Acute Ischaemic Stroke in Infective Endocarditis: Pathophysiology and Clinical Outcomes in Patients Treated with Reperfusion Therapy
Abstract
:1. Main Messages
- Management of acute ischaemic stroke patients with presumed or confirmed IE is challenging and treatment guidelines are far from optimal due to limited evidence.
- A comprehensive overview of acute stroke in the background of infective endocarditis is provided.
- Various clinical factors mediating outcomes and therapeutic strategies, specifically in the setting of reperfusion therapy, are also discussed.
2. Introduction
3. Epidemiology of Infective Endocarditis
4. Aetiology of Infective Endocarditis
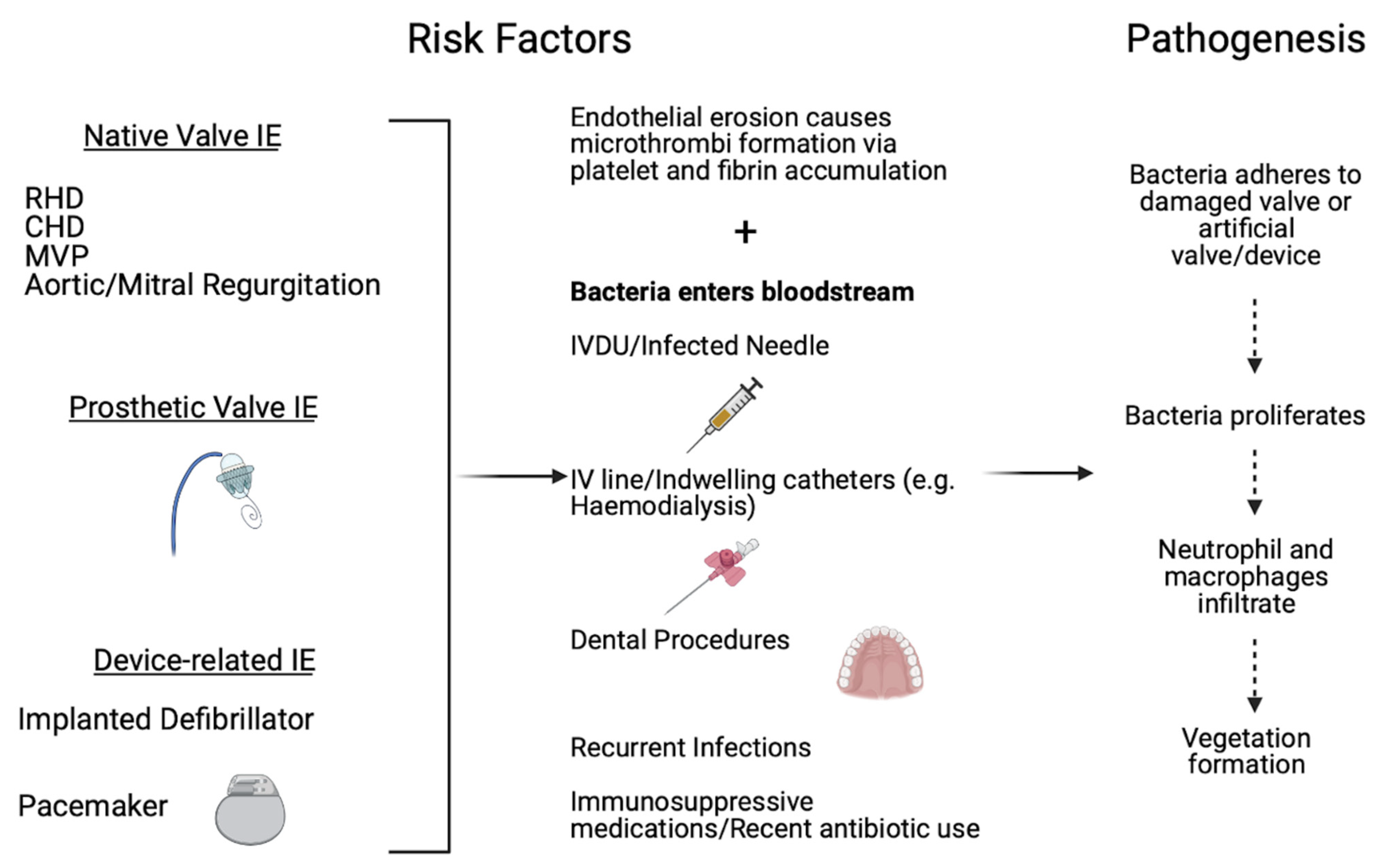
5. Infective Endocarditis Risk Factors and Pathophysiology
6. Epidemiology of Stroke
Risk Factors for Stroke
7. Pathophysiology of Acute Ischaemic Stroke
8. Aetiology of Stroke
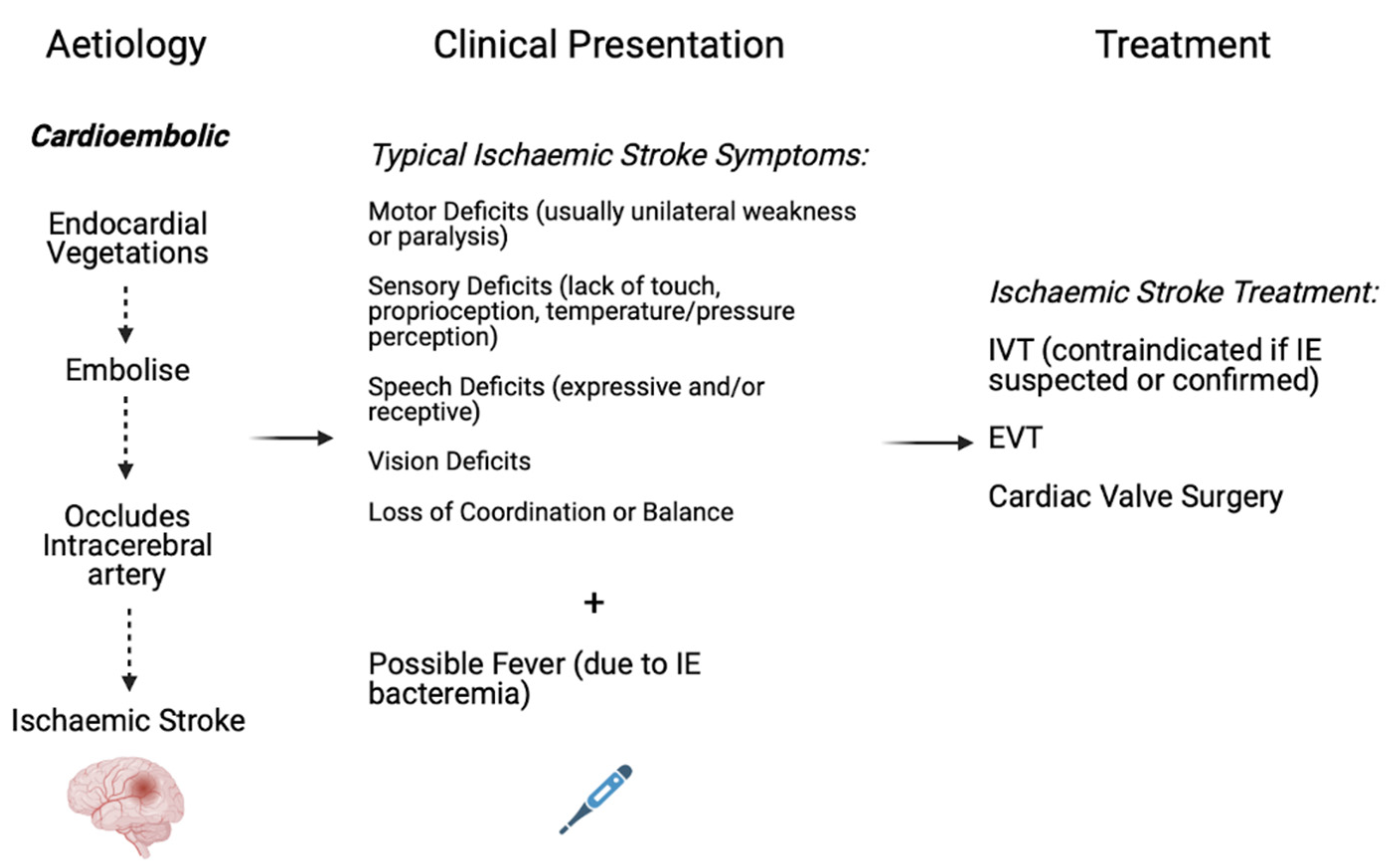
9. Pathophysiology of Acute Ischaemic Stroke in the setting of Infective Endocarditis
10. Aetiology of Acute Ischaemic Stroke in the Setting of Infective Endocarditis
11. Clinical Outcomes of Acute Ischaemic Stroke in Infective Endocarditis
12. Reperfusion Therapy in Acute Ischaemic Stroke Patients with Infective Endocarditis
13. Association with Good Functional Outcomes
14. Association with Mortality
15. Association with Adverse Events
15.1. Intracranial Haemorrhage
15.2. Recurrent Stroke
15.3. Association with Successful Reperfusion
16. Stroke Prevention in IE
17. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campbell, B.C.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Sonneville, R.; Mourvillier, B.; Bouadma, L.; Wolff, M. Management of neurological complications of infective endocarditis in ICU patients. Ann. Intensive Care 2011, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-C.; Wu, V.C.-C.; Chang, C.-H.; Chen, C.-T.; Hsieh, P.-C.; Liu, Z.-H.; Wong, H.-F.; Yang, C.-H.; Chou, A.