Abstract
Background: To date, no biomarkers are effective in predicting the risk of developing immune-related adverse events (irAEs) in patients treated with immune checkpoint inhibitors (ICIs). This study aims to evaluate the association between basal absolute eosinophil count (AEC) and irAEs during treatment with ICIs for solid tumors. Methods: We retrospectively evaluated 168 patients with metastatic melanoma (mM), renal cell carcinoma (mRCC), and non-small cell lung cancer (mNSCLC) receiving ICIs at our medical oncology unit. By combining baseline AEC with other clinical factors, we developed a mathematical model for predicting the risk of irAEs, which we validated in an external cohort of patients. Results: Median baseline AEC was 135/µL and patients were stratified into two groups accordingly; patients with high baseline AEC (>135/µL) were more likely to experience toxicity (p = 0.043) and have a better objective response rate (ORR) (p = 0.003). By constructing a covariance analysis model, it emerged that basal AEC correlated with the risk of irAEs (p < 0.01). Finally, we validated the proposed model in an independent cohort of 43 patients. Conclusions: Baseline AEC could be a predictive biomarker of ICI-related toxicity, as well as of response to treatment. The use of a mathematical model able to predict the risk of developing irAEs could be useful for clinicians for monitoring patients receiving ICIs.
1. Introduction
Immune checkpoint inhibitors (ICIs) improve the outcomes of patients with different types of cancers. However, not all patients respond to ICIs and these drugs are not devoid of adverse events (AEs). Due to their non-specific T cell activation mechanism, the major ICI toxicities are mediated by immunological and inflammatory tissue damage, collectively referred to as immune-related adverse events (irAEs) [1,2]. IrAEs are recorded in 60–85% of patients treated with anti-CTLA-4, in 57–85% of patients treated with anti-PD-1, and in about 95% of patients who received a combined block of CTLA- 4 and PD-1 [3]. Most irAEs are mild and can resolve spontaneously without treatment. However, more severe irAEs may require corticosteroid or immunosuppressive therapy, lead to discontinuation of therapy, and may increase mortality [4].
In some reports, the occurrence of irAEs correlated with favorable survival, while in others it did not [5,6,7,8]. To date, despite the wide use of ICIs for solid tumors and a large number of ongoing studies, no well-recognized predictive factors of response to ICIs have been identified, and there is a lack of information about clinical and blood markers associated with the development of irAEs. Biomarkers that can identify patients at highest risk of developing irAEs or lead to early detection of autoimmune toxicities could be crucial for optimizing patient selection for ICIs, closely monitoring high-risk patients, and early detection of irAEs [9,10]. Several potential baseline clinical–pathological risk factors for severe irAEs have been proposed, including family history of autoimmune diseases, tumor infiltration and location, previous viral infections such as HIV or hepatitis, and the concomitant use of drugs with known autoimmune toxicities such as antiarrhythmics, antibiotics, anticonvulsants, or antipsychotics [11]. Among the hematological biomarkers, a high neutrophil-to-lymphocyte ratio (NLR) was associated with a lower risk of developing irAEs in three retrospective studies [12,13,14] and a high baseline absolute lymphocyte count (ALC) was shown to be associated with an increased risk of developing irAEs in a single-center retrospective study [15]. In our study, an association between irAE ≥ 2 and a higher absolute eosinophil count (AEC) was also noted [15]. Nakamura et al. proved that baseline AEC > 240/μL was the most useful indicator to assess endocrine irAEs in patients with metastatic melanoma (mM) treated with ICIs, and that a higher relative eosinophil count (REC) after 1 month of therapy was significantly correlated with the occurrence of endocrine irAEs [16]. In the study by Krishnan et al., patients who experienced eosinophilia during treatment with ICIs were more likely to gain disease control and develop toxicity [17]. Other predictive biomarkers of toxicity investigated include subpopulations of lymphocytes [18,19,20], various cytokines, such as interleukin (IL) 6 and 17 [19,21,22], C-reactive protein (CRP) [23,24], multiple chemokines [25], autoantibodies [26,27,28,29,30,31], single-nucleotide polymorphisms (SNPs) [32,33], microRNA [34], the microbiome [19,35], and others [10]. However, none of these biomarkers are currently used in clinical practice to predict the risk of irAEs.
