Immune Responses Are Differentially Regulated by Root, Stem, Leaf, and Flower Extracts of Female and Male CBD Hemp (Cannabis sativa L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction and HPLC Analysis
2.3. Cell Culture
2.4. Cell Viability
2.5. Nitric Oxide Production
2.6. Gene Expression Analysis
2.7. Cellular Respiration Assay
2.8. Statistical Analysis
3. Results and Discussion
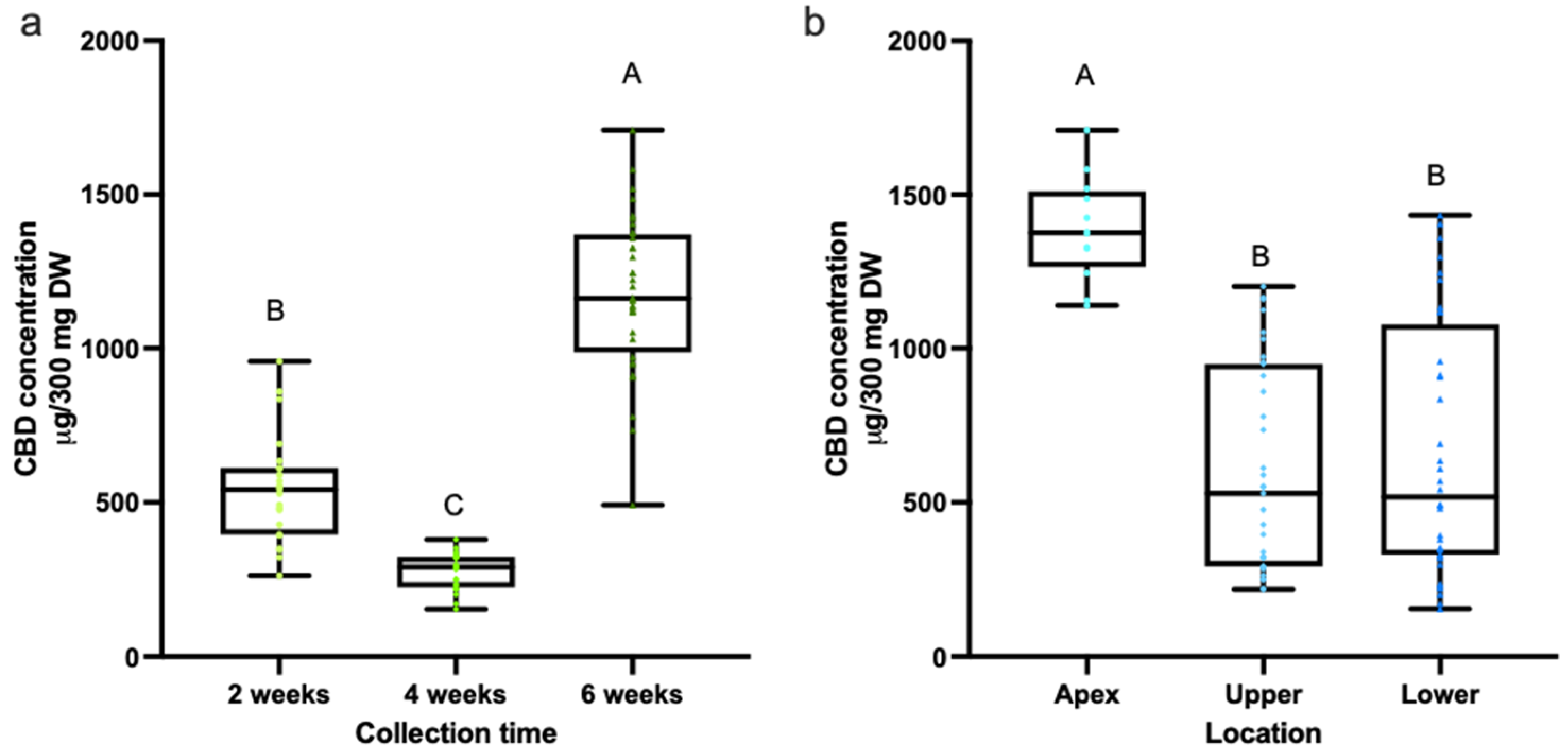
3.1. Quantification of Cannabinoids in CBD Hemp Tissues
3.2. Quantification of Cannabinoids in CBD Hemp Tissues
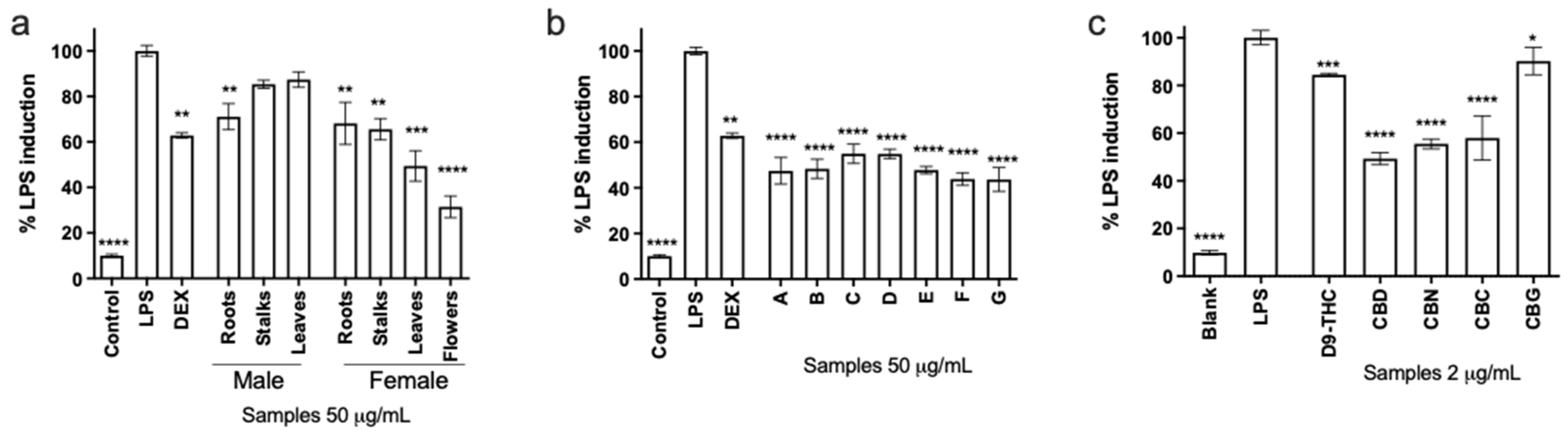
3.3. Nitric Oxide Production in Macrophages
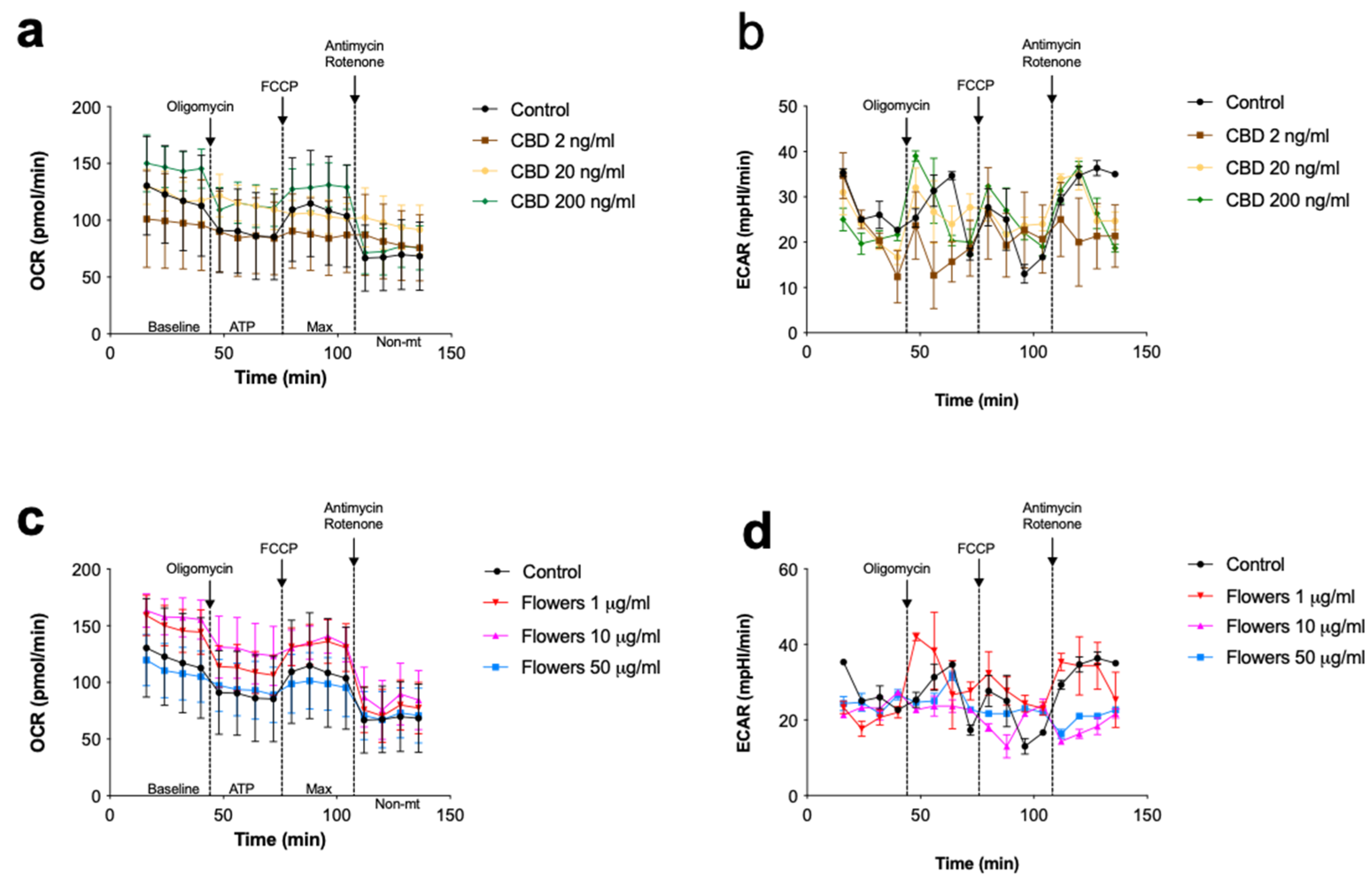
3.4. Changes in Cellular Respiration Associated with Cannabinoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef]
- Koutouki, K.; Lofts, K. Cannabis, reconciliation, and the rights of indigenous peoples: Prospects and challenges for cannabis legalization in Canada. Alta. Law Rev. 2019, 56, 709. [Google Scholar] [CrossRef]
- Parker, K.A.; Di Mattia, A.; Shaik, F.; Ortega, J.C.C.; Whittle, R. Risk management within the cannabis industry: Building a framework for the cannabis industry. Financ. Mark. Inst. Instrum. 2019, 28, 3–55. [Google Scholar] [CrossRef] [Green Version]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A review of hemp as food and nutritional supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Malcher-Lopes, R. Cannabinoids help to unravel etiological aspects in common and bring hope for the treatment of autism and epilepsy. Rev. Biol. 2014, 13, 43–59. [Google Scholar]