-H.; Chu, P.-H. Long-term outcome of neurological complications after infective endocarditis. Sci. Rep. 2020, 10, 3994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruitt, A.; Rubin, R.H.; Karchmer, A.; Duncan, G.W. Neurologic complications of bacterial endocarditis. Medicine 1978, 57, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Forestier, E.; Fraisse, T.; Roubaud-Baudron, C.; Selton-Suty, C.; Pagani, L. Managing infective endocarditis in the elderly: New issues for an old disease. Clin. Interv. Aging 2016, 11, 1199–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante-Mangoni, E.; Bradley, S.; Selton-Suty, C.; Tripodi, M.F.; Barsic, B.; Bouza, E.; Cabell, C.H.; Ramos, A.I.; Fowler, V., Jr.; Hoen, B.; et al. Current features of infective endocarditis in elderly patients: Results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch. Intern. Med. 2008, 168, 2095–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettencourt, S.; Ferro, J.M. Acute ischemic stroke treatment in infective endocarditis: Systematic review. J. Stroke Cerebrovasc. Dis. 2020, 29, 104598. [Google Scholar] [CrossRef]
- Bhaskar, S.; Cordato, D.; Cappelen-Smith, C.; Cheung, A.; Ledingham, D.; Celermajer, D.; Levi, C. Clarion call for histopathological clot analysis in “cryptogenic” ischemic stroke: Implications for diagnosis and treatment. Ann. Clin. Transl. Neurol. 2017, 4, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, S.; Saab, J.; Cappelen-Smith, C.; Killingsworth, M.; Wu, X.J.; Cheung, A.; Manning, N.; Aouad, P.; McDougall, A.; Hodgkinson, S.; et al. Clot Histopathology in Ischemic Stroke with Infective Endocarditis. Can. J. Neurol. Sci. 2019, 46, 331–336. [Google Scholar] [CrossRef]