Among the many factors under study, our interest has centered in on the role of eosinophils. As already mentioned above, the role of this white blood cell subpopulation as a potential cellular biomarker in cancer therapy has been highlighted in several studies [15,16,17,36,37,38,39,40,41,42,43].
This study aims to evaluate the association between AEC at baseline and irAEs, and to develop a mathematical model able to predict the risk of experiencing irAEs in patients treated with ICIs. Mathematical modeling is a powerful tool for describing complex biological systems and for examining the relative influence of various biological factors on the overall dynamic. Our goal is to create a model using routinely available clinical and blood parameters, in order to create a simple tool that can help clinicians in the decision-making process of the best treatment, and in monitoring patients receiving ICIs. To develop this model, we relied on retrospectively collected data from a monocentric cohort of patients treated with ICIs and, subsequently, we validated it in a control cohort.
2. Materials and Methods
We conducted a single-center, observational, and retrospective study at the Medical Oncology Unit, AOU Careggi, Firenze, Italy. The study included all cancer patients receiving ICIs as per clinical practice from 2013, and the prevalence and type of irAEs and the correlation with tumor response were evaluated.
The trial was examined and approved by the Regional Ethics Committee for Clinical Trials of the Tuscany Region (protocol code “17332_oss”).
The patients were divided into three cohorts: RCC, melanoma, and NSCLC. All the patients who received ICIs had stage IV disease.
For each cohort, we described the clinical–demographic data (age, sex), information about therapy (therapeutic plan, mono or combo, therapy line, treatment duration, and outcome), and toxicity severity (according to CTCAE version 4.03). The best response was defined based on RECIST ver. 1.1 as follows: complete response (CR) as disappearance of all lesions; partial response (PR) as more than 30% decrease in the sum of the longest diameter of lesions; progressive disease (PD) as more than 20% increase in the sum of the longest diameter of lesions or appearance of new lesions; and stable disease (SD) as neither sufficient reduction to qualify as PR nor sufficient increase to qualify as PD.
Based on this, we divided the patients into responders (CR, PR, and SD) and non-responders (PD) to immunotherapy. We considered the baseline AEC (N°/μL) in all patients.
Therefore, combining AEC with important clinical factors, including age, site of tumor, type of treatment, and toxicity, we developed a mathematical model to predict the risk of irAEs. To evaluate its validity, we used three external cohorts of patients with mRCC, mM, and mNSCLC who received ICIs as per clinical practice at the Medical Oncology Unit of the University Hospital of Pisa and at the Translational Oncology Unit of Careggi University Hospital. Multivariate logistic regression analyses were performed to explore the independent predictors for irAEs, in terms of type and number of events.
2.1. Statistical Analysis
We calculated the mean and median baseline AEC in patients with and without irAEs: the statistical comparison and the difference in the median between the two groups were evaluated using the parametric t-test.
In addition, descriptive analysis was performed regarding the irAEs observed in each cohort of patients affected by mRCC, mM, and mNSCLC. Statistical comparisons for categorical variables (baseline AEC > 135/μL or AEC < 135/μL and occurrence or not of all irAEs/ORR) were performed by contingency analysis with the χ2 test. All differences were considered statistically significant at p < 0.05.
2.2. Mathematical Model
We carried out a statistical analysis, through the construction of indicators and models, to explain the trend of toxicity, compared to the basal AEC.
3. Results
3.1. Patient Characteristics
Between April 2013 and May 2020, we enrolled 168 oncological patients treated with ICIs at our medical oncology unit, AOU-Careggi (Florence): 43 (26.0%) with mRCC, 61 (36.0%) with mM, and 64 (38.0%) with mNSCLC. Patient demographic and clinical characteristics are summarized in Table 1.

Table 1.
Patient characteristics.
Median age of enrolled patients at time of diagnosis was 65 years, ranging from 30 to 92 years; 69% (n = 116) were male, and 31% (n = 52) female. Among all patients, 93 (55.4%) received nivolumab, 40 (23.8%) pembrolizumab, 13 (7.7%) ipilimumab, 12 (7.1%) atezolizumab, and lastly 10 patients (6%) received nivolumab plus ipilimumab. Overall, 12 patients achieved CR (7.1%), 20 PR (11.9%), 50 SD (29.8%), and the remaining 86 experienced PD (51.8%). While almost half of patients (n = 86, 51.2%) were not responsive to treatment (defined as patients who experienced PD as best response), the percentage changed between different tumors: non-responders accounted for 58.1% (n = 25) of patients with mRCC, 56.3% (n = 36) of patients with mNSCLC, and 41.0% (n = 25) of patients with mM.
