Lymphocyte-to-Monocyte Ratio as a Marker for Endoscopic Activity in Ulcerative Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Disease Assessment
2.3. Endoscopic Assessment
2.4. FC Analysis
2.5. FIT Analysis
2.6. Statistical Analysis
2.7. Ethical Statement
3. Results
3.1. Patient Characteristics
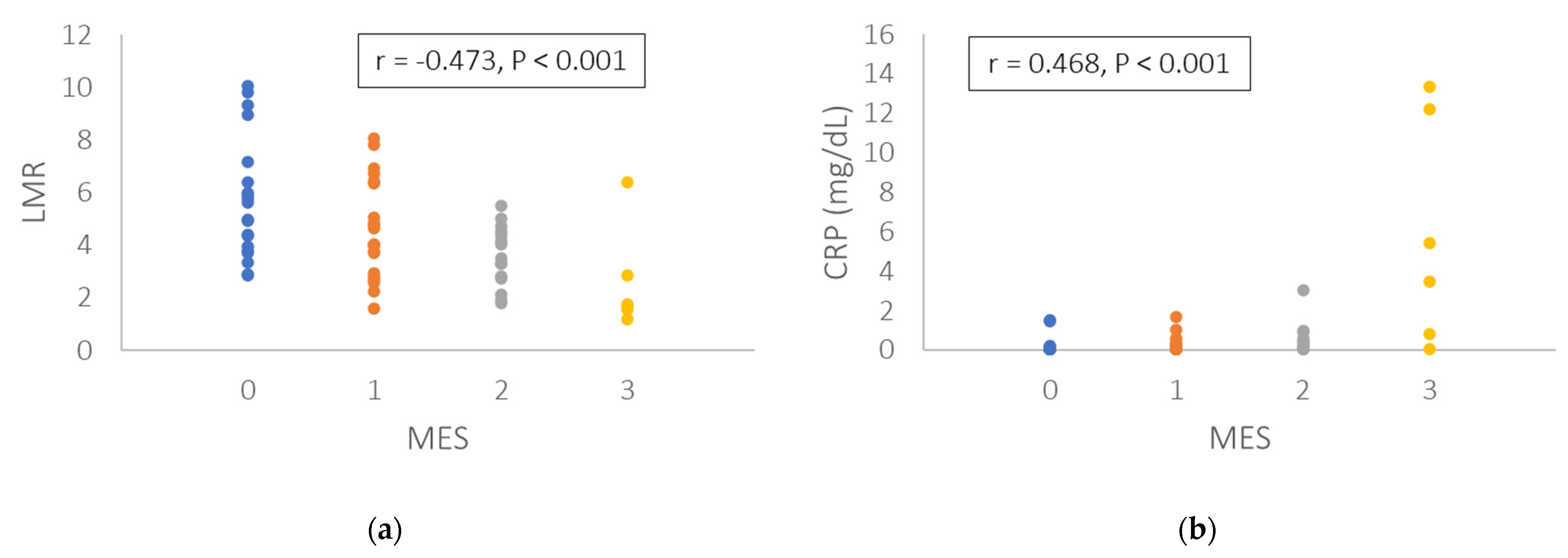
3.2. Correlation of Endoscopic Score with Leukocyte Subtypes and Biomarkers
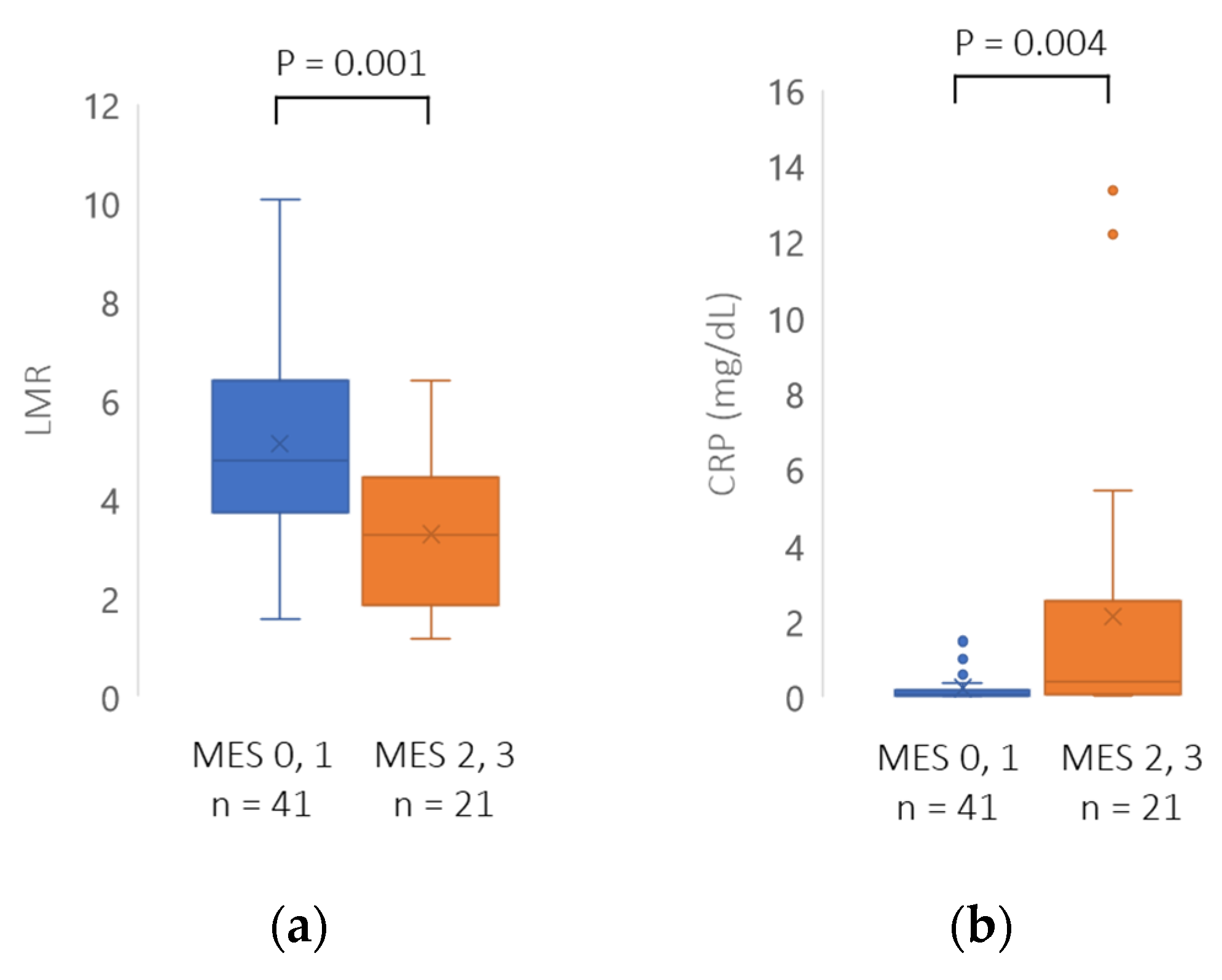
3.3. Comparison between Mucosal Healing and Non-Mucosal Healing Groups
3.4. Comparison of LMR and CRP in the Group of Patients without Immunomodulators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podolsky, D.K. Inflammatory Bowel Disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Colombel, J.F.; Rutgeerts, P.; Reinisch, W.; Esser, D.; Wang, Y.; Lang, Y.; Marano, C.W.; Strauss, R.; Oddens, B.J.; Feagan, B.G.; et al. Early Mucosal Healing with Infliximab Is Associated With Improved Long-term Clinical Outcomes in Ulcerative Colitis. Gastroenterology 2011, 141, 1194–1201. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Trummler, M.; Renzulli, P.; Seibold, F. Ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 1851–1858. [Google Scholar] [CrossRef]
- D’haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Nakarai, A.; Kato, J.; Hiraoka, S.; Kuriyama, M.; Akita, M.; Hirakawa, T.; Okada, H.; Yamamoto, K. Evaluation of Mucosal Healing of Ulcerative Colitis by a Quantitative Fecal Immunochemical Test. Am. J. Gastroenterol. 2013, 108, 83–89. [Google Scholar] [CrossRef]