- Abdulhak, A.A.B.; Baddour, L.M.; Erwin, P.J.; Hoen, B.; Chu, V.H.; Mensah, G.A.; Tleyjeh, I.M. Global and regional burden of infective endocarditis, 1990–2010: A systematic review of the literature. Glob. Heart 2014, 9, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Ambrosioni, J.; Hernandez-Meneses, M.; Téllez, A.; Pericàs, J.; Falces, C.; Tolosana, J.; Vidal, B.; Almela, M.; Quintana, E.; Llopis, J. The changing epidemiology of infective endocarditis in the twenty-first century. Curr. Infect. Dis. Rep. 2017, 19, 21. [Google Scholar] [CrossRef]
- Sy, R.W.; Kritharides, L. Health care exposure and age in infective endocarditis: Results of a contemporary population-based profile of 1536 patients in Australia. Eur. Heart J. 2010, 31, 1890–1897. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Cai, S.; Dai, H. Characteristics of infective endocarditis in a tertiary hospital in East China. PLoS ONE 2016, 11, e0166764. [Google Scholar] [CrossRef]
- Letaief, A.; Boughzala, E.; Kaabia, N.; Ernez, S.; Abid, F.; Chaabane, T.B.; Jemaa, M.B.; Boujnah, R.; Chakroun, M.; Daoud, M. Epidemiology of infective endocarditis in Tunisia: A 10-year multicenter retrospective study. Int. J. Infect. Dis. 2007, 11, 430–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreiros, E.; Nacinovich, F.; Casabé, J.H.; Modenesi, J.C.; Swieszkowski, S.; Cortes, C.; Hernan, C.A.; Kazelian, L.; Varini, S. Epidemiologic, clinical, and microbiologic profile of infective endocarditis in Argentina: A national survey. The Endocarditis Infecciosa en la República Argentina–2 (EIRA-2) Study. Am. Heart J. 2006, 151, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Salvador, V.B.D.; Chapagain, B.; Joshi, A.; Brennessel, D.J. Clinical risk factors for infective endocarditis in Staphylococcus aureus bacteremia. Tex. Heart Inst. J. 2017, 44, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis–Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [Green Version]