3.2. Immune-Related Adverse Events (irAEs) and Basal Absolute Eosinophil Count (AEC)
Of patients of our cohort, 67.3% (n = 113) experienced at least one irAE. We recorded a total of 196 irAEs; the median number of irAEs per patient was 1.35 (range 0–8), and it was lower for patients with mNSCLC compared to those with mRCC and mM (Table 2).

Table 2.
Number and CTCAE grade of irAEs.
Eighteen patients (10.7%) developed G3-G4 (“serious”) irAEs for a total of 21 irAEs while the total number of G1-G2 (“not serious”) irAEs was 175 (89.2%). In patients with mRCC, we registered n = 10 (15.9%) G3-G4 irAEs. The classification by type of irAE is reported in Supplementary Material Table S1. For each patient, we recorded the baseline absolute eosinophil count (AEC) (Figure 1).
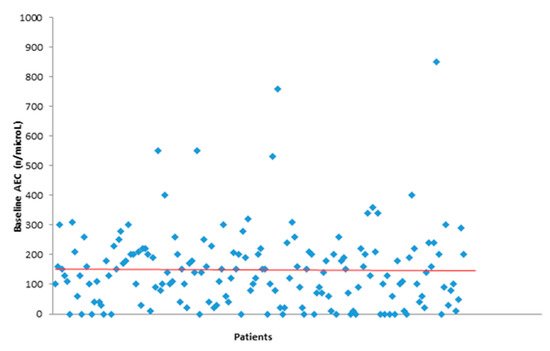
Figure 1.
Baseline absolute eosinophil count (AEC).
Median baseline AEC was 135/µL (Table 3). The median varies in different tumors: 105/µL in mNSCLC, 140/µL in mM, and 150/µL in mRCC groups of patients. For those who developed toxicity, the median AEC was higher than in patients who did not develop toxicity (150/µL vs. 90/µL).

Table 3.
Basal AEC in patients with irAEs and without irAEs.
Patients were divided in two groups based on the median basal AEC: one group with AEC > 135/µL and the other with <135/µL. We found a significant statistical difference between the two groups when related to irAE development: there was an association with a greater probability of developing toxicity (p = 0.043) in the group with high basal AEC (>135/µL).
Furthermore, the group with higher AEC was also positively associated with better outcome in terms of objective response rate (ORR) (p = 0.003). ORR percentages are summarized in Table 4.

Table 4.
ORR according to RECIST criteria 1.177 and basal AEC.
3.3. Mathematical Model for Prediction of Risk of irAEs
To corroborate the correlation between basal AEC values and risk of toxicity for patients treated with ICIs, we developed a covariance analysis model, with two explanatory variables (age and baseline AEC), a quadratic term (basal AEC), three factors (treatment, site, and toxicity), and one interaction (between age and treatment), as reported in Table 5 and Table 6. The summary table of the breakdown of variability data is given in Table 7.

Table 5.
Analysis model of covariance.

Table 6.
Proposed covariance analysis model to predict the toxicities as a function of the baseline AEC.

Table 7.
Summary table of the decomposition of the variability data.
The estimated values of the parameters have the task of providing us with predictions relating to the response variable “toxicity” by intervening in the explanatory variables. In addition to the estimate of the parameters, the table provides the estimate of the respective standard deviations, which indicates the variability of the parameters considering the universe of the samples.
The proposed model fits with the sequence of data (as can be observed from the values from the F significance tests with the respective p-values reported in Table 1 in the Materials and Methods section); for this reason, it is possible to explain the trend of the phenomenon studied and to obtain reliable prediction of toxicity based on basal AEC. Moreover, AEC values registered in patients treated with ICIs are able to precisely define the risk of occurrence of irAEs (p < 0.01) as per the mathematical model reported above, confirming the AEC value prediction ability.
Finally, we validated the proposed model in an independent cohort of oncological patients (n = 43) treated with ICIs: 13 (30.2%) at the Medical Oncology Unit of the University Hospital of Pisa with mM, and 30 at the Translational Oncology Unit of Careggi University Hospital, of which 21 (48.8%) had mNSCLC and 9 (21.0%) had mRCC.