- Takashima, S.; Kato, J.; Hiraoka, S.; Nakarai, A.; Takei, D.; Inokuchi, T.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; et al. Evaluation of Mucosal Healing in Ulcerative Colitis by Fecal Calprotectin Vs. Fecal Immunochemical Test. Am. J. Gastroenterol. 2015, 110, 873–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, J.; Hiraoka, S.; Nakarai, A.; Takashima, S.; Inokuchi, T.; Ichinose, M. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: Can it rival fecal calprotectin? Intest. Res. 2016, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Arihiro, S.; Matsuura, T.; Kato, T.; Matsuoka, M.; Saruta, M.; Mitsunaga, M.; Matsuura, M.; Fujiwara, M.; Okayasu, I.; et al. Prostaglandin E–Major Urinary Metabolite as a Reliable Surrogate Marker for Mucosal Inflammation in Ulcerative Colitis. Inflamm. Bowel Dis. 2014, 20, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Miyazu, T.; Takano, R.; Tamura, S.; Tani, S.; Kagami, T.; Yamade, M.; Hamaya, Y.; Iwaizumi, M.; Osawa, S.; et al. Prostaglandin E-major urinary metabolite versus fecal immunochemical occult blood test as a biomarker for patient with ulcerative colitis. BMC Gastroenterol. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Shinzaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Yasutomi, E.; Inokuchi, T.; Hiraoka, S.; Takei, K.; Igawa, S.; Yamamoto, S.; Ohmori, M.; Oka, S.; Yamasaki, Y.; Kinugasa, H.; et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, D.; Duan, S.; Zhao, Y.; Wu, C.; Zhu, F.; Chen, C.; Chen, Y. Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers: A meta-analysis. Cancer Cell Int. 2018, 18, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherfane, C.E.; Gessel, L.; Cirillo, D.; Zimmerman, M.B.; Polyak, S. Monocytosis and a Low Lymphocyte to Monocyte Ratio Are Effective Biomarkers of Ulcerative Colitis Disease Activity. Inflamm. Bowel Dis. 2015, 21, 