The Effects of Physical Activity on the Aging of Circulating Immune Cells in Humans: A Systematic Review
Abstract
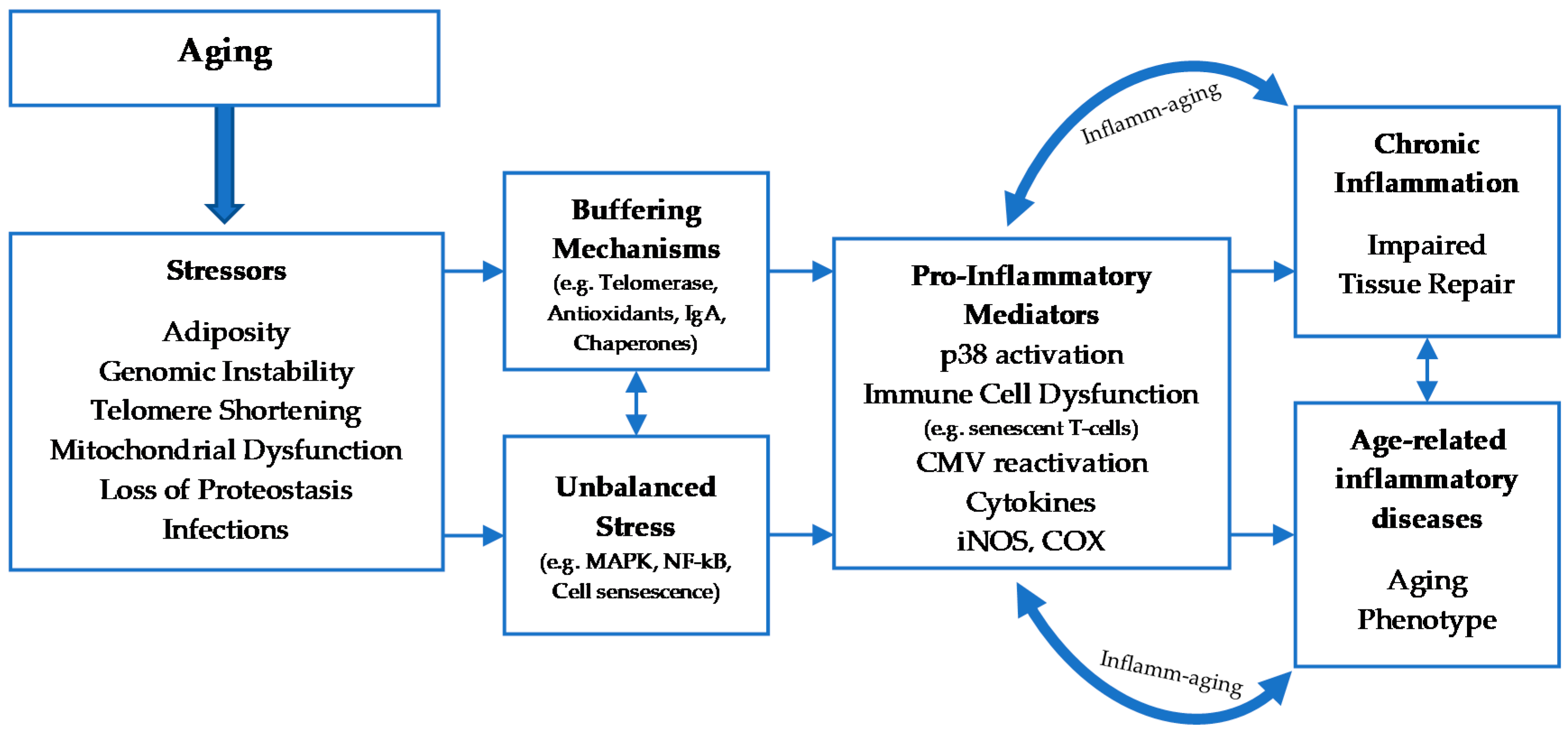
1. Introduction
2. Cellular Senescence
2.1. Classification and Mechanisms of Cellular Senescence
2.2. The Effects Of aging on Leukocyte Subsets
2.3. Physical Activity and Immune System
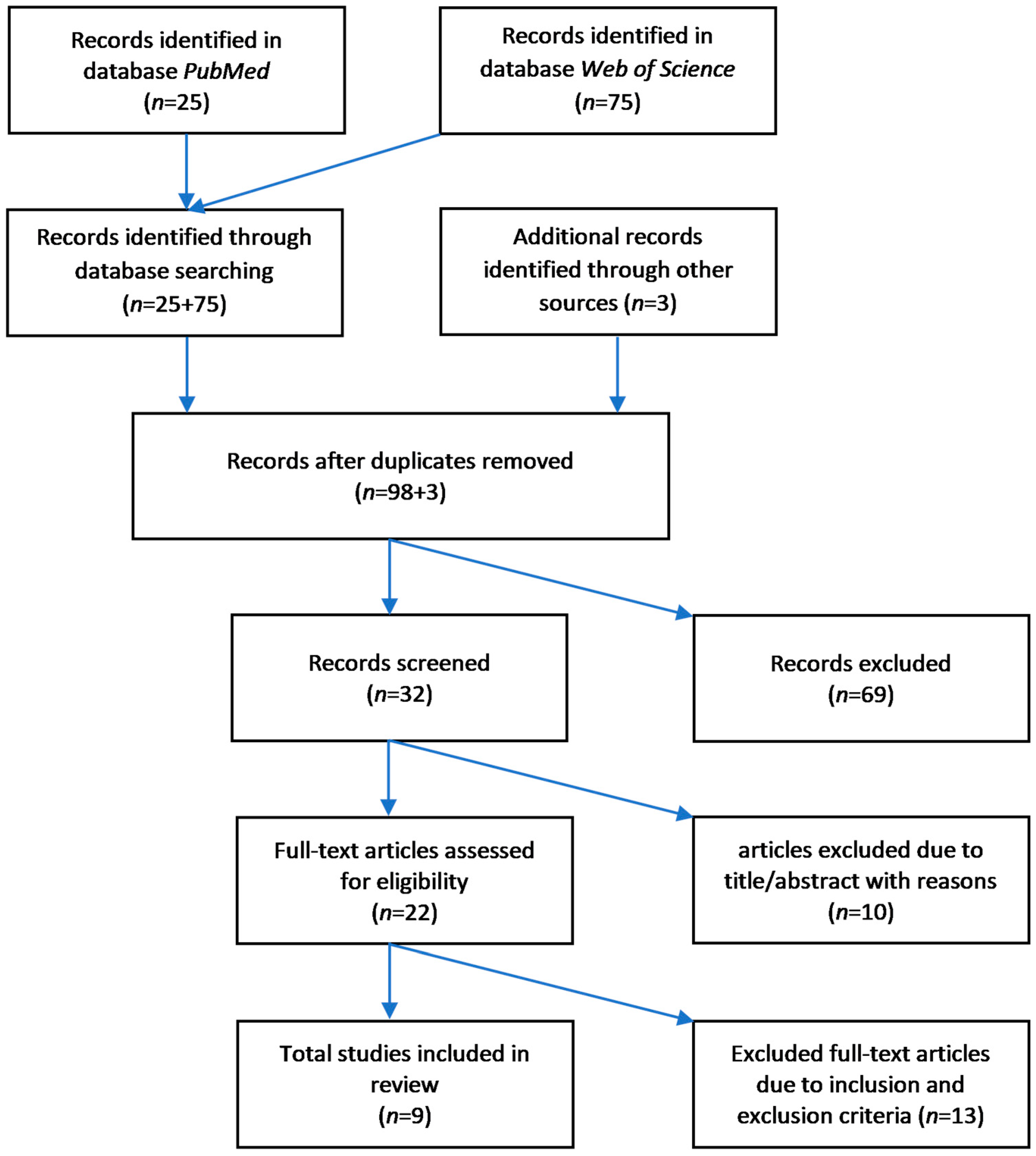
3. Methods
3.1. Search Strategy
3.2. Eligibilty Criteria
3.2.1. Inclusion of Studies
3.2.2. Exclusion of Studies
3.3. Study Quality Assessment
4. Results
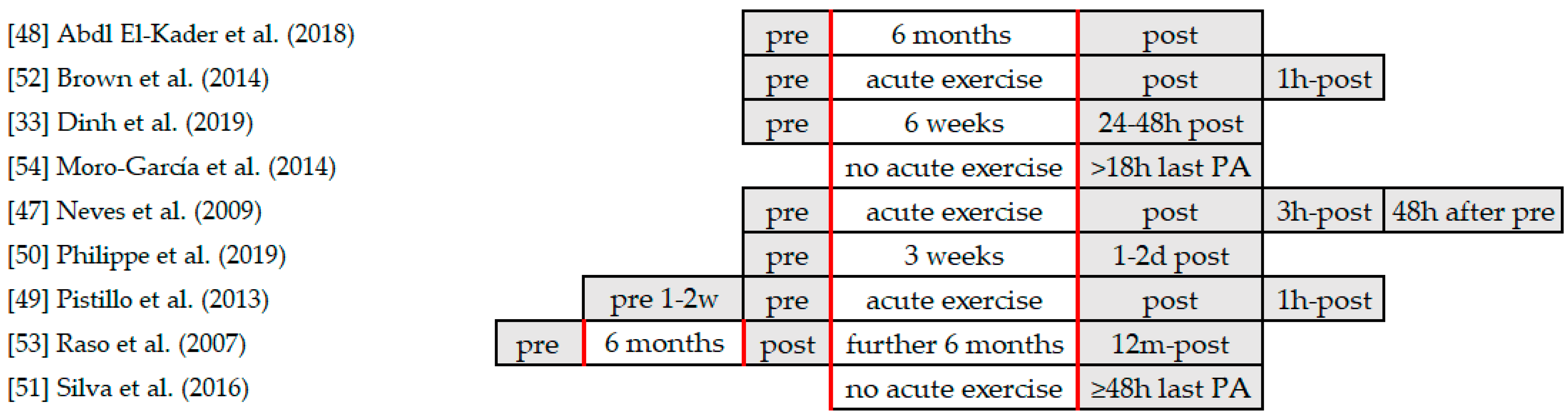
4.1. Exercise Interventions
4.2. Assessment of Leukocyte Subpopulations
4.3. Effects of Physical Activity on Cellular Senescence and Leukocyte Subpopulations
4.3.1. Trained vs. Untrained Subjects without Exercise Intervention
4.3.2. Endurance Exercise Intervention
4.3.3. Resistance Exercise Intervention
4.3.4. Aerobic Endurance vs. Resistance Exercise Intervention
4.4. Risk of Bias
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Date: 26.07.2020; Database: Pubmed | |||
|---|---|---|---|
| No. | Title | Authors | Year |
| 1 | Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly | Abd El-Kader, Shehab M.& Al-Shreef, Fadwa M. | 2018 |
| 2 | Strength Endurance Training but Not Intensive Strength Training Reduces Senescence-Prone T Cells in Peripheral Blood in Community-Dwelling Elderly Women | Cao Dinh, Hung; Njemini, Rose; Onyema, Oscar Okwudiri; Beyer, Ingo; Liberman, Keliane; Dobbeleer, Liza de; Renmans, Wim; Vander Meeren, Sam; Jochmans, Kristin; Delaere, Andreas; Knoop, Veerle; Bautmans, Ivan | 2019 |
| 3 | Resistance exercise sessions do not provoke acute immunosuppression in older women | Neves, Sergio da Cunha Jr; Lima, Ricardo Moreno; Simões, Herbert Gustavo; Marques, Mario C.; Reis, Victor Machado; Oliveira, Ricardo Jacó de | 2009 |
| Date: 27–29.07.2020; Database: Web of Science | |||
| 4 | Concentric and Eccentric Endurance Exercise Reverse Hallmarks of T-Cell Senescence in Pre-diabetic Subjects | Philippe, Marc; Gatterer, Hannes; Burtscher, Martin; Weinberger, Birgit; Keller, Michael; Grubeck-Loebenstein, Beatrix; Fleckenstein, Johannes; Alack, Katharina; Krüger, Karsten | 2019 |
| 5 | Moderate and intense exercise lifestyles attenuate the effects of aging on telomere length and the survival and composition of T cell subpopulations | Rodrigues Silva, Leia Cristina; Araujo, Adriana Ladeira de; Fernandes, Juliana Ruiz; Toledo Matias, Manuella de Sousa; Silva, Paulo Roberto; Duarte, Alberto J. S.; Garcez Leme, Luiz Eugenio; Benard, Gil | 2016 |
| 6 | The effects of age and viral serology on gamma delta T-cell numbers and exercise responsiveness in humans | Pistillo, Mira; Bigley, Austin B.; Spielmann, Guillaume; LaVoy, Emily C.; Morrison, Mark R.; Kunz, Hawley; Simpson, Richard J. | 2013 |
| 7 | Training status and sex influence on senescent T-lymphocyte redistribution in response to acute maximal exercise | Brown, Frankie F.; Bigley, Austin B.; Sherry, Chris; Neal, Craig M.; Witard, Oliver C.; Simpson, Richard J.; Galloway, Stuart D. R. | 2014 |
| 8 | Frequent participation in high volume exercise throughout life is associated with a more differentiated adaptive immune response | Moro-García, Marco Antonio; Fernández-García, Benjamín; Echeverría, Ainara; Rodríguez-Alonso, Manuel; Suárez-García, Francisco Manuel; Solano-Jaurrieta, Juan José; López-Larrea, Carlos; Alonso-Arias, Rebeca | 2014 |
| 9 | Effect of Resistance Training on Immunological Parameters of Healthy Elderly Women | Raso, Vagner; Benard, Gil; DA Silva Duarte, Alberto José; Natale, Valéria Maria | 2007 |
| Author | Age | Sex | CMV Serostatus | BMI | VO2max | HR | Training Volume | Lactate |
|---|---|---|---|---|---|---|---|---|
| [48] Abd El-Kader et al. | ✓ | x | x | ✓ | x | ✓ | ✓ | x |
| [52] Brown et al. | ✓ | ✓ | ✓ | x | x | ✓ | ✓ | ✓ |
| [33] Dinh et al. | ✓ | ✓ | ✓ | ✓ | x | x | ✓ | x |
| [54] Moro-García et al. | ✓ | ✓ | ✓ | ✓ | ✓ | x | ✓ | x |
| [47] Neves et al. | ✓ | ✓ | x | ✓ | x | x | ✓ | x |
| [50] Philippe et al. | ✓ | ✓ | x | x | x | x | ✓ | x |
| [49] Pistillo et al. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | x |
