Abstract
Dendritic cells (DC) play a major role during the priming phase of anti-tumor immunization, as they are required for an efficient tumor-associated antigens presentation. At least one dendritic cell-based therapy has already been successfully approved by regulators for clinical application in prostate cancer patients. Moreover, DC development is dependent on the granulocyte macrophage colony stimulating factor (GM-CSF), a cytokine that has been successfully used as a potent inducer of anti-tumoral immunity. To better understand the relation between DC and GM-CSF in anti-tumor immunity, we studied the DC function in mice lacking the cytokine receptor common subunit beta (βc-/-) for GM-CSF, IL-3 and IL-5 and immunized with irradiated tumor cells. Such immunization induces a protective, specific tumor immunization in wild-type mice, while βc-/- mice failed to mount an immune response. Upon in vitro stimulation, DC from βc-/- mice (DCβc-/-) are unable to undergo a full maturation level. In vivo experiments show that they lack the ability to prevent tumor growth, in contrast to DCWT. Moreover, matured DCWT rescued immunization in βc-/- mice. DC maturation is dependent on a functional pathway involving GM-CSF signaling through a biologically functional receptor. These findings may contribute to new strategies for efficient anti-tumor immunotherapies.
1. Introduction
Advances in understanding the complex mechanisms of immune regulation in cancer biology have provided a solid foundation for developing tumor immunotherapy. Thus, in the last decades, immunotherapy has emerged as a promising strategy for cancer treatment with a large number of approaches based on cancer immunization, monoclonal antibodies or cellular therapies.
As dendritic cells (DC) are responsible for processing and presenting tumor antigens for the priming of anti-tumor cytotoxic T lymphocytes, research efforts on exploiting their properties for cancer immunotherapy have been developed over the last decades [1,2,3]. In this way, autologous DC loaded with tumor-associated antigen (TAA) [4,5], DC matured ex vivo [6,7], DC genetically modified to express tumor antigen genes (such as PSA and MAGE-1) or DC transformed to express immunomodulatory molecule genes such as IL-12 and granulocyte macrophage colony stimulating factor (GM-CSF) [8] have been used to initiate anti-tumor immunotherapies.
In preclinical models, GM-CSF is among the most potent inducers of anti-tumoral immunity when produced locally at the immunization site [9]. Vaccination with irradiated autologous tumor cells engineered to express GM-CSF has been shown to stimulate a robust anti-tumoral immunity in patients with various solid and hematological malignancies, translating into prolonged survival in selected patients [10]. Moreover, early stage clinical studies and a mouse model using GM-CSF revealed a coordinated humoral and cellular immune response and a potent, specific and long-lasting anti-tumor immunity [11,12]. Phase III clinical trials have evaluated the efficacy of a therapeutic vaccine using autologous DC pulsed with a prostate acid phosphatase/GM-CSF fusion protein. Three studies reported prolonged survival in the DC vaccine arm compared to the placebo arm, although overall survival was not the primary endpoint [13,14]. More recently, a GM-CSF-based oncolytic therapeutic product (TVEC) has been approved for patients with metastatic melanoma [15].
In addition, GM-CSF was the first cytokine shown to efficiency differentiated in vitro DC from human and mouse hematopoietic progenitor cells [16,17]. In vivo, the evidence of its role is not so clear. Indeed, in the spleen and thymus of mice injected with GM-CSF or transgenic mice overexpressing GM-CSF, the number of DC was increased [18], whereas the mice lacking GM-CSF or the GM-CSF receptor displayed only a minor impairment in the development of DCs in spleen and lymph nodes [19]. In experimental models, DC generated with GM-CSF improves their phagocytic activity [20] and upregulates the expression of antigen-presenting molecules such as MHC classes I and II [21] and the expression of costimulatory molecules. While these studies examined the role of GM-CSF for DC maturation, the role of GM-CSF signaling for efficient anti-tumor immunization is not well understood [22]. The GM-CSF receptor is a heterodimer that consists of two subunits: an α-subunit specific for GM-CSF binding and a signaling β chain subunit that is shared with the receptors for interleukin 3 (IL-3) and interleukin 5 (IL-5). Knock-out mice of the beta subunit (βc-/-) of the GM-CSF, IL-3 and IL-5 receptor complex were created in order to study the impact of these cytokines and their receptor on anti-tumor immunity. In our previous works, we showed that tumor immunity obtained after efficient vaccination is not impaired in IL-3-/- and IL5-/- mice [23]. Moreover, because of an additional IL-3-specific β chain, it has been shown that both GM-CSF and IL-5 are abolished in βc-/- mice, whereas IL-3 activity is maintained [24,25]. Since the alpha chain of IL-5 receptor is only expressed on B cells, eosinophils and basophils [26], we excluded the impact of IL-5 on DC from βc-/- mice. These data suggest that IL-3 and IL-5 are not necessary to mount protective anti-tumoral immune responses. Thus, in an effort to further clarify the role of GM-CSF in the induction of a protective tumor immune response, we compared the immunization induced by DC from wild type mice or from βc-/- mice in βc-/- and WT-bearing tumors, by exploring the impact of GM-CSF on DC maturation and their ability to induce a specific immune response to prevent tumor growth.