1769–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okba, A.M.; Amin, M.M.; Abdelmoaty, A.S.; Ebada, H.; Kamel, A.H.; Allam, A.S.; Sobhy, O.M. Neutrophil/lymphocyte ratio and lymphocyte/monocyte ratio in ulcerative colitis as non-invasive biomarkers of disease activity and severity. Autoimmun. Highlights 2019, 10, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Cen, M.; Chen, X.; Chen, H.; Liu, X.; Cao, Q. Correlation between Serological Biomarkers and Disease Activity in Patients with Inflammatory Bowel Disease. BioMed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: A randomised trial. BMJ 1989, 298, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Jeong, Y.; Jeon, S.R.; Kim, H.G.; Moon, J.R.; Lee, T.H.; Jang, J.Y.; Cho, J.-H.; Park, J.S.; Park, H.; Lee, K.-H.; et al. The role of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in ulcerative colitis. Intest. Res. 2021, 19, 62–70. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Monteleone, I.; Fantini, M.C.; Monteleone, G. Regulation of Homeostasis and Inflammation in the Intestine. Gastroenterology 2011, 140, 1768–1775. [Google Scholar] [CrossRef]
- Nixon, J.; Riddell, R. Histopathology in ulcerative colitis. In Inflammatory Bowel Disease; Allan, R., Keighley, M., Alexander-Williams, C., Hawkins, C., Eds.; Churchill Livingstone: Edinburgh, UK, 1990; Volume 20, pp. 247–285. [Google Scholar]
- Sachar, D.B.; Taub, R.N.; Brown, S.M.; Present, D.H.; Korelitz, B.I.; Janowitz, H.D. Impaired Lymphocyte Responsiveness in Inflammatory Bowel Disease. Gastroenterology 1973, 64, 203–209. [Google Scholar] [CrossRef]
- Hermanowicz, A.; Gibson, P.R.; Jewell, D.P. The role of phagocytes in inflammatory bowel disease. Clin. Sci. 1985, 69, 241–249. [Google Scholar] [CrossRef]