- Camors, C.; Chavez, S.L.; Romi, A.M. The cannabis industry within the USA: The influence of gender on cannabis policy and sales. Sustain. Account. Manag. Policy J. 2020, 11, 1095–1126. [Google Scholar] [CrossRef]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R. Hemp as an Agricultural Commodity; Library of Congress Washington DC Congressional Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Rodriguez, C.E.B.; Ouyang, L.; Kandasamy, R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis. Behav. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids as key regulators of inflammasome signaling: A current perspective. Front. Immunol. 2021, 11, 613613. [Google Scholar] [CrossRef]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free. Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Zhai, X.-T.; Zhang, Z.-Y.; Jiang, C.-H.; Chen, J.-Q.; Ye, J.-Q.; Jia, X.-B.; Yang, Y.; Ni, Q.; Wang, S.-X.; Song, J.; et al. Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-κB signaling pathway. J. Ethnopharmacol. 2016, 183, 159–165. [Google Scholar] [CrossRef]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative stress and lipid mediators modulate immune cell functions in autoimmune diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Zhao, C.; Li, S.; Kong, L.; Wu, W.; Kong, W.; Liu, Y.; Wei, Y.; Zhu, J.-K.; et al. The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant. Cell Environ. 2016, 40, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.F.; Sa-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxidative Med. Cell. Longev. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Beji, C.; Loucif, H.; Telittchenko, R.; Olagnier, D.; Dagenais-Lussier, X.; Van Grevenynghe, J. Cannabinoid-Induced Immunomodulation during Viral Infections: A Focus on Mitochondria. Viruses 2020, 12, 875. [Google Scholar] [CrossRef]