2. Materials and Methods
2.1. Animals
Female BALB/c (H-2Kd), C57BL/6 (H-2Kb) and OT-1 (H-2Kb) mice were purchased from Charles River Laboratories (Saint-Germain sur L’Arbresle, France). BALB/c and C57BL/6 mice strains with a null mutation of the common beta subunit of the GM-CSF, IL-3 and IL-5 receptor (βc-/-) were reported by L. Robb [24]. βc-/- mice were backcrossed for at least nine generations with BALB/c or C57BL/6 mouse strains. The genotyping of all animals was confirmed by PCR analysis. All the animals used in this study were 8- to 12-week-old female mice and were grouped in a specific pathogen-free environment, with constant temperature and humidity and with lighting on a fixed light/dark cycle. All experiments were performed according with local care animal regulation. All animal studies were reviewed and approved by institutional and cantonal veterinary authorities in accordance with Swiss Federal law.
2.2. Cell Lines
The B16-F10 melanoma cell line, derived from a C57BL/6 mouse, and the Renca renal cortical adenocarcinoma cell line, derived from a BALB/c mouse, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The B16 melanoma cell line expressing cytoplasmic ovalbumin (B16-OVA) was provided by Dr Preynat-Seauve. B16-OVA and Renca cells were engineered to release murine GM-CSF (referred as B16-OVA-GM-CSF and Renca-GM-CSF, respectively) using a retroviral-mediated gene transfer, as previously described [27]. All cell lines were maintained in a culture medium containing DMEM (Gibco) supplemented with 10% heat inactivated fetal calf serum (FCS) (Gibco) and 1% penicillin-streptomycin (Gibco) at 37 °C in a humidified atmosphere of 5% CO2 and were confirmed to be mycoplasma-free using a Mycoplasma Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany).
2.3. Preparation of Bone Marrow-Derived Dendritic Cells
Bone marrow–derived DC were generated as previously described [28]. Briefly, bone marrow was harvested from femurs and tibias of wild type (WT), βc-/- of BALB/c and C57BL/6 mice and passed through a nylon mesh to remove small pieces of bone and debris. Bone marrow cells were seeded at a density of 5 × 105 cells per 24-well in a differentiating culture medium consisting of IMDM (Gibco) with 10% FCS and 1% penicillin-streptomycin supplemented with 50 ng/mL murine GM-CSF (Renca-GM-CSF supernatant), 50 ng/mL murine IL-4 (R&D systems, Abingdon, UK) and 10 ng/mL murine Flt3-ligand (Renca-FLT3-L supernatant). On days 2 and 5, non-adherent cells were gently removed, and a differentiating culture medium was added. On day 7, the medium was replaced by a fresh medium containing 10 μg/mL LPS (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) or 10 μg/mL CpG (Enzo Life Sciences AG, Lausen, Switzerland) to induce DC maturation. Alternatively, DC cells were pulsed with OVA by suspending 106 cells/mL in a differentiating medium containing 10 μM OVA peptide SIINFEKL-Kb. After a 4 h incubation period at 37 °C, with gentle shaking every 15 min, the OVA-pulsed DC were washed and resuspended in HBSS (Gibco) for injection in mice.
2.4. Animal Immunization and Tumor Challenge
Mice vaccinated with tumor cells were immunized by a ventral sub-cutaneous (s.c.) injection of 1 × 106 irradiated (3500 rads) B16-OVA, B16-OVA-GM-CSF or 5 × 105 Renca-GM-CSF cells and challenged seven days later by s.c. injection in the upper back of 1 × 105 live B16-OVA or 2.5 × 106 Renca cells. The immunization with loaded DC was performed by injecting 4 × 105 OVA-pulsed DC in the tail vein. The mice were challenged 7 days later with 1 × 105 non-irradiated B16-OVA cells injected s.c. in the upper back. The immunization with Renca-GM-CSF and immature DCWT was performed by s.c. injection of a mixed solution containing 4.5 × 105 DC and 5 × 105 irradiated Renca-GM-CSF cells. The animals were then challenged with 2.5 × 106 Renca cells s.c. injected in the upper back. The mice were either sacrificed 7 days after tumor challenge for tetramer analysis (see below) or observed for weeks for tumor development. The animals were sacrificed when tumors ulcerated or reached 10 mm in diameter.