- Torun, S.; Tunc, B.D.; Suvak, B.; Yildiz, H.; Tas, A.; Sayilir, A.; Ozderin, Y.O.; Beyazit, Y.; Kayacetin, E. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: A promising marker in predicting disease severity. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 491–497. [Google Scholar] [CrossRef]
- Mee, A.S.; Berney, J.; Jewell, D.P. Monocytes in inflammatory bowel disease: Absolute monocyte counts. J. Clin. Pathol. 1980, 33, 917–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celikbilek, M.; Dogan, S.; Ozbakır, O.; Zararsız, G.; Kücük, H.; Gürsoy, S.; Yurci, A.; Güven, K.; Yücesoy, M. Neutrophil-Lymphocyte Ratio as a Predictor of Disease Severity in Ulcerative Colitis. J. Clin. Lab. Anal. 2013, 27, 72–76. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N = 89 | |
|---|---|---|
| Age (year), mean (range) ± SD | 47.2 (17–77) ± 16.6 | |
| Sex, n (%) | Male | 54 (60.0) |
| Female | 36 (40.0) | |
| Disease extent, n (%) | Extensive colitis | 54 (60.7) |
| Left sided colitis | 28 (31.5) | |
| Proctitis | 7 (7.9) | |
| Disease duration (year), mean (range) ± SD | 11.1 (0.3–72) ± 10.6 | |
| CAI (Rachmilewitz index), mean (range) ± SD | 2.7 (0–17) ± 3.6 | |
| MES, n (%) | 0 | 30 (33.7) |
| 1 | 29 (32.6) | |
| 2 | 24 (27.0) | |
| 3 | 6 (6.7) | |
| FC (µg/g), mean (range) ± SD | 6294.8 (8.9–200,000) ± 22,543.1 | |
| FIT (ng/mL), mean (range) ± SD | 2902.4 (30–45,900) ± 7620.6 | |
| CRP (mg/dL), mean (range) ± SD | 0.74 (0.02–13.35) ± 2.15 | |
| Medication, n (%) | 5-ASA (%) | 57 (64.0) |
| Steroid enema (%) | 6 (6.7) | |
| Systemic steroid (%) | 13 (14.6) | |
| Advanced therapy (%) | 31 (34.8) | |
| Immunomodulators (%) | 27 (30.3) | |
| Variable | MES | Variable | MES | ||
|---|---|---|---|---|---|
| r | p | r | p | ||
| Neutrophil count | 0.343 | <0.001 | Neutrophil rate | 0.229 | 0.031 |