- Keynan, Y.; Rubinstein, E. Pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 2013, 15, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Barker-Collo, S.; Krishnamurthi, R.; Theadom, A.; Starkey, N. Epidemiology of ischaemic stroke and traumatic brain injury. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 485–494. [Google Scholar] [CrossRef]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: The GBD 2013 study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Li, P.; Guo, Q.; Shang, K.; Yan, D.; Du, S.; Lu, Y. Pathophysiology and biomarkers in acute ischemic stroke–a review. Trop. J. Pharm. Res. 2013, 12, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, R.R.; Baron, J.C. Pathophysiology of ischaemic stroke: Insights from imaging, and implications for therapy and drug discovery. Br. J. Pharmacol. 2008, 153, S44–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferro, J.M.; Massaro, A.R.; Mas, J.-L. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010, 9, 1085–1096. [Google Scholar] [CrossRef]
- Heiro, M.; Nikoskelainen, J.; Engblom, E.; Kotilainen, E.; Marttila, R.; Kotilainen, P. Neurologic manifestations of infective endocarditis: A 17-year experience in a teaching hospital in Finland. Arch. Intern. Med. 2000, 160, 2781–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruttmann, E.; Willeit, J.; Ulmer, H.; Chevtchik, O.; Höfer, D.; Poewe, W.; Laufer, G.; Müller, L.C. Neurological Outcome of Septic Cardioembolic Stroke After Infective Endocarditis. Stroke 2006, 37, 2094–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karchmer, A. Infective Endocarditis. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 7th ed.; WB Saunders: Philadelphia, PA, USA, 2005. [Google Scholar]
- Guzek, A.; Braksator, W.; Gąsior, Z.; Kuśmierczyk, M.; Różański, J.; Rybicki, Z. Infective endocarditis-can we treat it more effectively? Kardiochir. Torakochir. Pol. 2020, 17, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Brusch, J.L. Infective Endocarditis. Available online: https://emedicine.medscape.com/article/216650-overview#showall (accessed on 12 September 2021).