| [53] Raso et al. | ✓ | ✓ | ✓ | ✓ | x | x | ✓ | x |
| [51] Silva et al. | ✓ | x | x | ✓ | x | x | ✓ | x |
References
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 2017, 102, 977–988. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Investig. 2013, 123, 958–965. [Google Scholar] [CrossRef]
- de Araújo, A.L.; Silva, L.C.; Fernandes, J.R.; Benard, G. Preventing or reversing immunosenescence: Can exercise be an immunotherapy? Immunotherapy 2013, 5, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lowder, T.W.; Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Kunz, H. Exercise and the aging immune system. Ageing Res. Rev. 2012, 11, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Larbi, A.; Derhovanessian, E. Senescence of the human immune system. J. Comp. Pathol. 2010, 142 (Suppl. 1), 39–44. [Google Scholar] [CrossRef] [PubMed]
- Alack, K.; Pilat, C.; Krüger, K. Current knowledge and new challenges in exercise immunology. Dtsch. Z. Sportmed. 2019, 70, 250–260. [Google Scholar] [CrossRef]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of Acute and Chronic Exercise on Immunological Parameters in the Elderly Aged: Can Physical Activity Counteract the Effects of Aging? Front. Immunol. 2018, 9, 2187. [Google Scholar] [CrossRef]
- Kovaiou, R.D.; Grubeck-Loebenstein, B. Age-associated changes within CD4+ T cells. Immunol. Lett. 2006, 107, 8–14. [Google Scholar] [CrossRef]
- Wadhwa, R.; Kaul, Z.; Kaul, S.C. Cell Cycle Checkpoints and Senescence. In Cellular Ageing and Replicative Senescence; Rattan, S., Hayflick, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 145–167. [Google Scholar]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- LeBrasseur, N.K.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Frailty Pathophysiol. Phenotype Patient Care 2015, 83, 11–18. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Vargas, J.; Feltes, B.C.; Poloni Jde, F.; Lenz, G.; Bonatto, D. Senescence; an endogenous anticancer mechanism. Front. Biosci. 2012, 17, 2616–2643. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.; Biasini, C.; Zanni, F.; Zanlari, L.; Telera, A.; Lucchini, G.; Passeri, G.; Monti, D.; et al. The immune system in extreme longevity. Exp. Gerontol. 2008, 43, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Derhovanessian, E.; Larbi, A.; Strindhall, J.; Wikby, A. Cytomegalovirus and human immunosenescence. Rev. Med. Virol. 2009, 19, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Morrisette-Thomas, V.; Cohen, A.A.; Fülöp, T.; Riesco, É.; Legault, V.; Li, Q.; Milot, E.; Dusseault-Bélanger, F.; Ferrucci, L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014, 139, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, R.; Akbar, A.N.; Henson, S.M. The role of the T cell in age-related inflammation. Age 2013, 35, 563–572. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; ISBN 9789241599979. [Google Scholar]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Ockene, I.S.; Freedson, P.S.; Rosal, M.C.; Merriam, P.A.; Hebert, J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med. Sci. Sports Exerc. 2002, 34, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise, infection, and immunity. Int. J. Sports Med. 1994, 15, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Sha, W. Upper respiratory tract infection is reduced in physically fit and active adults. Br. J. Sports Med. 2011, 45, 987–992. [Google Scholar] [CrossRef]
- Krüger, K.; Mooren, F.-C.; Pilat, C. The Immunomodulatory Effects of Physical Activity. Curr. Pharm. Des. 2016, 22, 3730–3748. [Google Scholar] [CrossRef]
- Simpson, R.J.; Guy, K. Coupling aging immunity with a sedentary lifestyle: Has the damage already been done?—A mini-review. Gerontology 2010, 56, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Chilton, W.L.; Marques, F.Z.; West, J.; Kannourakis, G.; Berzins, S.P.; O’Brien, B.J.; Charchar, F.J. Acute exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PLoS ONE 2014, 9, e92088. [Google Scholar] [CrossRef]