- Young, C.; Clifford, B. The quantitative determination of phytocannabinoids in hemp oils using HPLC with UV detection. Cannabis Sci. Technol. 2018, 1, 38–43. [Google Scholar]
- Hoskin, R.T.; Xiong, J.; Esposito, D.; Lila, M.A. Blueberry polyphenol-protein food ingredients: The impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Esposito, D.; Rathinasabapathy, T.; Poulev, A.; Komarnytsky, S.; Raskin, I. Akt-dependent anabolic activity of natural and synthetic brassinosteroids in rat skeletal muscle cells. J. Med. Chem. 2011, 54, 4057–4066. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Grace, M.H.; Esposito, D.; Komarnytsky, S.; Wang, F.; Lila, M.A. Polyphenols isolated from Acacia mearnsii bark with anti-inflammatory and carbolytic enzyme inhibitory activities. Chin. J. Nat. Med. 2017, 15, 816–824. [Google Scholar] [CrossRef]
- Fernandez, B.E.; Peterseil, V.; Hackl, G.; Menges, S.; Meijer, E.; Staginnus, C. Distribution of chemical phenotypes (chemotypes) in European agricultural hemp (cannabis sativa L.) cultivars. J. Forensic Sci. 2019, 65, 715–721. [Google Scholar] [CrossRef]
- Pagano, S.; Coniglio, M.; Valenti, C.; Federici, M.I.; Lombardo, G.; Cianetti, S.; Marinucci, L. Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature. Biomed. Pharmacother. 2020, 132, 110728. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS–) vs. alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Xiong, J.; Matta, F.V.; Grace, M.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Phenolic content, anti-inflammatory properties, and dermal wound repair properties of industrially processed and non-processed acai from the Brazilian Amazon. Food Funct. 2020, 11, 4903–4914. [Google Scholar] [CrossRef]