- Morris, N.A.; Matiello, M.; Lyons, J.L.; Samuels, M.A. Neurologic complications in infective endocarditis: Identification, management, and impact on cardiac surgery. Neurohospitalist 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Kamel, H.; Healey, J.S. Cardioembolic stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef]
- García-Cabrera, E.; Fernández-Hidalgo, N.; Almirante, B.; Ivanova-Georgieva, R.; Noureddine, M.; Plata, A.; Lomas, J.M.; Gálvez-Acebal, J.; Hidalgo-Tenorio, C.; Ruíz-Morales, J.; et al. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation 2013, 127, 2272–2284. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, I.; Hunter, M.D.; Sundheim, K.; Klein, B.; Dunn, L.; Sorabella, R.; Han, S.M.; Willey, J.; George, I.; Gutierrez, J. Clinical risk factors for acute ischaemic and haemorrhagic stroke in patients with infective endocarditis. Intern. Med. J. 2018, 48, 1072–1080. [Google Scholar] [CrossRef]
- Schirone, L.; Iaccarino, A.; Saade, W.; D’Abramo, M.; De Bellis, A.; Frati, G.; Sciarretta, S.; Mestres, C.-A.; Greco, E. Cerebrovascular complications and infective endocarditis: Impact of available evidence on clinical outcome. BioMed Res. Int. 2018, 2018, 4109358. [Google Scholar] [CrossRef]
- Deprele, C.; Berthelot, P.; Lemetayer, F.; Comtet, C.; Fresard, A.; Cazorla, C.; Fascia, P.; Cathébras, P.; Chaumentin, G.; Convert, G. Risk factors for systemic emboli in infective endocarditis. Clin. Microbiol. Infect. 2004, 10, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grecu, N.; Cristina, T.; Terecoasa, E.; Bajenaru, O. Endocarditis and stroke. Maedica 2014, 9, 375. [Google Scholar]
- Kang, D.-H.; Kim, Y.-J.; Kim, S.-H.; Sun, B.J.; Kim, D.-H.; Yun, S.-C.; Song, J.-M.; Choo, S.J.; Chung, C.-H.; Song, J.-K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuny, F.; Avierinos, J.-F.; Tribouilloy, C.; Giorgi, R.; Casalta, J.-P.; Milandre, L.; Brahim, A.; Nadji, G.; Riberi, A.; Collart, F.; et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur. Heart J. 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.V.; Killingsworth, M.C.; Bhaskar, S. Cerebral collaterals in acute ischaemia: Implications for acute ischaemic stroke patients receiving reperfusion therapy. Eur. J. Neurosci. 2021, 53, 1238–1261. [Google Scholar] [CrossRef]
- Rastogi, A.; Weissert, R.; Bhaskar, S.M.M. Emerging role of white matter lesions in cerebrovascular disease. Eur. J. Neurosci. 2021, 54, 5531–5559. [Google Scholar] [CrossRef]
- Rastogi, A.; Weissert, R.; Bhaskar, S.M.M. Leukoaraiosis severity and post-reperfusion outcomes in acute ischaemic stroke: A meta-analysis. Acta Neurol. Scand. 2021. [Google Scholar] [CrossRef]
- Shi, C.; Killingsworth, M.C.; Bhaskar, S.M.M. Prognostic capacity of hyperdense middle cerebral artery sign in anterior circulation acute ischaemic stroke patients receiving reperfusion therapy: A systematic review and meta-analysis. Acta Neurol. Belg. 2021, 1–13. [Google Scholar] [CrossRef]
- Baskar, P.S.; Chowdhury, S.Z.; Bhaskar, S.M.M. In-hospital systems interventions in acute stroke reperfusion therapy: A meta-analysis. Acta Neurol. Scand. 2021, 144, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Santana Baskar, P.; Cordato, D.; Wardman, D.; Bhaskar, S. In-hospital acute stroke workflow in acute stroke-Systems-based approaches. Acta Neurol. Scand. 2021, 143, 111–120. [Google Scholar] [CrossRef]