4. Discussion
ICIs are becoming the standard of care for many different cancers [1,44]. It is well known that ICIs can cause peculiar immunologic toxicities called irAEs. The mechanism of ICIs enhances the activity of T cells against antigens present both in tumors and healthy tissues and increases the level of pre-existing autoantibodies and inflammatory mediators, leading to a series of irAEs [45]. IrAEs are usually mild and, when rapidly recognized and treated, patients can often continue immunotherapy. On the other hand, severe irAEs can be life-threatening, therefore biomarkers able to predict irAE onset are crucial. However, research on the mechanisms of irAEs is still in the early stage, and there are no recognized biomarkers able to predict the development. Recently, the use of baseline blood cell counts used as biomarkers has been growing as they are easy, affordable, accessible, and usually routinely requested in clinical practice [46,47].
The relationship between eosinophil count and toxicity has not been widely researched yet. It has been suggested that AEC in patients with mM is associated with both the response to treatment with ICIs as well as the risk of developing irAEs. This is because eosinophils may have antitumoral effects in the tumor microenvironment by promoting NK cell and T cell recruitment and direct cytotoxicity by production of granzymes and other cytotoxic proteins [47]. A similar report highlighted that patients experiencing treatment toxicity were more likely to have eosinophilia during the course of treatment [17]. Interestingly, Jodai et al. reported that the interaction between PD-1 receptors with both programmed cell death receptor ligand 1 (PD-L1) and PD-L2 on lung dendritic cells might explain the mechanism of eosinophilic pneumonia: the binding of PD-1 to PD-L2 on the dendritic cells may activate pulmonary inflammation induced by Th2 cells which produces interleukin (IL) 4, 5, and 13, eventually resulting in eosinophilic activation [48]. Furthermore, another paper showed that a baseline feature of a high AEC (≥0.125 × 109 cells/L) was associated with an increased risk of ICI pneumonitis in patients with NSCLC [49].
As described in previous studies, eosinophils play both regulator and effector roles in multiple immune functions, such as activation of T cells by carrying out antigen-presenting functions and attraction of tumor-specific CD8+ T cells [43,47]. Preclinical data evidenced that eosinophils regulate pulmonary T cell responses [50]. These evidences show that eosinophils are closely related to the occurrence of immune pneumonitis in ICI-treated patients.
With the present study, we aimed to explore the correlation between AEC and occurrence of irAEs in a cohort of patients affected by solid tumors and treated with ICIs. We found that there is an association between basal AEC > 135/µL and risk of irAEs.
Furthermore, we also noticed a positive correlation between baseline AEC and outcome: patients with basal AEC > 135/µL showed a higher ORR. Clinically, tumor-associated eosinophilia has been reported in many studies to be correlated to a good prognosis, for example, in gastrointestinal cancers, head and neck cancer, bladder cancer, and prostate cancer. The role of activated eosinophils in directing the immune response mediated by CD8+ T cells was detected in a recent study; moreover, eosinophils also contribute to modifying the tumor microenvironment and improving vascularization. All these mechanisms are known to promote tumor regression and survival [43].
The relationship between AEC and immunotherapy response has been studied, especially in melanoma and NSCLC. A baseline signature of low LDH, absolute monocyte counts (AMCs), high AEC, Tregs, and relative lymphocyte counts (RLCs) is associated with favorable outcome following ipilimumab in patients with mM [36]. Another clinical study showed that both absolute and relative eosinophil counts were significantly associated with improved OS in patients with mM treated with ipilimumab [37]. In a retrospective study of patients with mM treated with ipilimumab, there was an increase in AEC after the first ipilimumab infusion in responding patients compared to non-responders [42]. Similar results were found in patients with mRCC and mNSCLC treated with nivolumab. For patients with mRCC, a higher (>4.2) baseline neutrophil–lymphocyte ratio (NLR) was associated with an increased risk of progression, whereas a higher (>0.1 k/uL) baseline AEC was associated with a lower risk of progression [38]. In mNSCLC, a low absolute neutrophil count (ANC), high absolute count of lymphocytes (ALC), and high AEC were significantly and independently associated with both better progression-free survival and overall survival [39].