- El-Gayar, S.; Thüring-Nahler, H.; Pfeilschifter, J.; Röllinghoff, M.; Bogdan, C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J. Immunol. 2003, 171, 4561–4568. [Google Scholar] [CrossRef] [Green Version]
- Giacoppo, S.; Gugliandolo, A.; Trubiani, O.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Cannabinoid CB2 receptors are involved in the protection of RAW264.7 macrophages against the oxidative stress: An in vitro study. Eur. J. Histochem. 2017, 61, 61. [Google Scholar] [CrossRef] [Green Version]
- Schultze, N.; Wanka, H.; Zwicker, P.; Lindequist, U.; Haertel, B. Mitochondrial functions of THP-1 monocytes following the exposure to selected natural compounds. Toxicology 2017, 377, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Rahman, I. Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Duncan, R. Regulatory effects of cannabidiol on mitochondrial functions: A review. Cells 2021, 10, 1251. [Google Scholar] [CrossRef] [PubMed]


| Yield (µg/g DW) | Male Palnts | Female Plants | |||||
|---|---|---|---|---|---|---|---|
| Roots | Stalks | Leaves | Roots | Stalks | Leaves | Flowers | |
| CBDV | ND | ND | 68.6 ± 2.9 | ND | ND | 69.8 ± 2.1 | 108 ± 5.3 |
| CBDA | 2.7 ± 0.8 | 84.3 ± 19.9 | 7212 ± 273 | 5.3 ± 0.7 | 238 ± 28.6 | 10953 ± 284 | 16862 ± 651 |
| CBGA | ND | 1.5 ± 0.1 | 192 ± 12.3 | ND | 3.4 ± 0.0 | 222 ± 9.4 | 374 ± 41.5 |
| CBG | ND | 0.9 ± 0.0 | 42.1 ± 0.4 | ND | 1.3 ± 0.1 | 52.7 ± 2.5 | 84.1 ± 9.3 |
| CBD | ND | 4.5 ± 0.7 | 473 ± 9.1 | ND | 8.7 ± 1.0 | 703 ± 22.6 | 1594 ± 89.8 |
| THCV | ND | 2.1 ± 0.4 | 45.1 ± 4.1 | ND | 0.96 ± 0.2 | 36.4 ± 1.7 | 38.3 ± 2.1 |
| CBN | ND | ND | 28.6 ± 0.3 | ND | ND | 28.5 ± 0.2 | 30.3 ± 0.6 |
| ∆9-THC | ND | 2.0 ± 0.5 | 175 ± 5.0 | ND | 3.3 ± 0.3 | 213 ± 14.8 | 510 ± 48.7 |