| Eosinophil count | 0.114 | 0.289 | Eosinophil rate | −0.014 | 0.893 |
| Basophil count | 0.020 | 0.851 | Basophil rate | −0.122 | 0.256 |
| Lymphocyte count | −0.049 | 0.651 | Lymphocyte rate | −0.308 | 0.003 |
| Monocyte count | 0.361 | <0.001 | Monocyte rate | 0.119 | 0.268 |
| NLR | 0.280 | 0.008 | FC | 0.704 | <0.001 |
| NMR | −0.039 | 0.717 | FIT | 0.735 | <0.001 |
| LMR | −0.359 | <0.001 | CRP | 0.504 | <0.001 |
| Variable | MES 0, 1 n = 59 | MES 2, 3 n = 30 | p |
|---|---|---|---|
| Neutrophil count (/µL), mean ± SD | 3595.5 ± 1596.6 | 5132.2 ± 2980.9 | 0.002 |
| Eosinophil count (/µL), mean ± SD | 182.5 ± 155.9 | 211.5 ± 191.4 | 0.446 |
| Basophil count (/µL), mean ± SD | 43.8 ± 26.3 | 42.0 ± 27.7 | 0.762 |
| Lymphocyte count (/µL), mean ± SD | 1647.5 ± 800.6 | 1419.66 ± 554.6 | 0.166 |
| Monocyte count (/µL), mean ± SD | 355.3 ± 176.7 | 455.19 ± 227.1 | 0.025 |
| Neutrophil rate (%), mean ± SD | 61.4 ± 8.2 | 67.0 ± 13.7 | 0.017 |
| Eosinophil rate (%), mean ± SD | 3.1 ± 2.4 | 3.1 ± 2.3 | 0.959 |
| Basophil rate (%), mean ± SD | 0.8 ± 0.3 | 0.7 ± 0.4 | 0.361 |
| Lymphocyte rate (%), mean ± SD | 28.5 ± 8.0 | 22.5 ± 11.2 | 0.005 |
| Monocyte rate (%), mean ± SD | 6.3 ± 2.0 | 6.6 ± 2.4 | 0.429 |
| NLR, mean ± SD | 2.42 ± 1.09 | 4.45 ± 3.58 | <0.001 |
| NMR, mean ± SD | 10.8 ± 4.2 | 12.9 ± 9.4 | 0.151 |
| LMR, mean ± SD | 5.01 ± 2.09 | 3.49 ± 1.42 | 0.001 |
| FC (µg/g), mean ± SD | 1368.1 ± 2637.0 | 15,983.9 ± 37,171.2 | 0.003 |
| FIT (ng/mL), mean ± SD | 738.8 ± 2418.9 | 7157.7 ± 11,673.2 | <0.001 |
| CRP (mg/dL), mean ± SD | 0.18 ± 0.35 | 1.83 ± 3.46 | <0.001 |
| Variable | Cut-Off Value | AUC [95% CI] | Sensitivity | Specificity |
|---|---|---|---|---|
| Neutrophil count | 4662.0 | 0.642 [0.510–0.775] | 53.3 | 78.0 |