As an additional step, we tried to build a model to predict toxicity based on our results. In the present study, we developed a mathematical model able to define the risk of developing irAEs in patients treated with ICIs depending on age, AEC, treatment, and tumor location. In the era of personalized oncology, prediction models are becoming increasingly useful instruments in clinical practice. As we move towards clinical oncology in which we want to carefully individualize treatment, care, and monitoring as much as possible, it is imperative to collect information on an individual’s risk profile. Prediction tools for chemotherapy toxicity have been reported [51,52] but models for predicting the risk of developing irAEs in immunotherapy are not yet published.
Our model integrates variables such as AEC, age, drugs, and tumor type and could be a useful tool to determine baseline risk of toxicity. This information can be used in the decision-making process, especially for fragile patients. With regard to toxicity, a basal value of AEC > 135 µL would suggest the importance of closer monitoring of the patient due to a higher risk of developing an irAE. In addition, an early toxicity assessment, provided by our model, could be associated with better treatment adherence and outcomes.
There are a number of limitations in the current study that need to be addressed. The main limitations are the retrospective design of the study and the small sample size. A further limitation is the selection of patients, which is more selective in clinical trials, due to inclusion and exclusion criteria, than in real-life clinical practice. In addition, we analyzed a non-homogeneous cohort in terms of tumor types (mRCC, mM, and mNSCLC) and treatments (different single ICIs or combinations of ICIs). Owing to the inherent potential biases of our analysis, these results can only be viewed as a hypothesis generator and should be confirmed in future prospective studies.
Conversely, the strength of this research is the external validation of the model. Despite its shortcomings, our model allowed the development of a system for identifying patients at high risk prior to starting immunotherapy. Prospective external validation is also being planned.
5. Conclusions
In our experience, baseline AEC is suggested to be a predictive biomarker of ICIs related toxicity and response to immunotherapy. The possibility to use a mathematical model to predict the risk of onset of irAES could be useful to individualize treatments, and to monitor patients during therapy, to achieve the best compliance and adherence to ICIs and to better manage adverse events.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/immuno1030017/s1, Table S1: Total irAEs divided by type and tumor.
Author Contributions
Conceptualization, M.M.-C.; Data curation, R.G., A.P., E.P., A.M. and G.R.; Investigation, E.G. (Elisa Giommoni), E.G. (Elisabetta Gambale) and R.M.; Methodology, D.C.; Supervision, A.A.; Validation, D.C.; Writing—original draft, R.G. and S.P.; Writing—review & editing, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana (protocol code 17332_oss, 19 May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azoury, S.C.; Straughan, D.M.; Shukla, V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr. Cancer Drug Targets 2015, 15, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharm. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Chandra, S.; Sosman, J.A. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA 2018, 320, 1702–1703. [Google Scholar] [CrossRef]
- Suo, A.; Chan, Y.; Beaulieu, C.; Kong, S.; Cheung, W.Y.; Monzon, J.G.; Smylie, M.; Walker, J.; Morris, D.; Cheng, T. Anti-PD1-Induced Immune-Related Adverse Events and Survival Outcomes in Advanced Melanoma. Oncologist 2020, 25, 438–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judd, J.; Zibelman, M.; Handorf, E.; O’Neill, J.; Ramamurthy, C.; Bentota, S.; Doyle, J.; Uzzo, R.G.; Bauman, J.; Borghaei, H.; et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated with Programmed Cell Death 1 Inhibitors. Oncologist 2017, 22, 1232–1237. [Google Scholar] [CrossRef] [Green Version]
