuji, I.; Mizuno, N.; Iiyama, T.; Kanno, A.; Tachibana, Y.; Ito, T.; Kamisawa, T.; et al. Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut 2017, 66, 487–494. [Google Scholar] [CrossRef]
- Luo, S.; Zhu, R.; Yu, T.; Fan, H.; Hu, Y.; Mohanta, S.K.; Hu, D. Chronic Inflammation: A Common Promoter in Tertiary Lymphoid Organ Neogenesis. Front. Immunol. 2019, 10, 2938. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.W.; Hill, D.G.; Jones, S.A. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front. Immunol. 2016, 7, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domblides, C.; Rochefort, J.; Riffard, C.; Panouillot, M.; Lescaille, G.; Teillaud, J.L.; Mateo, V.; Dieu-Nosjean, M.C. Tumor-Associated Tertiary Lymphoid Structures: From Basic and Clinical Knowledge to Therapeutic Manipulation. Front. Immunol. 2021, 12, 698604. [Google Scholar] [CrossRef]
- Maehara, T.; Moriyama, M.; Nakashima, H.; Miyake, K.; Hayashida, J.N.; Tanaka, A.; Shinozaki, S.; Kubo, Y.; Nakamura, S. Interleukin-21 contributes to germinal centre formation and immunoglobulin G4 production in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Ann. Rheum. Dis. 2012, 71, 2011–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasashima, S.; Zen, Y.; Kawashima, A.; Konishi, K.; Sasaki, H.; Endo, M.; Matsumoto, Y.; Kawakami, K.; Kasashima, F.; Moriya, M.; et al. Inflammatory abdominal aortic aneurysm: Close relationship to IgG4-related periaortitis. Am. J. Surg. Pathol. 2008, 32, 197–204. [Google Scholar] [CrossRef]
- Zen, Y.; Nakanuma, Y. IgG4-related disease: A cross-sectional study of 114 cases. Am. J. Surg. Pathol. 2010, 34, 1812–1819. [Google Scholar] [CrossRef]
- Miyanaga, T.; Mizuguchi, K.; Hara, S.; Zoshima, T.; Inoue, D.; Nishioka, R.; Mizushima, I.; Ito, K.; Fuji, H.; Yamada, K.; et al. Tertiary lymphoid tissue in early-stage IgG4-related tubulointerstitial nephritis incidentally detected with a tumor lesion of the ureteropelvic junction: A case report. BMC Nephrol. 2021, 22, 34. [Google Scholar] [CrossRef]
- Cargill, T.; Makuch, M.; Sadler, R.; Lighaam, L.C.; Peters, R.; van Ham, M.; Klenerman, P.; Bateman, A.; Rispens, T.; Barnes, E.; et al. Activated T-Follicular Helper 2 Cells Are Associated with Disease Activity in IgG4-Related Sclerosing Cholangitis and Pancreatitis. Clin. Transl. Gastroenterol. 2019, 10, e00020. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Moreno, J.; Hartson, L.; Navarro, C.; Gaxiola, M.; Selman, M.; Randall, T.D. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J. Clin. Investig. 2006, 116, 3183–3194. [Google Scholar] [CrossRef] [Green Version]
- Turesson, C.; Matteson, E.L.; Colby, T.V.; Vuk-Pavlovic, Z.; Vassallo, R.; Weyand, C.M.; Tazellaar, H.D.; Limper, A.H. Increased CD4+ T cell infiltrates in rheumatoid arthritis-associated interstitial pneumonitis compared with idiopathic interstitial pneumonitis. Arthritis Rheum. 2005, 52, 73–79. [Google Scholar] [CrossRef]
- Atkins, S.R.; Turesson, C.; Myers, J.L.; Tazelaar, H.D.; Ryu, J.H.; Matteson, E.L.; Bongartz, T. Morphologic and quantitative assessment of CD20+ B cell infiltrates in rheumatoid arthritis-associated nonspecific interstitial pneumonia and usual interstitial pneumonia. Arthritis Rheum. 2006, 54, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kaneko, Y.; Yamaoka, K.; Kondo, H.; Takeuchi, T. Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: A retrospective, case-control study. Rheumatol. Int. 2016, 36, 881–889. [Google Scholar] [CrossRef]
- Akiyama, M.; Suzuki, K.; Yasuoka, H.; Kaneko, Y.; Yamaoka, K.; Takeuchi, T. Follicular helper T cells in the pathogenesis of IgG4-related disease. Rheumatology 2018, 57, 236–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M.; Suzuki, K.; Kaneko, Y.; Takeuchi, T. IgG4-Related Disease: A Growing Appreciation of Follicular Helper T Cell Expansion. Clin. Transl. Gastroenterol. 2019, 10, e00076. [Google Scholar] [CrossRef]