2.5. Antibodies
All monoclonal antibodies (mAb) and isotype controls were purchased from BD Pharmingen (San Diego, CA, USA). Phycoerythrin (PE)-conjugated antibodies included anti-CD11c (hamster IgG, clone HL3) and an isotype control (hamster IgG, clone A19-3). Biotin-labeled mAb included anti-MHC class II (I-A/I-E, rat IgG2a, clone 2G9), anti-CD40 (rat IgG2a, clone 3/23), anti-CD80 (hamster IgG, clone 16-A10A1), anti-CD86 (rat IgG2a, clone GL1) and isotype controls (hamster IgG, clone B81-8; rat IgG2a, clone R35–95). The secondary antibody streptavidin-conjugated allophycocyanin (APC) was used as a second step for the detection of biotin-labeled mAb.
2.6. Flow Cytometric Analysis
Cultured DC or peripheral blood lymphocyte (PBL) suspensions were incubated with the indicated antibodies. Live cells were identified using the non-permeant DNA dye 7-amino-actinomycin D (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland). For the quantitative detection of cytokines, the BD™ CBA (Cytometric Bead Array) Mouse Inflammation Kit (Becton-Dickinson, Mountain View, CA, USA) was used. Data were acquired with CellQuest™ software (BD) on a BD FACS Calibur™ (Becton-Dickinson, Mountain View, CA, USA) instrument and analyzed with BD FACS Diva™ and FlowJo™ Software.
2.7. Peripheral Blood Lymphocyte Analysis
Blood was collected 7 days after immunization and tumor challenge. Peripheral blood lymphocytes (PBL) were isolated using Lympholyte®-Mammal solution (Tebu-bio, Le Perray en Yvelines, France) according to the manufacturer’s instructions and incubated for 15 min with mouse IgG2a-biotin (25 μL of 2.4.G2 cells supernatant) to block Fc receptors. For the OVA-specific CD8+ T cells detection, PBL were then incubated for 45 min with tetramer OVA-conjugated PE provided by Alena Donda’s laboratory platform and CD8 APC (BD biosciences Pharmingen, Allschwil, Switzerland) before washing and FACS analysis.
2.8. Statistical Analysis
Data analyses were performed using GraphPad Prism (GraphPad Software). Data are expressed as mean ± standard error of the mean (SEM). A two-way ANOVA with the Tukey’s test was used for the analysis of multiple groups, and a Student’s t-test was used to compare the two groups. Kaplan–Meier survival curves of mice in the tumor studies were analyzed by the log rank-survival test. Differences regarded as statistically significant between the groups are presented as follows: * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
3. Results
3.1. Mice without a Functional GM-CSF Signaling Do Not Mount Anti-Tumor Immunity
As previously reported [23], the immunization of C57BL/6 WT mice with B16-OVA-GM-CSF extended the survival and conferred a clear and efficient immune response against tumor challenge, with the absence of tumor development in 15/21 mice (71%) (Figure 1). To evaluate the role of GM-CSF signaling in the anti-tumor immunization process, C57BL/6 WT mice and C57BL/6 βc-/- mice were immunized with an irradiated B16-OVA cell line. Seven days later, the animals were challenged with non-irradiated B16-OVA cells and observed over time for tumor development.
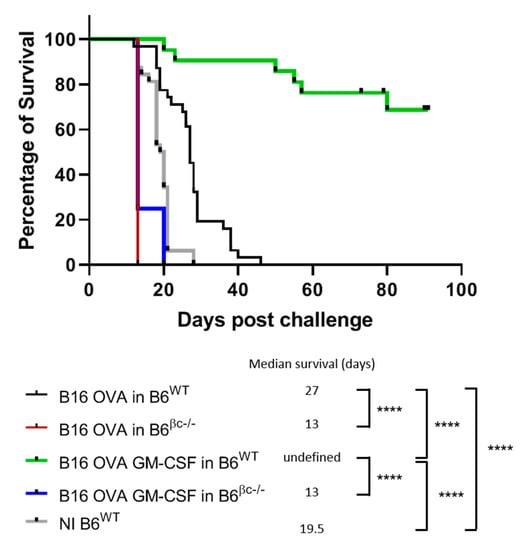
Figure 1.