| Monocyte count | 389.6 | 0.657 [0.537–0.777] | 56.7 | 69.5 |
| Neutrophil rate | 68.8 | 0.630 [0.490–0.770] | 50.0 | 83.1 |
| Lymphocyte rate | 23.0 | 0.677 [0.545–0.809] | 56.7 | 76.3 |
| NLR | 3.73 | 0.659 [0.524–0.795] | 43.3 | 91.5 |
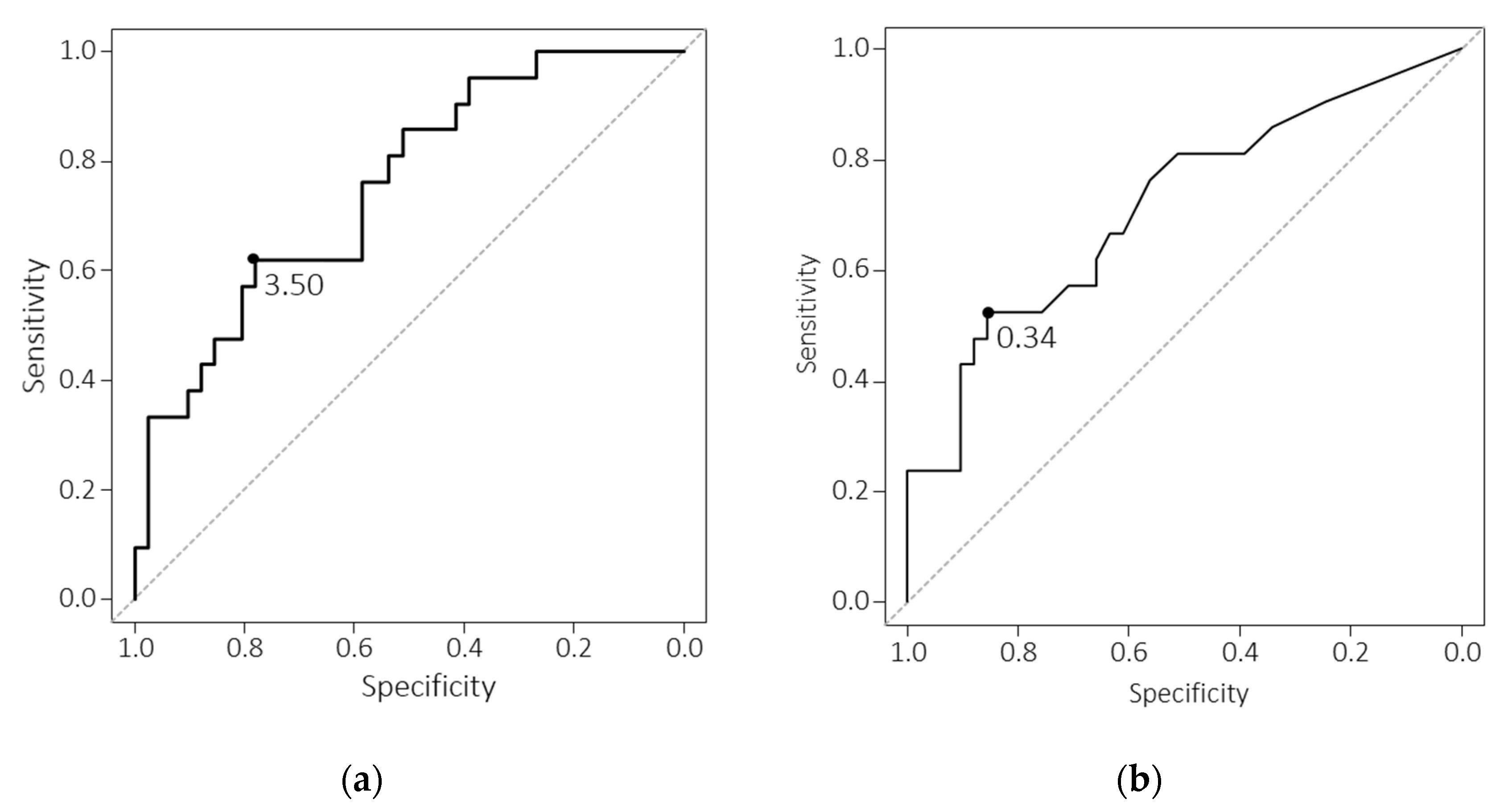
| LMR | 4.35 | 0.712 [0.604–0.821] | 76.7 | 61.0 |
| FC | 2510.0 | 0.860 [0.784–0.937] | 76.7 | 81.4 |
| FIT | 261.0 | 0.908 [0.848–0.968] | 93.3 | 79.7 |
| CRP | 0.27 | 0.769 [0.661–0.876] | 56.7 | 88.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, N.; Takahashi, S.; Asai, Y.; Miyazu, T.; Tamura, S.; Tani, S.; Yamade, M.; Iwaizumi, M.; Hamaya, Y.; Osawa, S.; et al. Lymphocyte-to-Monocyte Ratio as a Marker for Endoscopic Activity in Ulcerative Colitis. Immuno 2021, 1, 360-368. https://doi.org/10.3390/immuno1040024
Ishida N, Takahashi S, Asai Y, Miyazu T, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Osawa S, et al. Lymphocyte-to-Monocyte Ratio as a Marker for Endoscopic Activity in Ulcerative Colitis. Immuno. 2021; 1(4):360-368. https://doi.org/10.3390/immuno1040024
Chicago/Turabian StyleIshida, Natsuki, Satoru Takahashi, Yusuke Asai, Takahiro Miyazu, Satoshi Tamura, Shinya Tani, Mihoko Yamade, Moriya Iwaizumi, Yasushi Hamaya, Satoshi Osawa, and et al. 2021. "Lymphocyte-to-Monocyte Ratio as a Marker for Endoscopic Activity in Ulcerative Colitis" Immuno 1, no. 4: 360-368. https://doi.org/10.3390/immuno1040024
APA StyleIshida, N., Takahashi, S., Asai, Y., Miyazu, T., Tamura, S., Tani, S., Yamade, M., Iwaizumi, M., Hamaya, Y., Osawa, S., Furuta, T., & Sugimoto, K. (2021). Lymphocyte-to-Monocyte Ratio as a Marker for Endoscopic Activity in Ulcerative Colitis. Immuno, 1(4), 360-368. https://doi.org/10.3390/immuno1040024