- Paderi, A.; Giorgione, R.; Giommoni, E.; Mela, M.M.; Rossi, V.; Doni, L.; Minervini, A.; Carini, M.; Pillozzi, S.; Antonuzzo, L. Association between Immune Related Adverse Events and Outcome in Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Cancers 2021, 13, 860. [Google Scholar] [CrossRef]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Vareki, S.M.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. 2018, 25, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Hommes, J.W.; Verheijden, R.J.; Suijkerbuijk, K.P.M.; Hamann, D. Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events-A Comprehensive Review. Front. Oncol. 2021, 10, 585311. [Google Scholar] [CrossRef]
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2016, 27, 559–574. [Google Scholar] [CrossRef]
- Eun, Y.; Kim, I.Y.; Sun, J.M.; Lee, J.; Cha, H.S.; Koh, E.M.; Kim, H.; Lee, J. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci. Rep. 2019, 9, 14039. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.; Calvetti, L.; Dal Maso, A.; Attili, I.; Del Bianco, P.; Pasello, G.; Guarneri, V.; Aprile, G.; Conte, P.; Bonanno, L. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 2019, 24, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehl, A.; Yarchoan, M.; Hopkins, A.; Jaffee, E.; Grossman, S.A. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 2017, 8, 114268–114280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Tanaka, R.; Maruyama, H.; Ishitsuka, Y.; Okiyama, N.; Watanabe, R.; Fujimoto, M.; Fujisawa, Y. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn. J. Clin. Oncol. 2019, 49, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, T.; Tomita, Y.; Roberts-Thomson, R. A retrospective analysis of eosinophilia as a predictive marker of response and toxicity to cancer immunotherapy. Future Sci. OA 2020, 6, FSO608. [Google Scholar] [CrossRef]
- Damuzzo, V.; Solito, S.; Pinton, L.; Carrozzo, E.; Valpione, S.; Pigozzo, J.; Arboretti Giancristofaro, R.; Chiarion-Sileni, V.; Mandruzzato, S. Clinical implication of tumor-associated and immunological parameters in melanoma patients treated with ipilimumab. Oncoimmunology 2016, 5, e1249559. [Google Scholar] [CrossRef] [Green Version]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Oh, D.Y.; Cham, J.; Zhang, L.; Fong, G.; Kwek, S.S.; Klinger, M.; Faham, M.; Fong, L. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell Repertoire. Cancer Res. 2017, 77, 1322–1330. [Google Scholar] [CrossRef] [Green Version]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J. Transl. Med. 2018, 16, 94. [Google Scholar] [CrossRef] [Green Version]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abolhassani, A.R.; Schuler, G.; Kirchberger, M.C.; Heinzerling, L. C-reactive protein as an early marker of immune-related adverse events. J. Cancer Res. Clin. Oncol. 2019, 145, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef]
- Khan, S.; Khan, S.A.; Luo, X.; Fattah, F.J.; Saltarski, J.; Gloria-McCutchen, Y.; Lu, R.; Xie, Y.; Li, Q.; Wakeland, E.; et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br. J. Cancer 2019, 120, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Maekura, T.; Naito, M.; Tahara, M.; Ikegami, N.; Kimura, Y.; Sonobe, S.; Kobayashi, T.; Tsuji, T.; Minomo, S.; Tamiya, A.; et al. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In Vivo 2017, 31, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Kimbara, S.; Fujiwara, Y.; Iwama, S.; Ohashi, K.; Kuchiba, A.; Arima, H.; Yamazaki, N.; Kitano, S.; Yamamoto, N.; Ohe, Y. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018, 109, 3583–3590. [Google Scholar] [CrossRef] [Green Version]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Bomze, D.; Ring, S.S.; Berner, F.; Fässler, M.; Diem, S.; Abdou, M.T.; Hammers, C.; Emtenani, S.; Braun, A.; et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J. Am. Acad. Dermatol. 2020, 82, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Tahir, S.A.; Gao, J.; Miura, Y.; Blando, J.; Tidwell, R.S.S.; Zhao, H.; Subudhi, S.K.; Tawbi, H.; Keung, E.; Wargo, J.; et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc. Natl. Acad. Sci. USA 2019, 116, 22246–22251. [Google Scholar] [CrossRef]