- Venkat, A.; Cappelen-Smith, C.; Askar, S.; Thomas, P.R.; Bhaskar, S.; Tam, A.; McDougall, A.J.; Hodgkinson, S.J.; Cordato, D.J. Factors Associated with Stroke Misdiagnosis in the Emergency Department: A Retrospective Case-Control Study. Neuroepidemiology 2018, 51, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Bivard, A.; Stanwell, P.; Parsons, M.; Attia, J.R.; Nilsson, M.; Levi, C. Baseline collateral status and infarct topography in post-ischaemic perilesional hyperperfusion: An arterial spin labelling study. J. Cereb. Blood Flow Metab. 2017, 37, 1148–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gariel, F.; Lapergue, B.; Bourcier, R.; Berge, J.; Barreau, X.; Mazighi, M.; Kyheng, M.; Labreuche, J.; Fahed, R.; Blanc, R. Mechanical thrombectomy outcomes with or without intravenous thrombolysis: Insight from the ASTER randomized trial. Stroke 2018, 49, 2383–2390. [Google Scholar] [CrossRef]
- D’Anna, L. Endovascular treatment of ischemic large-vessel stroke due to infective endocarditis: Case series and review of the literature. Neurol. Sci. 2020, 41, 3517–3525. [Google Scholar] [CrossRef]
- Marquardt, R.J.; Cho, S.-M.; Thatikunta, P.; Deshpande, A.; Wisco, D.; Uchino, K. Acute ischemic stroke therapy in infective endocarditis: Case series and systematic review. J. Stroke Cerebrovasc. Dis. 2019, 28, 2207–2212. [Google Scholar] [CrossRef]
- Asaithambi, G.; Adil, M.M.; Qureshi, A.I. Thrombolysis for ischemic stroke associated with infective endocarditis: Results from the nationwide inpatient sample. Stroke 2013, 44, 2917–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosioni, J.; Urra, X.; Hernández-Meneses, M.; Almela, M.; Falces, C.; Tellez, A.; Quintana, E.; Fuster, D.; Sandoval, E.; Vidal, B. Mechanical thrombectomy for acute ischemic stroke secondary to infective endocarditis. Clin. Infect. Dis. 2018, 66, 1286–1289. [Google Scholar] [CrossRef] [Green Version]
- Ramos, C.; Mayo, P.; Trillo, S.; Gómez-Escalonilla, C.; Caniego, J.L.; Moreu, M.; Vega, J.; Rosati, S.; Simal, P.; Carrillo, Á.X. Management of Large Vessel Occlusion Stroke Related to Infective Endocarditis: Is Mechanical Thrombectomy a Safe Option? J. Stroke Cerebrovasc. Dis. 2020, 29, 105248. [Google Scholar] [CrossRef] [PubMed]
- Feil, K.; Küpper, C.; Tiedt, S.; Dimitriadis, K.; Herzberg, M.; Dorn, F.; Liebig, T.; Dieterich, M.; Kellert, L.; for the GSR Investigators. Safety and efficacy of mechanical thrombectomy in infective endocarditis: A matched case–control analysis from the German Stroke Registry–Endovascular Treatment. Eur. J. Neurol. 2021, 28, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Marnat, G.; Sibon, I.; Gory, B.; Richard, S.; Olindo, S.; Consoli, A.; Bourcier, R.; Kyheng, M.; Labreuche, J.; Darganzali, C. Safety and outcomes of mechanical thrombectomy for acute stroke related to infective endocarditis: A case–control study. Int. J. Stroke 2020, 16, 585–592. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; Van Den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [Green Version]
- Sardar, P.; Chatterjee, S.; Giri, J.; Kundu, A.; Tandar, A.; Sen, P.; Nairooz, R.; Huston, J.; Ryan, J.J.; Bashir, R.; et al. Endovascular therapy for acute ischaemic stroke: A systematic review and meta-analysis of randomized trials. Eur. Heart J. 2015, 36, 2373–2380. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, J.M.; Liebeskind, D.S.; Slater, L.-A.; Nogueira, R.G.; Clark, W.; Dávalos, A.; Bonafé, A.; Jahan, R.; Fischer, U.; Gralla, J. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: A pooled analysis of the SWIFT and STAR studies. JAMA Neurol. 2017, 74, 268–274. [Google Scholar] [CrossRef]
- Dickerman, S.A.; Abrutyn, E.; Barsic, B.; Bouza, E.; Cecchi, E.; Moreno, A.; Doco-Lecompte, T.; Eisen, D.P.; Fortes, C.Q.; Fowler, V.G., Jr.; et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: An analysis from the ICE Prospective Cohort Study (ICE-PCS). Am. Heart J. 2007, 154, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Tackling, G.; Lala, V. Endocarditis Antibiotic Regimens. [Updated 2021 April 21]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542162/ (accessed on 12 September 2021).