- Akiyama, M.; Kaneko, Y. Comment on: Persistence of circulating T-follicular helper cells after rituximab is associated with relapse of IgG4-related disease. Rheumatology 2021, keab687, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Nakamura, T.; Kurose, N.; Kawanami, T.; Nakamura, T.; Iwao-Kawanami, H.; Nakajima, A.; Miki, M.; Sakai, T.; Fujita, Y.; Tanaka, M.; et al. CD14+ follicular dendritic cells in lymphoid follicles may play a role in the pathogenesis of IgG4-related disease. Biomed. Res. 2015, 36, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Ohta, M.; Moriyama, M.; Maehara, T.; Gion, Y.; Furukawa, S.; Tanaka, A.; Hayashida, J.N.; Yamauchi, M.; Ishiguro, N.; Mikami, Y.; et al. DNA Microarray Analysis of Submandibular Glands in IgG4-Related Disease Indicates a Role for MARCO and Other Innate Immune-Related Proteins. Medicine 2016, 95, e2853. [Google Scholar] [CrossRef] [Green Version]
- Takanashi, S.; Kikuchi, J.; Sasaki, T.; Akiyama, M.; Yasuoka, H.; Yoshimoto, K.; Seki, N.; Sugahara, K.; Chiba, K.; Kaneko, Y.; et al. Lymphadenopathy in IgG4-related disease: A phenotype of severe activity and poor prognosis, with eotaxin-3 as a new biomarker. Rheumatology 2021, 60, 967–975. [Google Scholar] [CrossRef]
- Crotty, S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015, 15, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Le Gros, G.; Coyle, A.J.; Kosco-Vilbois, M.; Brombacher, F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol. Rev. 1995, 148, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Schaerli, P.; Willimann, K.; Lang, A.B.; Lipp, M.; Loetscher, P.; Moser, B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000, 192, 1553–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Rott, L.S.; Clark-Lewis, I.; Campbell, D.J.; Wu, L.; Butcher, E.C. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 2001, 193, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Försteret, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Rao, S.; Tsai, L.M.; Lee, S.K.; He, Y.; Sutcliffe, E.L.; Srivastava, M.; Linterman, M.; Zheng, L.; Simpson, N.; et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009, 31, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef] [Green Version]
- Morita, R.; Schmitt, N.; Bentebibel, S.E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.H.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, M.; Suzuki, K.; Yamaoka, K.; Yasuoka, H.; Takeshita, M.; Kaneko, Y.; Kondo, H.; Kassai, Y.; Miyazaki, T.; Morita, R.; et al. Number of Circulating Follicular Helper 2 T Cells Correlates with IgG4 and Interleukin-4 Levels and Plasmablast Numbers in IgG4-Related Disease. Arthritis Rheumatol. 2015, 67, 2476–2481. [Google Scholar] [CrossRef] [Green Version]
- Mattoo, H.; Mahajan, V.S.; Della-Torre, E.; Sekigami, Y.; Carruthers, M.; Wallace, Z.S.; Deshpande, V.; Stone, J.H.; Pillai, S. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J. Allergy Clin. Immunol. 2014, 134, 679–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M.; Yasuoka, H.; Yamaoka, K.; Suzuki, K.; Kaneko, Y.; Kondo, H.; Kassai, Y.; Koga, K.; Miyazaki, T.; Morita, R.; et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res. Ther. 2016, 18, 167. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, M.; Kaneko, Y.; Yamaoka, K.; Hayashi, Y.; Yasuoka, H.; Suzuki, K.; Takeuchi, T. Subclinical labial salivary gland involvement in IgG4-related disease affected with vital organs. Clin. Exp. Rheumatol. 2015, 33, 949–950. [Google Scholar] [PubMed]
- Kamekura, R.; Takano, K.; Yamamoto, M.; Kawata, K.; Shigehara, K.; Jitsukawa, S.; Nagaya, T.; Ito, F.; Sato, A.; Ogasawara, S.; et al. Cutting Edge: A Critical Role of Lesional T Follicular Helper Cells in the Pathogenesis of IgG4-Related Disease. J. Immunol. 2017, 199, 2624–2629. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, W.; Yang, H.; Wang, M.; Zhang, P.; Feng, R.; Chen, H.; Peng, L.; Zhang, X.; Zhao, Y.; et al. Aberrant Expansion and Function of Follicular Helper T Cell Subsets in IgG4-Related Disease. Arthritis Rheumatol. 2018, 70, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Yasuoka, H.; Yoshimoto, K.; Takeuchi, T. Interleukin-4 contributes to the shift of balance of IgG subclasses toward IgG4 in IgG4-related disease. Cytokine 2018, 110, 416–419. [Google Scholar] [CrossRef]