Mice without a functional GM-CSF receptor do not mount anti-tumor immunity. C57BL/6 wild type (B6WT) and C57BL/6 βc-/- (B6βc-/-) mice were immunized with irradiated B16-OVA (n = 31 for B6WT and n = 4 for B6βc-/-) or B16-OVA GM-CSF secreting cells (n = 21 for B6WT and n = 4 for B6βc-/-). Non-immunized (NI) B6WT (n = 32) mice were used as a negative control. Seven days later, the mice were then challenged with a contro-lateral s.c. injection of live B16-OVA tumor cells. Following the injection, the mice were monitored for survival and were sacrificed when tumors ulcerated or reached 10 mm in diameter. The survival of the animals was plotted as a Kaplan–Meier curve for each group and was analyzed by the log rank-survival test. Differences regarded as statistically significant between the groups are presented as follows: * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
The C57BL/6 βc-/- group showed a slight decrease in the median overall survival compared to the C57BL/6 WT group, with, respectively, 13 and 27 days (Figure 1). With these models, we can demonstrate that both the release of GM-CSF at the vaccine site and functional GM-CSF receptors are needed for a potent anti-tumor immune response.
3.2. DCβc-/- Are Not Able to Undergo Full Maturation upon TLR Stimulation
To understand the failure of C57BL/6 βc-/- to mount a protective anti-tumor immune response, we compared the maturation profile of DC derived from mice lacking a functional GM-CSF receptor with that from wild-type controls. The percentage of CD11c + DC was evaluated by flow cytometry in each culture before stimulation, and no difference was observed (data not shown). After stimulation with LPS (Figure 2) or CpG (data not shown), the percentage of positive cells and the level of expression for activation molecules were analyzed by flow cytometry.

Figure 2.
The maturation profile of DCβc-/- is different from that of DCWT. DC generated from WT or βc-/- BalbC mice were stimulated with LPS during two days. Co-stimulatory (CD40, CD80 and CD86) and MCH II expression is evaluated by flow cytometry. Histograms are representative from one out of eleven different experiments. Scatter dot plots are the cumulative results of the percentage of positive cells among CD11c+ cells and the mean of MFI for each markers. Differences regarded as statistically significant between the groups are presented as follows: * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
Compared to DCWT, DCβc-/- failed to upregulate CD80/CD86 expression, whereas no differences between both DC were observed for CD40 molecules. The proportion of MHC class II positive cells was significantly increased for the DCWT compared to DCβc-/-, which shows a high basal proportion level. The same trend between DCβc-/- and DCWT generated in the absence of GM-CSF was observed (Supplementary Figure S1), suggesting the importance of the GM-CSF molecule and pathway during DC differentiation for their maturation. Moreover, after LPS stimulation, the IL-6 and MCP-1 production was lower in DCβc-/- than in DCWT (Figure 3).

Figure 3.
The IL-6 and MCP-1 production by DCβc-/- is decreased compared to DCWT. DCWT and DCβc-/- were stimulated during two days with LPS. The IL-6 and MCP-1 concentration in supernatants were then quantified with a BD™ CBA (Cytometric Bead Array) Mouse Inflammation Kit and analyzed by flow cytometry. The graphs are the cumulative results of the two experiments (the black bar is the non-stimulated condition, and the grey bar is the LPS stimulation). Differences regarded as statistically significant between the groups are presented as follows: * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
In the counterpart, IL-10, IL12p70 and TNF-α production was not altered (data not shown). These results show that mature DCβc-/- display a phenotype that is different from that of mature DCWT, with a reduced upregulation of CD80/CD86. This suggests that the GM-CSF receptor plays a driving role in the ability of DC to undergo maturation changes. Such data were not seen in IL-3 -/- or IL-5 -/- mice [23].
3.3. Immunization with DCβc-/- Is Unable to Mount a Specific Immune Response or to Prevent Tumor Growth
To assess the ability of DCβc-/- and DCWT to prime peripheral CD8 T cells, mature DCs were pulsed with OVA peptide prior to being injected into C57BL/6 WT mice. Just before the injection, the proportion of CD11c positive cells was evaluated. We noticed a higher percentage of CD11c positive cells in the C57BL/6 βc-/- group (data not shown), suggesting that the absence of GM-CSF functional signaling did not reduce the number of dendritic cells generated in vitro. OT-1 mice were used as the positive control, and non-injected mice as the negative control. The ability of DC to elicit a specific immune response was evaluated by tetramer analysis of OVA-specific CD8+ T cells in peripheral blood. The results showed that OVA-pulsed DCβc-/- failed to induce any expansion of OVA-specific CD8 + T cells (Mean ± SEM: 2.225 ± 0.125%), similarly to the negative control (Mean ± SEM: 3.048 ± 0.4848%), while OVA-pulsed DCWT were able to promote a specific immune response with 18.59 ± 2.136% (mean ± SEM) OVA-specific CD8 + T cells (Figure 4a,b).

Figure 4.