- Vilacosta, I.; Graupner, C.; San Román, J.A.; Sarriá, C.; Ronderos, R.; Fernández, C.; Mancini, L.; Sanz, O.; Sanmartín, J.V.; Stoermann, W. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J. Am. Coll. Cardiol. 2002, 39, 1489–1495. [Google Scholar] [CrossRef] [Green Version]
- Tornos, P.; Almirante, B.; Mirabet, S.; Permanyer, G.; Pahissa, A.; Soler-Soler, J. Infective endocarditis due to Staphylococcus aureus: Deleterious effect of anticoagulant therapy. Arch. Intern. Med. 1999, 159, 473–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, G.; Hoen, B.; Tornos, P.; Thuny, F.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; de Jesus Antunes, M.; Thilen, U.; Lekakis, J.; et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur. Heart J. 2009, 30, 2369–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoen, B.; Duval, X. Infective Endocarditis. N. Engl. J. Med. 2013, 368, 1425–1433. [Google Scholar] [CrossRef]
- Chan, K.L.; Dumesnil, J.G.; Cujec, B.; Sanfilippo, A.J.; Jue, J.; Turek, M.A.; Robinson, T.I.; Moher, D. A randomized trial of aspirin on the risk of embolic events in patients with infective endocarditis. J. Am. Coll. Cardiol. 2003, 42, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Anavekar, N.S.; Tleyjeh, I.M.; Anavekar, N.S.; Mirzoyev, Z.; Steckelberg, J.M.; Haddad, C.; Khandaker, M.H.; Wilson, W.R.; Chandrasekaran, K.; Baddour, L.M. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin. Infect. Dis. 2007, 44, 1180–1186. [Google Scholar] [CrossRef]
| AIS with a History of IE | AIS without a History of IE | |||||
|---|---|---|---|---|---|---|
| IVT Only | EVT Only | IVT + EVT | IVT Only | EVT Only | IVT + EVT | |
| Mortality (mRS = 6 at three months post-treatment) | 5/18 (28%) [7] | 5/22 (23%) [7] 4/21 (19%) [50] 3/6 (50%) [52] 5/10 (50%) [53] | 3/10 (30%) [7] * 4/19 (21%) [50] * 33/55 (60%) [54] * 7/27 (26%) [55] 1/2 (50%) [53] | 59/267 (22%) [56] 7/35 (20%) [57] 28/147 (19%) [58] 12/97 (12%) [59] 16/103 (16%) [60] 191/1107 (17%) [61] | 16/131 (12%) [62] | * 30/104 (29%) [54] * 15/77 (20%) [55] * 49/233 (21%) [56] * 3/35 (9%) [57] * 17/164 (10%) [58] * 9/98 (9%) [59] * 19/103 (18%) [60] * 213/1312 (16%) [61] 13/160 (8%) [62] |
| Total | 5/18 (28%) | 17/59 (29%) | 48/113 (42%) | 313/1756 (18%) | 16/131 (12%) | 368/2286 (16%) |
| ICH (post-treatment) | 11/18 (61%) [7] | 0/22 (0%) [7] 3/17 (18%) [50] 0/6 (0%) [52] 4/10 (40%) [53] | 4/10 (40%) [7] * 12/19 (63%) [50] * 44/222 (20%) [51] * 17/55 (31%) [54] * 10/25 (40%) [55] 2/2 (100%) [53] | 17/267 (6%) (sICH) [56] 2/35 (6%) (sICH) [57] 4/150 (3%) (sICH) [58] 53/1110 (5%) (sICH) [61] | No studies | * 8730/134,048 (7%) [51] * 22/104 (21%) [54] * 38/77 (49%) [55] * 18/233 (8%) (sICH) [56] * 0/35 (0%) (sICH) [57] * 6/165 (4%) (sICH) [58] * 66/1313 (5%) (sICH) [61] |