- Yajima, H.; Yamamoto, M.; Shimizu, Y.; Sakurai, N.; Suzuki, C.; Naishiro, Y.; Imai, K.; Shinomura, Y.; Takahashi, H. Loss of interleukin-21 leads to atrophic germinal centers in multicentric Castleman’s disease. Ann. Hematol. 2016, 95, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Yasuoka, H.; Yoshimoto, K.; Takeuchi, T. CC-chemokine ligand 18 is a useful biomarker associated with disease activity in IgG4-related disease. Ann. Rheum. Dis. 2018, 77, 1386–1387. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Moriyama, M.; Tanaka, A.; Maehara, T.; Tsuboi, H.; Iizuka, M.; Hayashida, J.N.; Ohta, M.; Saeki, T.; Notohara, K.; et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Clin. Immunol. 2015, 156, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, H.; Iizuka-Koga, M.; Asashima, H.; Takahashi, H.; Kudo, H.; Ono, Y.; Honda, F.; Iizuka, A.; Segawa, S.; Abe, S.; et al. Upregulation and pathogenic roles of CCL18-CCR8 axis in IgG4-related disease. Mod. Rheumatol. 2020, 30, 729–737. [Google Scholar] [CrossRef]
- Mancuso, G.; Jofra, T.; Lanzillotta, M.; Aiuti, A.; Cicalese, M.P.; di Colo, G.; Dagna, L.; Fousteri, G.; Della-Torre, E. Persistence of circulating T-follicular helper cells after rituximab is associated with relapse of IgG4-related disease. Rheumatology 2021, 60, 3947–3949. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Dis. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Long, D.; Chen, Y.; Wu, H.; Zhao, M.; Lu, Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J. Autoimmun. 2019, 99, 1–14. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Suzuki, K.; Yoshimoto, K.; Yasuoka, H.; Kaneko, Y.; Takeuchi, T. Peripheral TIGIT+ T Follicular Helper Cells That Produce High Levels of Interleukin-21 via OX40 Represent Disease Activity in IgG4-Related Disease. Front. Immunol. 2021, 12, 651357. [Google Scholar] [CrossRef] [PubMed]
- Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; Debatin, N.F.; et al. Patterns of TIGIT Expression in Lymphatic Tissue, Inflammation, and Cancer. Dis. Markers 2019, 2019, 5160565. [Google Scholar] [CrossRef]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; Cella, M.; Colonna, M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahiliani, V.; Hutchinson, T.E.; Abboud, G.; Croft, M.; Salek-Ardakani, S. OX40 Cooperates with ICOS to Amplify Follicular Th Cell Development and Germinal Center Reactions during Infection. J. Immunol. 2017, 198, 218–228. [Google Scholar] [CrossRef]
- Fu, N.; Xie, F.; Sun, Z.; Wang, Q. The OX40/OX40L Axis Regulates T Follicular Helper Cell Differentiation: Implications for Autoimmune Diseases. Front. Immunol. 2021, 12, 670637. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Ravens, I.; Kremmer, E.; Maier, M.K.; Hadis, U.; Hardtke, S.; Forster, R.; Bernhardt, G. Abundance of follicular helper T cells in Peyer’s patches is modulated by CD155. Eur. J. Immunol. 2009, 39, 3160–3170. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyama, M.; Kaneko, Y. Recent Advances in Understanding the Role of TIGIT+ Follicular Helper T Cells in IgG4-Related Disease. Immuno 2021, 1, 380-390. https://doi.org/10.3390/immuno1040026
Akiyama M, Kaneko Y. Recent Advances in Understanding the Role of TIGIT+ Follicular Helper T Cells in IgG4-Related Disease. Immuno. 2021; 1(4):380-390. https://doi.org/10.3390/immuno1040026
Chicago/Turabian StyleAkiyama, Mitsuhiro, and Yuko Kaneko. 2021. "Recent Advances in Understanding the Role of TIGIT+ Follicular Helper T Cells in IgG4-Related Disease" Immuno 1, no. 4: 380-390. https://doi.org/10.3390/immuno1040026
APA StyleAkiyama, M., & Kaneko, Y. (2021). Recent Advances in Understanding the Role of TIGIT+ Follicular Helper T Cells in IgG4-Related Disease. Immuno, 1(4), 380-390. https://doi.org/10.3390/immuno1040026