- Cosgrove, C.; Galloway, S.D.R.; Neal, C.; Hunter, A.M.; McFarlin, B.K.; Spielmann, G.; Simpson, R.J. The impact of 6-month training preparation for an Ironman triathlon on the proportions of naive, memory and senescent T cells in resting blood. Eur. J. Appl. Physiol. 2012, 112, 2989–2998. [Google Scholar] [CrossRef]
- Dinh, H.C.; Bautmans, I.; Beyer, I.; Onyema, O.O.; Liberman, K.; De Dobbeleer, L.; Renmans, W.; Vander Meeren, S.; Jochmans, K.; Delaere, A.; et al. Six weeks of strength endurance training decreases circulating senescence-prone T-lymphocytes in cytomegalovirus seropositive but not seronegative older women. Immun. Ageing 2019, 16, 17. [Google Scholar] [CrossRef]
- Dinh, H.C.; Njemini, R.; Onyema, O.O.; Beyer, I.; Liberman, K.; De Dobbeleer, L.; Renmans, W.; Vander Meeren, S.; Jochmans, K.; Delaere, A.; et al. Strength Endurance Training but Not Intensive Strength Training Reduces Senescence-Prone T Cells in Peripheral Blood in Community-Dwelling Elderly Women. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1870–1878. [Google Scholar] [CrossRef]
- Kapasi, Z.F.; Ouslander, J.G.; Schnelle, J.F.; Kutner, M.; Fahey, J.L. Effects of an exercise intervention on immunologic parameters in frail elderly nursing home residents. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Jabbar, B. Effects of hypobaric Endurance Training on Graded Exercise Induced Lymphocyte Mobilization, Senescence and Their Surface Thiol Levels in Elite Male Athletes. Int. J. Appl. Exerc. Physiol. 2018, 7, 48–55. [Google Scholar] [CrossRef]
- Kohut, M.L.; Senchina, D.S.; Madden, K.S.; Martin, A.E.; Felten, D.L.; Moynihan, J.A. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp. Gerontol. 2004, 39, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.P.; Peixoto, F.M.; Soares, J.F.; Figueiredo, P.A.; Leitao, J.C.; Gaivao, I.; Duarte, J.A. Influence of aerobic fitness on age-related lymphocyte DNA damage in humans: Relationship with mitochondria respiratory chain and hydrogen peroxide production. AGE 2010, 32, 337–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ai-jun, N.; Yan-qun, W.; Jin-long, L. Effect of healthy Qigong “WuQinXi” exercise on peripheral blood T-cell subgroups in middle-aged subjects. Afr. J. Biotechnol. 2010, 9, 4620–4623. [Google Scholar]
- Reidy, P.T.; Lindsay, C.C.; McKenzie, A.I.; Fry, C.S.; Supiano, M.A.; Marcus, R.L.; LaStayo, P.C.; Drummond, M.J. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp. Gerontol. 2018, 107, 37–49. [Google Scholar] [CrossRef]
- Soares, J.P.; Mota, M.P.; Duarte, J.A.; Collins, A.; Gaivao, I. Age-related increases in human lymphocyte DNA damage: Is there a role of aerobic fitness? Cell Biochem. Funct. 2013, 31, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, G.; McFarlin, B.K.; O’Connor, D.P.; Smith, P.J.W.; Pircher, H.; Simpson, R.J. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav. Immun. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Wang, H.Y.; Bashore, T.R.; Tran, Z.V.; Friedman, E. Age-related decreases in lymphocyte protein kinase C activity and translocation are reduced by aerobic fitness. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, 545–551. [Google Scholar] [CrossRef]
- Wang, J.-S.; Chen, W.-L.; Weng, T.-P. Hypoxic exercise training reduces senescent T-lymphocyte subsets in blood. Brain Behav. Immun. 2011, 25, 270–278. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, i4919. [Google Scholar] [CrossRef]
- Palmowski, J.; Reichel, T.; Boßlau, T.K.; Krüger, K. The effect of acute running and cycling exercise on T cell apoptosis in humans: A systematic review. Scand. J. Immunol 2019, e12834. [Google Scholar] [CrossRef]
- da Cunha Neves, S., Jr.; Lima, R.M.; Simões, H.G.; Marques, M.C.; Reis, V.M.; de Oliveira, R.J. Resistance exercise sessions do not provoke acute immunosuppression in older women. J. Strength Cond. Res. 2009, 23, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; Al-Shreef, F.M. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr. Health Sci. 2018, 18, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Pistillo, M.; Bigley, A.B.; Spielmann, G.; LaVoy, E.C.; Morrison, M.R.; Kunz, H.; Simpson, R.J. The effects of age and viral serology on gamma delta T-cell numbers and exercise responsiveness in humans. Cell. Immunol. 2013, 284, 91–97. [Google Scholar] [CrossRef]