The lack of a specific immune response to prevent tumor growth in the absence of functional GM-CSF signaling is rescued with OVA-pulsed DCWT. DCWT or DCβc-/- were pulsed with OVA peptide and injected in C57BL/6 WT or βc-/- mice. After five days, PBL were isolated from blood punction and analyzed by flow cytometry for OVA-specific CD8 + T cells. (A) Representative dot plot of staining for OVA-specific CD8 + T cells tetramer. (B) Histograms of the percentage of OVA-specific CD8 + T cells in the peripheral blood of non-immunized mice (n = 12), OT-1 positive mice (n = 2) or B6 WT mice immunized with OVA-pulsed DCWT (n = 7) or OVA-pulsed DCβc-/- (n = 2), or B6 βc-/- mice immunized with OVA-pulsed DCWT (n = 3). (C) C57BL/6 WT mice were immunized with OVA-pulsed DCWT (n = 16) or OVA-pulsed DCβc-/- (n = 2), and C57BL/6 βc-/- mice were immunized with OVA-pulsed DCWT (n = 3). Non-immunized B6WT mice were used as the negative control (n = 16). Seven days later, the mice were then challenged with a contro-lateral s.c. injection of live B16-OVA tumor cells. Following the injection, the mice were monitored for survival and were sacrificed when tumors ulcerated or reached 10 mm in diameter. The survival of the animals was plotted as a Kaplan–Meier curve for each group and analyzed by the log rank-survival test. Differences regarded as statistically significant between the groups are presented as fol-lows: * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
In order to further test the role of DC maturation in tumor immunization, the survival of mice injected with OVA-pulsed DCWT or OVA-pulsed DCβc-/- was evaluated after B16-OVA cells challenge. As suggested by the OVA staining data, the median survival of C57BL/6 WT mice immunized with OVA-pulsed DCβc-/- at day 16 was the same as the non-immunized mice (Figure 4c). In contrast, a marked delay in tumor appearance and a longer median overall survival of 38 days was observed after immunization with OVA-pulsed DCWT (Figure 4c). These results indicate that signaling through the β chain of the GM-CSF receptor is essential for the priming of T cells and for promoting an efficient anti-tumor response.
3.4. OVA-Pulsed DCWT Are Able to Rescue the Lack of a Specific Anti-Tumoral Immune Response
As C57BL/6 βc-/- mice present deficient functional DCs and are unable to mount proper anti-tumor immune responses when immunized with irradiated B16-OVA-GM-CSF cells, the potential of immunization using DCWT in those mice was investigated. Firstly, the ability of OVA-pulsed DCWT to prime CD8 T cells in C57BL/6 WT or βc-/- mice was tested. OT-1 mice were used as positive controls and non-injected mice as negative controls. The ability of DCs to elicit a specific immune response was evaluated by a tetramer analysis of OVA-specific CD8 + T cells in peripheral blood. The results showed that OVA-pulsed DCWT promoted a clear expansion of the OVA-specific CD8 + T cell population in C57BL/6 βc-/- mice (mean ± SEM: 18.99 ± 1.623%), in the same extent as in C57BL/6 WT (mean ± SEM: 18.59 ± 2.136%) (Figure 4a,b). Then, the ability of OVA-pulsed DCWT to induce an efficient immune response in C57BL/6 βc-/- mice following the challenge with B16-OVA cells was evaluated.
Both wild-type and deficient mice showed a protective response against tumor growth with a median overall survival of 38 and 42 days, respectively (Figure 4c). These results demonstrate that DCWT can promote an efficient T cell priming in both WT and βc-/- mice, showing that fully functional DCs are sufficient for T-cell priming in βc-/-mice.
3.5. Immunization of BALB/c βc-/- Mice with a Combination of DCWT and Renca-GM-CSF Cells Restores the Protective Anti-Tumoral Response
As previously shown in an immunotherapy model using BALB/c mice, the immunization with Renca-GM-CSF cells was able to induce an efficient immune response against tumor cell challenge in BALB/c WT mice but failed to protect BALB/c βc-/- mice [23]. Based on these results, we decided to validate the powerful effect of DCs on immune induction in the model described previously. BALB/c βc-/- mice were immunized with irradiated Renca-GM-CSF cells in combination with either DCWT or DCβc-/-. After the challenge with live Renca cells, no tumor was observed in the group immunized with fully functional DCWT (Figure 5).
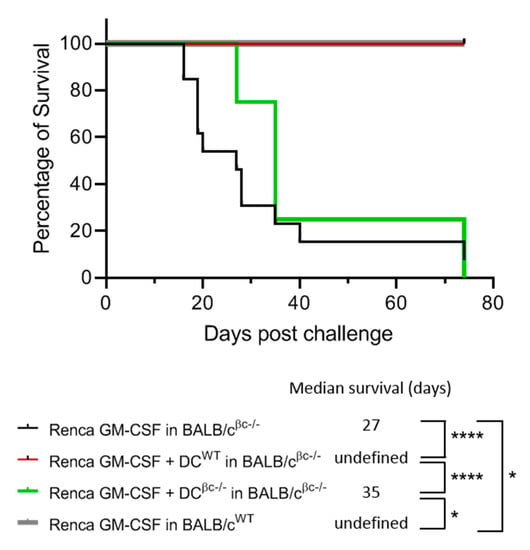
Figure 5.