- Gowen, M.F.; Giles, K.M.; Simpson, D.; Tchack, J.; Zhou, H.; Moran, U.; Dawood, Z.; Pavlick, A.C.; Hu, S.; Wilson, M.A.; et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J. Transl. Med. 2018, 16, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bins, S.; Basak, E.A.; El Bouazzaoui, S.; Koolen, S.L.W.; Oomen-de Hoop, E.; van der Leest, C.H.; van der Veldt, A.A.M.; Sleijfer, S.; Debets, R.; van Schaik, R.H.N.; et al. Association between single-nucleotide polymorphisms and adverse events in nivolumab-treated non-small cell lung cancer patients. Br. J. Cancer 2018, 118, 1296–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refae, S.; Gal, J.; Ebran, N.; Otto, J.; Borchiellini, D.; Peyrade, F.; Chamorey, E.; Brest, P.; Milano, G.; Saada-Bouzid, E. Germinal Immunogenetics predict treatment outcome for PD-1/PD-L1 checkpoint inhibitors. Investig. New Drugs 2020, 38, 160–171. [Google Scholar] [CrossRef]
- Marschner, D.; Falk, M.; Javorniczky, N.R.; Hanke-Müller, K.; Rawluk, J.; Schmitt-Graeff, A.; Simonetta, F.; Haring, E.; Dicks, S.; Ku, M.; et al. MicroRNA-146a regulates immune-related adverse events caused by immune checkpoint inhibitors. JCI Insight 2020, 5, e132334. [Google Scholar] [CrossRef] [Green Version]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef] [Green Version]
- Martens, A.; Wistuba-Hamprecht, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [Green Version]
- Schindler, K.; Harmankaya, K.; Postow, M.A.; Frantal, S.; Bello, D.; Ariyan, C.E.; Michielin, O.A.; Hoeller, C.; Pehamberger, H.; Wolchok, J.D. Pretreatment levels of absolute and relative eosinophil count to improve overall survival (OS) in patients with metastatic melanoma under treatment with ipilimumab, an anti CTLA-4 antibody. J. Clin. Oncol. 2013, 31, abstr 9024. [Google Scholar] [CrossRef]
- Zahoor, H.; Barata, P.C.; Jia, X.; Martin, A.; Allman, K.D.; Wood, L.S.; Gilligan, T.D.; Grivas, P.; Ornstein, M.C.; Garcia, J.A.; et al. Patterns, predictors and subsequent outcomes of disease progression in metastatic renal cell carcinoma patients treated with nivolumab. J. Immunother. Cancer 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, N.; Qian, L.; Wang, X.; Fan, P.; Kuai, J.; Lin, S.; Liu, C.; Jiang, W.; Qin, S.; et al. CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Int. J. Cancer 2020, 146, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Sevko, A.; Jiang, H.; Lichtenberger, R.; Reith, M.; Tarnanidis, K.; Holland-Letz, T.; Umansky, L.; Beckhove, P.; Sucker, A.; et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin. Cancer Res. 2015, 21, 5453–5459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Lavacchi, D.; Pellegrini, E.; Palmieri, V.E.; Doni, L.; Mela, M.M.; Di Maida, F.; Amedei, A.; Pillozzi, S.; Carini, M.; Antonuzzo, L. Immune Checkpoint Inhibitors in the Treatment of Renal Cancer: Current State and Future Perspective. Int. J. Mol. Sci. 2020, 21, 4691. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Rowland, A.; Kichenadasse, G.; Wiese, M.D.; Gurney, H.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br. J. Cancer 2017, 117, 913–920. [Google Scholar] [CrossRef]
- Simon, S.C.S.; Utikal, J.; Umansky, V. Opposing roles of eosinophils in cancer. Cancer Immunol. Immunother. 2019, 68, 823–833. [Google Scholar] [CrossRef]
- Jodai, T.; Yoshida, C.; Sato, R.; Kakiuchi, Y.; Sato, N.; Iyama, S.; Kimura, T.; Saruwatari, K.; Saeki, S.; Ichiyasu, H.; et al. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti-PD-1 immune checkpoint antibody in a lung cancer patient. Immun. Inflamm. Dis. 2019, 7, 3–6. [Google Scholar] [CrossRef]
- Chu, X.; Zhao, J.; Zhou, J.; Zhou, F.; Jiang, T.; Jiang, S.; Sun, X.; You, X.; Wu, F.; Ren, S.; et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer 2020, 150, 76–82. [Google Scholar] [CrossRef]
- Jacobsen, E.A.; Ochkur, S.I.; Pero, R.S.; Taranova, A.G.; Protheroe, C.A.; Colbert, D.C.; Lee, N.A.; Lee, J.J. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med. 2008, 205, 699–710. [Google Scholar] [CrossRef]
- Coller, J.K.; White, I.A.; Logan, R.M.; Tuke, J.; Richards, A.M.; Mead, K.R.; Karapetis, C.S.; Bowen, J.M. Predictive model for risk of severe gastrointestinal toxicity following chemotherapy using patient immune genetics and type of cancer: A pilot study. Support. Care Cancer 2015, 23, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Dranitsaris, G.; Shah, A.; Spirovski, B.; Vincent, M. Severe diarrhea in patients with advanced-stage colorectal cancer receiving FOLFOX or FOLFIRI chemotherapy: The development of a risk prediction tool. Clin. Colorectal. Cancer 2007, 6, 367–373. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).