- Philippe, M.; Gatterer, H.; Burtscher, M.; Weinberger, B.; Keller, M.; Grubeck-Loebenstein, B.; Fleckenstein, J.; Alack, K.; Krueger, K. Concentric and Eccentric Endurance Exercise Reverse Hallmarks of T-Cell Senescence in Pre-diabetic Subjects. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.R.; de Araujo, A.L.; Fernandes, J.R.; Matias, M.D.; Silva, P.R.; Duarte, A.J.S.; Garcez Leme, L.E.; Benard, G. Moderate and intense exercise lifestyles attenuate the effects of aging on telomere length and the survival and composition of T cell subpopulations. AGE 2016, 38, 24. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.F.; Bigley, A.B.; Sherry, C.; Neal, C.M.; Witard, O.C.; Simpson, R.J.; Galloway, S.D.R. Training status and sex influence on senescent T-lymphocyte redistribution in response to acute maximal exercise. Brain Behav. Immun. 2014, 39, 152–159. [Google Scholar] [CrossRef]
- Raso, V.; Benard, G.; DA Silva Duarte, A.J.; Natale, V.M. Effect of Resistance Training on Immunological Parameters of Healthy Elderly Women. Med. Sci. Sports Exerc. 2007, 39, 2152–2159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moro-García, M.A.; Fernández-García, B.; Echeverría, A.; Rodríguez-Alonso, M.; Suárez-García, F.M.; Solano-Jaurrieta, J.J.; López-Larrea, C.; Alonso-Arias, R. Frequent participation in high volume exercise throughout life is associated with a more differentiated adaptive immune response. Brain Behav. Immun. 2014, 39, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Pawelec, G.; Koch, S.; Franceschi, C.; Wikby, A. Human immunosenescence: Does it have an infectious component? Ann. N. Y. Acad. Sci. 2006, 1067, 56–65. [Google Scholar] [CrossRef]
- Shearer, G.M. Th1/Th2 changes in aging. Mech. Ageing Dev. 1997, 94, 1–5. [Google Scholar] [CrossRef]
- Saygin, O.; Karacabey, K.; Ozmerdivenli, R.; Zorba, E.; Ilhan, F.; Bulut, V. Effect of chronic exercise on immunoglobin, complement and leukocyte types in volleyball players and athletes. Neuro Endocrinol. Lett. 2006, 27, 271–276. [Google Scholar] [PubMed]
- McFarlin, B.K.; Flynn, M.G.; Phillips, M.D.; Stewart, L.K.; Timmerman, K.L. Chronic resistance exercise training improves natural killer cell activity in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1315–1318. [Google Scholar] [CrossRef]
- Krüger, K.; Alack, K.; Ringseis, R.; Mink, L.; Pfeifer, E.; Schinle, M.; Gindler, K.; Kimmelmann, L.; Walscheid, R.; Muders, K.; et al. Apoptosis of T-Cell Subsets after Acute High-Intensity Interval Exercise. Med. Sci. Sports Exerc. 2016, 48, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Anane, L.H.; Edwards, K.M.; Burns, V.E.; Zanten, J.J.; Drayson, M.T.; Bosch, J.A. Phenotypic characterization of gammadelta T cells mobilized in response to acute psychological stress. Brain Behav. Immun. 2010, 24, 608–614. [Google Scholar] [CrossRef]
- Dimitrov, S.; Lange, T.; Born, J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010, 184, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Leosco, D.; Parisi, V.; Femminella, G.D.; Formisano, R.; Petraglia, L.; Allocca, E.; Bonaduce, D. Effects of exercise training on cardiovascular adrenergic system. Front. Physiol. 2013, 4, 348. [Google Scholar] [CrossRef]
- Krüger, K.; Lechtermann, A.; Fobker, M.; Völker, K.; Mooren, F.C. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav. Immun. 2008, 22, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J. Aging, persistent viral infections, and immunosenescence: Can exercise “make space”? Exerc. Sport Sci. Rev. 2011, 39, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Emmons, R.; Niemiro, G.M.; De, L.M. Hematopoiesis with Obesity and Exercise: Role of the Bone Marrow Niche. Exerc. Immunol. Rev. 2017, 23, 82–95. [Google Scholar] [PubMed]
- Szade, A.; Szade, K.; Nowak, W.N.; Bukowska-Strakova, K.; Muchova, L.; Gońka, M.; Żukowska, M.; Cieśla, M.; Kachamakova-Trojanowska, N.; Rams-Baron, M.; et al. Cobalt protoporphyrin IX increases endogenous G-CSF and mobilizes HSC and granulocytes to the blood. EMBO Mol. Med. 2019, 11, e09571. [Google Scholar] [CrossRef]