Immunization of BALB/c βc-/- mice with a combination of DCWT and Renca-GM-CSF cells restores the protective anti-tumoral response. DCWT (n = 13) or DCβc-/- (n = 4) were pulsed with OVA peptide and injected in BALB/cβc-/- mice. BALB/cWT (n = 4) and BALB/c βc-/- mice (n = 13) immunized with only RENCA-GM-CSF were used as positive and negative controls, respectively. Eight days after, the mice were challenged with a contro-lateral s.c. injection of live Renca tumor cells. Following the injection, the mice were monitored for survival and were sacrificed when tumors ulcerated or reached 10 mm in diameter. The survival of the animals was plotted as a Kaplan–Meier curve for each group and analyzed by the log rank-survival test. Differences regarded as statistically significant between the groups are presented as follows: * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
These results demonstrate that DCWT in the presence of GM-CSF can restore the protective anti-tumor response in BALB/c βc-/- mice lacking GM-CSF signaling, showing the crucial role of GM-CSF signaling in mounting an anti-tumoral response.
4. Discussion
Efficient cell-based immunotherapy requires that each step leading to tumor rejection, from tumor antigen processing and presentation by APC, to the maturation of APC and the initiation and polarization of T cell-mediated immune responses, be fully functional.
In the present study, we showed that the local release of GM-CSF by irradiated tumor cells at the injection site greatly increases the efficiency of a cell-based immunization model using OVA-expressing tumor cells. However, protective immunity against tumor challenge with B16 and B16-OVA tumor cells not only requires GM-CSF-secreting cells but also a functional GM-CSF signaling pathway. Indeed, despite the expression of a strong antigen, mice lacking the common beta subunit receptor for GM-CSF, IL-3 and IL-5 are unable to reject a subsequent tumor challenge.
The improved immunogenicity of GM-CSF-secreting tumor cells has been previously described in several tumor models [12,23]. Although the beta subunit shares a common receptor activity with GM-CSF and IL-5, we showed that immunized IL5-/- mice did not have any lack in anti-tumor immunity [23], indicating that IL-5 signaling is not necessary for protective anti-tumor immunization. From these observations, we postulated that tumor cell immunogenicity may be related to the local release of GM-CSF, which induces a favorable local environment for tumor antigens processing by DCs and triggers an optimal immune response.
The observation that βc-/- mice are unable to mount an efficient anti-tumoral immune response can be explained by the fact that any changes in the GM-CSF pathways profoundly affect the quantity of dermal CD103 + DCs and CD11b + DCs in the lung, skin and lamina propria, establishing that GM-CSF is required for normal non-lymphoid tissue DCs homeostasis [29,30,31].
It has been well described that mature DCs enhance the expression of costimulatory molecules, produce the cytokines and chemokines necessary for the efficient activation of T cell responses [32] and can migrate to the lymphoid tissues [33]. In contrast, immature DCs fail to induce antigen-specific responses [34] and may in fact induce the differentiation of regulatory T cells [35,36]. In summary, the anti-tumor immune response depends on the activation/maturation status of DCs.
In this study, the in vitro stimulation of DCβc-/- showed a change in the DC’s maturation profile in the absence of GM-CSF signaling, with a clear defect in the upregulation of CD80/CD86 co-stimulatory molecules, suggesting that the GM-CSF signaling pathway may be involved in CD80 and CD86 expression. A study has shown that the transcription factor PU.1, also implicated in the GM-CSF signaling pathway, plays a key role in the gene expression of CD80 and CD86 in bone-marrow-derived DCs [37], suggesting that the lower CD80/CD86 expression on DC βc-/- can be explained by a probable defect in this signaling pathway. Nevertheless, after LPS stimulation, the expression, especially of CD86, is induced on almost all DCβc-/-, similarly to DCWT. This observation indicates that other signaling pathways may be involved in DC maturation. Indeed, other transcription factors involved in the LPS-induced expression of CD80 or CD86, as the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and IRFs, could take over.