| Total | 11/18 (61%) | 7/55 (13%) | 89/333 (27%) | 76/1562 (5%) | N/A | 8880/135,975 (7%) |
| Reperfusion Status (mTICI 2b-3) | N/A | 5/6 (83%) [52] 4/10 (40%) [53] | * 41/55 (75%) [54] * 24/28 (86%) [55] 2/2 (100%) [53] | N/A | No studies | * 91/104 (88%) [54] * 80/84 (95%) [55] * 115/196 (59%) [56] * 25/29 (86%) [57] * 113/156 (72%) [58] * 73/83 (88%) [59] * 67/102 (66%) [60] |
| Total | N/A | 9/16 (56%) | 30/85 (35%) | N/A | N/A | 564/754 (75%) |
| Good Functional Outcome (mRS 0–2 at three-month follow-up) | 7/17 (41%) [7] | 15/22 (68%) [7] 13/21 (62%) [50] 3/6 (50%) [52] 3/10 (30%) [53] | * 23/222 (10%) (discharge into home or self-care) [51] 6/10 (60%) [7] * 7/19 (37%) [50] * 11/55 (20%) [54] * 7/27 (26%) [55] 1/2 (50%) [53] | 51/267 (1%) [56] 14/35 (40%) [57] 43/147 (29%) [58] 33/93 (35%) [59] 29/103 (28%) [60] 351/1107 (32%) [61] | 61/128 (48%) [62] | * 49,572/134,048 (37%) (discharge into home or self-care) [51] * 45/104 (43%) [54] * 39/77 (51%) [55] * 76/233 (33%) [56] * 25/35 (71%) [57] * 87/164 (53%) [58] * 59/98 (60%) [59] * 45/103 (44%) [60] * 557/1312 (42%) [61] 90/156 (58%) [62] |
| Total | 7/17 (41%) | 34/59 (58%) | 55/335 (16%) | 521/1752 (30%) | 61/128 (48%) | 50,595/136,330 (37%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maheshwari, R.; Wardman, D.; Cordato, D.J.; Bhaskar, S.M.M. Acute Ischaemic Stroke in Infective Endocarditis: Pathophysiology and Clinical Outcomes in Patients Treated with Reperfusion Therapy. Immuno 2021, 1, 347-359. https://doi.org/10.3390/immuno1040023
Maheshwari R, Wardman D, Cordato DJ, Bhaskar SMM. Acute Ischaemic Stroke in Infective Endocarditis: Pathophysiology and Clinical Outcomes in Patients Treated with Reperfusion Therapy. Immuno. 2021; 1(4):347-359. https://doi.org/10.3390/immuno1040023
Chicago/Turabian StyleMaheshwari, Rohan, Daniel Wardman, Dennis John Cordato, and Sonu Menachem Maimonides Bhaskar. 2021. "Acute Ischaemic Stroke in Infective Endocarditis: Pathophysiology and Clinical Outcomes in Patients Treated with Reperfusion Therapy" Immuno 1, no. 4: 347-359. https://doi.org/10.3390/immuno1040023
APA StyleMaheshwari, R., Wardman, D., Cordato, D. J., & Bhaskar, S. M. M. (2021). Acute Ischaemic Stroke in Infective Endocarditis: Pathophysiology and Clinical Outcomes in Patients Treated with Reperfusion Therapy. Immuno, 1(4), 347-359. https://doi.org/10.3390/immuno1040023