- Bujko, K.; Cymer, M.; Adamiak, M.; Ratajczak, M.Z. An Overview of Novel Unconventional Mechanisms of Hematopoietic Development and Regulators of Hematopoiesis—A Roadmap for Future Investigations. Stem Cell Rev. Rep. 2019, 15, 785–794. [Google Scholar] [CrossRef]
- Mooren, F.C.; Krüger, K. Apoptotic lymphocytes induce progenitor cell mobilization after exercise. J. Appl. Physiol. 2015, 119, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.G.; Fahlman, M.; Braun, W.A.; Lambert, C.P.; Bouillon, L.E.; Brolinson, P.G.; Armstrong, C.W. Effects of resistance training on selected indexes of immune function in elderly women. J. Appl. Physiol. 1999, 86, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K.; Mastro, A.M.; Miles, M.P.; Bush, J.A.; Grove, D.S.; Leach, S.K.; Volek, J.S.; Nindl, B.C.; Marx, J.O.; Gotshalk, L.A.; et al. Lymphocyte proliferation in response to acute heavy resistance exercise in women: Influence of muscle strength and total work. Eur. J. Appl. Physiol. 2001, 85, 367–373. [Google Scholar] [CrossRef]
- Thompson, B.; Almarjawi, A.; Sculley, D.; de Jonge, X.J. The Effect of the Menstrual Cycle and Oral Contraceptives on Acute Responses and Chronic Adaptations to Resistance Training: A Systematic Review of the Literature. Sports Med. 2020, 50, 171–185. [Google Scholar] [CrossRef]
- Ghosh, M.; Rodriguez-Garcia, M.; Wira, C.R. The Immune System in Menopause: Pros and Cons of Hormone Therapy. J. Steroid Biochem. Mol. Biol. 2014, 142, 171–175. [Google Scholar] [CrossRef]
- Wong, G.C.L.; Narang, V.; Lu, Y.; Camous, X.; Nyunt, M.S.Z.; Carre, C.; Tan, C.; Xian, C.H.; Chong, J.; Chua, M.; et al. Hallmarks of improved immunological responses in the vaccination of more physically active elderly females. Exerc. Immunol. Rev. 2019, 25, 20–33. [Google Scholar] [PubMed]
- Minuzzi, L.G.; Rama, L.; Chupel, M.U.; Rosado, F.; Dos Santos, J.V.; Simpson, R.; Martinho, A.; Paiva, A.; Teixeira, A.M. Effects of lifelong training on senescence and mobilization of T lymphocytes in response to acute exercise. Exerc. Immunol. Rev. 2018, 24, 72–84. [Google Scholar]
- Werner, C.; Fürster, T.; Widmann, T.; Pöss, J.; Roggia, C.; Hanhoun, M.; Scharhag, J.; Büchner, N.; Meyer, T.; Kindermann, W.; et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 2009, 120, 2438–2447. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Blackburn, E.; O’Donovan, A.; Adler, N.; Epel, E. The power of exercise: Buffering the effect of chronic stress on telomere length. PLoS ONE 2010, 5, e10837. [Google Scholar] [CrossRef] [PubMed]
- Najarro, K.; Nguyen, H.; Chen, G.; Xu, M.; Alcorta, S.; Yao, X.; Zukley, L.; Metter, E.J.; Truong, T.; Lin, Y.; et al. Telomere Length as an Indicator of the Robustness of B- and T-Cell Response to Influenza in Older Adults. J. Infect. Dis. 2015, 212, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2019, 40, 34–46. [Google Scholar] [CrossRef]
- Clements, S.J.; Carding, S.R. Diet, the intestinal microbiota, and immune health in aging. Crit. Rev. Food Sci. Nutr. 2018, 58, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Pecht, T.; Gutman-Tirosh, A.; Bashan, N.; Rudich, A. Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes. Rev. 2014, 15, 322–337. [Google Scholar] [CrossRef]
- Valdes, A.M.; Glass, D.; Spector, T.D. Omics technologies and the study of human ageing. Nat. Rev. Genet. 2013, 14, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Damiot, A.; Pinto, A.J.; Turner, J.E.; Gualano, B. Immunological Implications of Physical Inactivity among Older Adults during the COVID-19 Pandemic. Gerontology 2020, 66, 431–438. [Google Scholar] [CrossRef] [PubMed]
| Innate Immunity | Changes | Adaptive Immunity | Changes |
|---|---|---|---|
| Phagocytosis | ↓ ↔ | Naïve cell number | ↓ |
| Free radical production | ↑ ↓ | Memory cell number | ↑ |
| Chemotaxis | ↓ | T-regulatory cell number | ↑ |
| Cytokine production | ↑ | T-regulatory cell function | ↓ |
| Myeloid cell number | ↑ | Proliferation | ↓ |
| IL-2 | ↓ | ||
| B-regulatory cell number/function | ↓ | ||
| B-cell immunoglobulin production | ↓ | ||