Some reports have suggested that both of these co-stimulatory molecules may have a different function due to their difference in temporal expression [38,39]. Another study has clearly described the role of the CD80 and CD86 expressed by DCs in the polarization of type 1 (Th1) or type 2 (Th2) CD4 + T helper cells. A full DC maturation induces robust IFN-gamma-producing lymphocytes Th1, CD4 cells and CD8 cytotoxic cells [40]. Since DCβc-/- showed differences in the maturation profile in comparison with DCWT, we hypothesize that their ability to stimulate a potent T-cell mediated response is less efficient. It is supported by the results showing that the immunization with OVA-pulsed DCβc-/- failed to induce a strong expansion of OVA-specific CD8+ T cells. Another study using a βc-/- chimeric mice model showed that these mice were unable to mount antigen-specific CD8+ effector T cells in the lung and the lung-draining LNs upon immunization with particulate antigens injected into the trachea [30]. The authors hypothesized that these results are probably due to the strong reduction of lung CD103+ DCs [30]. In addition, it was shown that GM-CSF controls DC cross-presentation and cross-priming function in vitro [41], suggesting that the defect in anti-tumoral immunity may be due to a lack of cross-presentation by these cells [42].
The defect in the DC homeostasis of βc-/- mice can be counteracted by the injection of fully functional DCs, as shown by the restoration of the protective effect against tumor challenging done by the immunization of βc-/- mice with OVA-pulsed DCWT. These data support the rationale of a DC-based vaccine using mature, rather than immature, tumor antigen-pulsed DCs [43]. Antigen-loaded DCs were shown to induce a robust tumor-specific T-cell response and a complete tumor regression [44].
Other approaches using GM-CSF-transduced tumor cells revealed an improvement in their immunogenicity, with the generation of an anti-tumor CD8+ T-cell immunity [12,23,45,46]. The mechanisms that control the clinical potency of this vaccine are not clearly established, but it is critical to examine whether these vaccines improve anti-tumor immunity, partly through their ability to promote the activation and/or the Ag-presenting function of DCs. In order to analyze the full potency of these cells for a clinical use; the in vitro generation of clinical grade cell population must be standardized. As shown in this study, an efficient anti-tumor immune response relies on co-stimulation under DC mediation enhanced by GM-CSF signaling, suggesting that a combination of both approaches can be successful. The various anti-tumor immunotherapies that have been described demonstrate that no single immunotherapeutic modality can effectively cure an established cancer. A combination of standard chemotherapy with the enhanced cross-presentation of tumor-associated antigens from apoptotic or necrotic tumor cells, the depletion of immunosuppressive lymphocytes, the inhibition of cancer-induced immunosuppression and therapeutic vaccines is likely to further improve cancer immunotherapy in the future.
5. Conclusions
In conclusion, this study showed that immunization with irradiated tumor cells doesn’t induce a protective, specific tumor immunization in βc-/- mice with non-functional GM-CSF receptor, compared to wild-type mice. The absence of anti-tumoral response is linked to the fact that DC from βc-/- mice (DCβc-/-) are unable to undergo a full maturation level, showing the importance of GM-CSF for the priming phase of anti-tumor immunity. These findings may contribute to new strategies for efficient anti-tumor immunotherapies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2673-5601/1/3/16/s1, Figure S1: Maturation profiles of DCWT generated in the absence of GM-CSF and DCβc-/- are similar DC.
Author Contributions
Conceptualization, F.S. and N.M.; methodology, F.S., P.L. and A.D.; validation, F.S.; formal analysis, F.S. and E.C.; investigation, F.S. and P.L.; resources, A.D.; writing—original draft preparation, F.S., E.C. and R.V.; writing—review and editing, E.C., R.V. and N.M.; visualization, E.C.; supervision, N.M.; funding acquisition, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swiss National Science Foundation, grant number PNR37, the Ligue genevoise contre le cancer, the Foundation Coromandel, the Philanthropy Settlement and the Fondation pour la lutte contre le cancer.