| ∆8-THC | ND | ND | ND | ND | ND | ND | ND |
| CBC | ND | ND | 56.8 ± 1.9 | ND | 1.0 ± 0.1 | 51.2 ± 1.1 | 88.2 ± 6.6 |
| THCA | 2.1 ± 0.1 | 18.7 ± 4.1 | 1366 ± 51.3 | 2.9 ± 0.2 | 46.8 ± 8.1 | 2008 ± 76 | 3445 ± 297 |
| Total | 4.8 ± 0.9 | 114 ± 24.8 | 9661 ± 351 | 8.2 ± 0.9 | 303 ± 38.1 | 14337 ± 399 | 23134 ± 1111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, L.G.A.; Overbaugh, E.; Xiong, J.; Rathinasabapathy, T.; Komarnytsky, S.; da Silva, D.J.H.; Esposito, D.A. Immune Responses Are Differentially Regulated by Root, Stem, Leaf, and Flower Extracts of Female and Male CBD Hemp (Cannabis sativa L.) Plants. Immuno 2021, 1, 369-379. https://doi.org/10.3390/immuno1040025
Esposito LGA, Overbaugh E, Xiong J, Rathinasabapathy T, Komarnytsky S, da Silva DJH, Esposito DA. Immune Responses Are Differentially Regulated by Root, Stem, Leaf, and Flower Extracts of Female and Male CBD Hemp (Cannabis sativa L.) Plants. Immuno. 2021; 1(4):369-379. https://doi.org/10.3390/immuno1040025
Chicago/Turabian StyleEsposito, Laura G. A., Ezekial Overbaugh, Jia Xiong, Thirumurugan Rathinasabapathy, Slavko Komarnytsky, Derly José Henriques da Silva, and Debora A. Esposito. 2021. "Immune Responses Are Differentially Regulated by Root, Stem, Leaf, and Flower Extracts of Female and Male CBD Hemp (Cannabis sativa L.) Plants" Immuno 1, no. 4: 369-379. https://doi.org/10.3390/immuno1040025
APA StyleEsposito, L. G. A., Overbaugh, E., Xiong, J., Rathinasabapathy, T., Komarnytsky, S., da Silva, D. J. H., & Esposito, D. A. (2021). Immune Responses Are Differentially Regulated by Root, Stem, Leaf, and Flower Extracts of Female and Male CBD Hemp (Cannabis sativa L.) Plants. Immuno, 1(4), 369-379. https://doi.org/10.3390/immuno1040025