| B-cell autoantibody production | ↑ |
| Headings | Search Terms |
|---|---|
| Physical activity | exercise, physical exercise, physical activity, physical fitness, training, exercise training, activity, aerobic training, aerobic fitness, strength training, resistance training, physical endurance, and strength endurance training |
| Cellular senescence | senescence, cellular senescence, cell aging, senescent cells, immunosenescence, and aging |
| Leukocyte subpopulations | leukocyte, leukocytes, leukocyte subpopulations, leukocyte populations, lymphocytes, T-cells, B-cells, monocytes, macrophages, dendritic cells, natural killer cells, neutrophils, and immune system |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| healthy subjects (no manifested chronic illness, immune disease or no acute infection) | ill subjects(especially HIV+, cancer, or diagnosed DM) |
| human study | animal study |
| elderly subjects (≥50); elderly compared to young subjects (18–85 years) | younger subjects (<50 years); except elderly (>85 years) compared to young subjects |
| acute exercise, if not at least lifelong training and/or 2 cohorts (trained/sedentary or aerobic/resistance exercise or pre-/post-intervention) | no exercise, no comparison of sedentary and active subjects, or no sufficient survey of physical activity |
| some kind of control group or 2 intervention groups or longitudinal analysis | no control group, only one intervention group, or cross-sectional analysis without controls |
| aerobic, anaerobic, or resistance exercise | exercise with a focus on coordination or flexibility (as intervention group) |
| investigation of any kind of leukocyte subpopulations (except eosinophilic and basophilic granulocytes) or telomere length through blood samples | eosinophilic and basophilic granulocytes, change in insulin sensitivity, ROS production, DNA damage, tissue biopsy, macrophages in skeletal muscle as an indicator for rehabilitation after exercise |
| effect of acute or chronic physical activity on leukocyte subpopulations | effects of vaccination or nutritional strategies (like CR or consumption of supplements/vitamins) on immune response |
| Author (Year) | Reason for Exclusion |
|---|---|
| [30] Chilton et al. (2014) | outcome measures → focus on miRNA, SIRT, TERT, and TL |
| [31] Cosgrove et al. (2012) | cohort → age of subjects does not meet inclusion criteria (<50 years) |
| [32] Dinh et al. (2019) | cohort → same cohort as in Dinh et al. (2019) [33]; focus on CMV |
| [34] Kapasi et al. (2003) | cohort → frail subjects outcome measure → cytokine activity exercise program → wheelchair endurance |
| [35] Karim et al. (2018) | exercise program → focus on hypobaric exercise, not senescence insufficient description of results |
| [36] Kohut et al. (2004) | outcome measure → focus on macrophage stimulation and infection with HSV-1 |
| [37] Mota et al. (2010) | insufficient focus on senescence of cells cohort → young subjects |
| [38] Niu Ai-jun et al. (2010) | insufficient and inconsistent information |
| [39] Reidy et al. (2018) | outcome measure → focus on macrophages and insulin sensitivity, not senescent cells |
| [40] Soares et al. (2013 | outcome measure → correlation between age and DNA strand breaks/FPG in general, not cell-specific |
| [41] Spielmann et al. (2011) | outcome measure → no intervention, no cohorts, and wide age span |
| [42] Wang et al. (2000) | outcome measure → mainly cytosolic and membranous PKC activity |
| [43] Wang et al. (2011) | exercise program → hypoxic compared with not hypoxic; enhancing aerobic fitness |
| Pre-Intervention Risk of Bias | |
|---|---|
| Bias due to confounding | no supplementation before the intervention, immune function not affected, homogeneous groups, education about intervention, serostatus (CMV), physical status, time of blood sample, and training load |
| Bias in the selection of participants into the study | selection into the study based on participant characteristics observed after the start of the intervention |
| At-intervention risk of bias | |
| Bias in the classification of interventions | Clearly defined intervention groups, sedentary or active, what kind of exercise (aerobic or resistance)? Frequency? Intensity? Does classification infect outcome? |
| Bias due to deviations from intended interventions | Effect of assignment to intervention or effect of starting and adhering to intervention, co-intervention balanced? (Exercise additional to intervention?) |
| Post-intervention risk of bias | |
| Bias due to missing data | outcome data available for all or nearly all participants? Participants excluded due to missing or incomplete data? |
| Bias in measurement of outcome | Outcome influenced by knowledge of intervention? Not possible to blind participants or outcome assessors due to the design of studies or systematic errors in measurement? |
| Bias in selection of the reported result | Only one or a subset of measurements reported? Pre-specification of methods used for analysis? |
| Author (Year) | Design | Cohort | Duration | Exercise |
|---|---|---|---|---|
| [48] Abd El-Kader et al. (2018) | Non-RCT (no CG, 2 IG) | 60 sedentary elderly subjects 61–67 yrs aerobic group (n = 30) resistance group (n = 30) | 6 months 3 days/week | aerobic exercise 40 min treadmill resistance exercise 40 min, 9 resistance machines supervised, monitored |
| [52] Brown et al. (2014) | Non-RCT | 32 adults trained soccer players (n = 16) 8 males, 8 females, 18.3 ± 1.7 yrs untrained controls (n = 16) 8 males, 8 females, 19.3 ± 2.0 yrs | acute exercise | treadmill running test (to volitional exhaustion) starting at 10 km/h (males)/8 km/h (females) 1 km/h every 3 min |
| [33] Dinh et al. (2019) | RCT (2 IG, 1 CG) | 100 women (≥ 65 yrs) not performing for past 6 months IST (n = 31, 69.18 ± 5.12 yrs) SET (n = 33, 69.02 ± 6.05 yrs) CON (n = 36, 70.31 ± 5.15 yrs) | 6 weeks 2–3 times/week | IST 3 × 10 reps at 80% 1RM, 1 min rest SET 2 × 30 reps at 40% 1RM, 1 min rest CON (stretching) 3 × 10–12 stretching exercises, passive-static (30 s) |
| [54] Moro-García et al. (2014) | retrospective cohort study | 95 adults 32.6 ± 9.9–75.5 ± 4.2 yrs young non-athletes (n = 30) young athletes (n = 27) elderly non-athletes (n = 26) elderly athletes (n = 12) | no acute exercise subjects not performing 18 h before blood samples were taken | young athletes water rowing, running, resistance training 6.2 ± 1 days a week 125.3 ± 41.5 min a day for 13 ± 4.2 yrs elderly athletes endurance, stretching, body core, resistance training |
| [47] Neves et al. (2009) | RCT (2 IG, 1 CG) | 15 elderly women 67.5 ± 3.9 yrs 2 resistance exercise sessions 1 control session | acute exercise 4 measurement moments T1, T2, T3, and T4 | resistance exercise session 50% 1RM 80% 1RM control session |
| [50] Philippe et al. (2019) | Non-RCT (no CG, 2 IG) | 16 male older adults 56.9 ± 5.1 yrs CE (n = 8); EE: (n = 8) Pre-diabetic subjects (IGT) | 3 weeks 3 days/week (M, W, and F) | walking exercise CE (uphill) EE (downhill) |
| [49] Pistillo et al. (2013) | Non-RCT | 34 physicaly active males young males (n = 17, 23–35 yrs) older males (n = 17, 50–64 yrs) | acute exercise pre and post blood samples | cycling exercise 30 min cycle ergometer at 80–85% PP |
| [53] Raso et al. (2007) | RCT (IG, CG) | 42 healthy sedentary females 60–77 yrs IG, CG | 12 months 3×/week | resistance training program light to moderate intensity, supervised 3 × 12 reps at 54.9% ± 2.4% 1RM control group 1RM at baseline, 6 months, and 12 months |
| [51] Silva et al. (2016) | retrospective cohort study | 46 elderly subjects 65–85 yrs UTr (n = 15) mTr (n = 16) iTr (n = 15) | no acute exercise regular training for ≥5 yrsrunning or ball sports | UTr never trained MTr ball sports or running < 6 km, 2–3×/week ITr ≥5 days/week (>50 km/week) |
| Author (Year) | Cohort | Exercise Intervention | Duration | Main Outcome Measures | Main Results |
|---|---|---|---|---|---|
| [48] Abd El-Kader et al. (2018) | sedentary elderly subjects (n = 60) | aerobic endurance Group A resistance training Group B | 6 months | CD3+ CD4+, and CD8+ TNFα, IL-6, and IL-10 | ↓ TNFα; IL-6 (AE) * ↑ IL-10 (AE) * ↓ CD3+ (AE) * ↓ CD4+; CD8+(AE) * ↓ CD4+/CD8+(AE) * |
| [52] Brown et al. (2014) | male/female subjects (n = 32) Tr soccer players UTr | aerobic/anaerobic endurance (treadmill running test to volitional exhaustion) | single bout of exercise | CD4+, CD8+, CD57+, and CD28 (sen), CD57, CD28+ (naïve) | ↓ CD4+ sen (Tr) * ↑ CD4+ naïve (Tr) ↓ CD8+ sen (Tr) * ↑ CD8+ naïve (Tr) * ↑ CD4+ sen, CD8+ sen (m) * ↓ CD4+ naïve, CD8+ naïve (m) * |
| [33] Dinh et al. (2019) | elderly women (n = 100) | resistance training IST, SET stretching CON | 6 weeks | CD8+ CD8− CD28, and CD57 | ↓ CD8+ and CD57+ (SET) ** ↓ CD8+, CD28−, and CD57+ (SET) ** ↓ CD8− and CD57+ (SET) * ↓ CD8−, CD28−, CD57+ (SET) * ↑ CD8+ memory * ↑ CD8− memory * |
| [54] Moro-García et al. (2014) | athletes/non-athletes (n = 95) | no intervention athletes performing aerobic/anaerobic endurance, resistance training | - | VO2max physical activity score TREC content CD4+, CD8+, CD16+, and CD56+ CD19+ and CD69 | ↓ CD4+ (A) * ↑ CD8+ (A) * ↑ NK cells (A) ** ↓ TREC/CD8+ (YA) * ↓ Neutrophils, Lymphocytes (A) * |
| [47] Neves et al. (2009) | elderly women (n = 15) | resistance exercise (S50%, S80%, SC) | acute exercise | LT CT, IgA Total Lymphocytes CD4+, CD8+ | ↓ CT (T3; S50%, S80%, SC) * ↑ LT (T2; SC) * ↑ IgA (T2 S50%; T2, T3 S80%) * ↑ Total Lymphocytes (T3) * ↑ CD4+ (T3, S80%, SC) * ↑ CD8+ (T3, SC) * |
| [50] Philippe et al. (2019) | older adults (n = 16) | aerobic endurance (walking exercise) | 3 weeks | TNFα, IL-6 CD3+, CD4+, CD8+ CD45RO, CCR7 CD16+, CD19+ CD25+ | ↑ CD4+/CD8+ * ↑ CD4+/CD3+ * ↓ CD8+/CD3+ * ↑ CD4+/CCR7+/CD45RO+ * ↓ CD4+/CCR7−/CD45RO− * ↓ CD16+* ↑ CD8+/CCR7+/CD45RO+ * ↑ CD8+/CCR7+/CD45RO− * ↓ CD8+/CCR7−/CD45RO− * |
| [49] Pistillo et al. (2013) | active males (n = 34) | aerobic endurance (cycle ergometer) | single bout of exercise | viral serostatus Total Lymphocytes CD3+ and γδ T-cells KLRG1 | ↓ γδ T-cells (old)*** ↑ γδ T-cells CMV+ (young) ** ↑ γδ T-cells (post exercise) ** ↑ KLRG1+ γδ T-cells (young) ** |
| [53] Raso et al. (2007) | elderly sedentary women | resistance exercise (light to moderate, IG) no exercise CG | 12 months | CD4+, CD8+ CD45RA+, and CD45RO+ NKCA CD56dim and CD56bright Total Lymphocytes | ↑ CD4+/CD45RA+ (IG) ↑ CD8+/CD45RA+ (IG) ↓ CD4+/CD45RO+ (IG) ↓ CD8+/CD45RO+ (IG) ↑ CD56dim/CD56bright (no significant differences) |
| [51] Silva et al. (2016) | elderly subjects (n = 46) UTr, MTr, ITr | no intervention aerobic endurance ball sports | ≥5 years | Questionnaires (e.g., SF-36) CD3+, CD4+, and CD8+ CD45RO and CD28 TL (CD4+, CD8+) | ↓ CD8+ TEMRA (MTr, ITr) * ↓ CD4+ TEMRA (ITr) ** ↑ CD4+ CM (MTr) * ↑ T-cell TL (MTr, ITr) * ↑ CD8+ EM (ITr) * 1/3 (CD4+ TEMRA/CD8+ TEMRA) |
| Author | Confounding | Selection Participants | Classification Intervention | Deviation Intended Intervention | Missing Data | Outcome Measurement | Selection Reported Results | Ø |
|---|---|---|---|---|---|---|---|---|
| [48] Abd El-Kader et al. | + | + | + | ± | + | ± | + | ± |
| [52] Brown et al. | + | + | + | + | + | ± | ± | ± |
| [54] Moro-García et al. | ± | ± | + | + | + | ± | + | ± |
| [50] Philippe et al. | + | + | + | ± | ? | ± | + | ± |
| [49] Pistillo et al. | ± | + | ± | ? | ? | ± | + | × |
| [51] Silva et al. | ± | ± | + | + | + | ± | + | ± |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brauer, L.; Krüger, K.; Weyh, C.; Alack, K. The Effects of Physical Activity on the Aging of Circulating Immune Cells in Humans: A Systematic Review. Immuno 2021, 1, 132-159. https://doi.org/10.3390/immuno1030009
Brauer L, Krüger K, Weyh C, Alack K. The Effects of Physical Activity on the Aging of Circulating Immune Cells in Humans: A Systematic Review. Immuno. 2021; 1(3):132-159. https://doi.org/10.3390/immuno1030009
Chicago/Turabian StyleBrauer, Lara, Karsten Krüger, Christopher Weyh, and Katharina Alack. 2021. "The Effects of Physical Activity on the Aging of Circulating Immune Cells in Humans: A Systematic Review" Immuno 1, no. 3: 132-159. https://doi.org/10.3390/immuno1030009
APA StyleBrauer, L., Krüger, K., Weyh, C., & Alack, K. (2021). The Effects of Physical Activity on the Aging of Circulating Immune Cells in Humans: A Systematic Review. Immuno, 1(3), 132-159. https://doi.org/10.3390/immuno1030009