Institutional Review Board Statement
All animal experiments were performed in accordance with the Swiss Federal and local laws, policies, regulations and standards in effect at the time of their conduct (approval code N° 31.1.1064/1556).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. N.M. is a founder and minority stock holder of MaxiVAX SA, E.C. is an employee of MaxiVAX SA. MaxiVAX SA is a Geneva-based biotech company involved in cell encapsulation technology that is not relevant for this manuscript. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic Cell-Based Immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Martinek, J.; Wu, T.-C.; Cadena, D.; Banchereau, J.; Palucka, K. Interplay between dendritic cells and cancer cells. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 348, pp. 179–215. ISBN 978-0-12-818351-9. [Google Scholar]
- Lin, C.-C.; Wang, T.-E.; Liu, C.-Y.; Lin, C.-P.; Liu, T.-P.; Chen, M.-J.; Chang, W.-H.; Lin, J.-C.; Chang, K.-M.; Chu, C.-H.; et al. Potentiation of the Immunotherapeutic Effect of Autologous Dendritic Cells by Pretreating Hepatocellular Carcinoma with Low-Dose Radiation. Clin. Investig. Med. 2008, 31, E150–E159. [Google Scholar] [CrossRef]
- Wang, Q.-T.; Nie, Y.; Sun, S.-N.; Lin, T.; Han, R.-J.; Jiang, J.; Li, Z.; Li, J.-Q.; Xiao, Y.-P.; Fan, Y.-Y.; et al. Tumor-Associated Antigen-Based Personalized Dendritic Cell Vaccine in Solid Tumor Patients. Cancer Immunol. Immunother. 2020, 69, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Dhodapkar, K.M.; Palucka, A.K. Interactions of Tumor Cells with Dendritic Cells: Balancing Immunity and Tolerance. Cell Death Differ. 2008, 15, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Banchereau, J. Taking Dendritic Cells into Medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cranmer, L.D.; Trevor, K.T.; Hersh, E.M. Clinical Applications of Dendritic Cell Vaccination in the Treatment of Cancer. Cancer Immunol. Immunother. 2004, 53, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. GM-CSF-Based Cancer Vaccines. Immunol. Rev. 2002, 188, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. GM-CSF-Secreting Melanoma Vaccines. Oncogene 2003, 22, 3188–3192. [Google Scholar] [CrossRef]
- Curry, W.T.; Gorrepati, R.; Piesche, M.; Sasada, T.; Agarwalla, P.; Jones, P.S.; Gerstner, E.R.; Golby, A.J.; Batchelor, T.T.; Wen, P.Y.; et al. Vaccination with Irradiated Autologous Tumor Cells Mixed with Irradiated GM-K562 Cells Stimulates Antitumor Immunity and T Lymphocyte Activation in Patients with Recurrent Malignant Glioma. Clin. Cancer Res. 2016, 22, 2885–2896. [Google Scholar] [CrossRef]
- Dranoff, G.; Jaffee, E.; Lazenby, A.; Golumbek, P.; Levitsky, H.; Brose, K.; Jackson, V.; Hamada, H.; Pardoll, D.; Mulligan, R.C. Vaccination with Irradiated Tumor Cells Engineered to Secrete Murine Granulocyte-Macrophage Colony-Stimulating Factor Stimulates Potent, Specific, and Long-Lasting Anti-Tumor Immunity. Proc. Natl. Acad. Sci. USA 1993, 90, 3539–3543. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Wei, X.X.; Chan, S.; Kwek, S.; Lewis, J.; Dao, V.; Zhang, L.; Cooperberg, M.R.; Ryan, C.J.; Lin, A.M.; Friedlander, T.W.; et al. Systemic GM-CSF Recruits Effector T Cells into the Tumor Microenvironment in Localized Prostate Cancer. Cancer Immunol. Res. 2016, 4, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Caux, C.; Vanbervliet, B.; Massacrier, C.; Dezutter-Dambuyant, C.; de Saint-Vis, B.; Jacquet, C.; Yoneda, K.; Imamura, S.; Schmitt, D.; Banchereau, J. CD34+ Hematopoietic Progenitors from Human Cord Blood Differentiate along Two Independent Dendritic Cell Pathways in Response to GM-CSF+TNF Alpha. J. Exp. Med. 1996, 184, 695–706. [Google Scholar] [CrossRef]
- Hopewell, E.L.; Cox, C. Manufacturing Dendritic Cells for Immunotherapy: Monocyte Enrichment. Mol. Ther. Methods Clin. Dev. 2020, 16, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Daro, E.; Pulendran, B.; Brasel, K.; Teepe, M.; Pettit, D.; Lynch, D.H.; Vremec, D.; Robb, L.; Shortman, K.; McKenna, H.J.; et al. Polyethylene Glycol-Modified GM-CSF Expands CD11b(High)CD11c(High) but NotCD11b(Low)CD11c(High) Murine Dendritic Cells in Vivo: A Comparative Analysis with Flt3 Ligand. J. Immunol. 2000, 165, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Vremec, D.; Lieschke, G.J.; Dunn, A.R.; Robb, L.; Metcalf, D.; Shortman, K. The Influence of Granulocyte/Macrophage Colony-Stimulating Factor on Dendritic Cell Levels in Mouse Lymphoid Organs. Eur. J. Immunol. 1997, 27, 40–44. [Google Scholar] [CrossRef]
- Shen, Z.; Reznikoff, G.; Dranoff, G.; Rock, K.L. Cloned Dendritic Cells Can Present Exogenous Antigens on Both MHC Class I and Class II Molecules. J. Immunol. 1997, 158, 2723–2730. [Google Scholar]
- Zou, G.M.; Tam, Y.K. Cytokines in the Generation and Maturation of Dendritic Cells: Recent Advances. Eur. Cytokine Netw. 2002, 13, 186–199. [Google Scholar]
- Hong, I.-S. Stimulatory versus Suppressive Effects of GM-CSF on Tumor Progression in Multiple Cancer Types. Exp. Mol. Med. 2016, 48, e242. [Google Scholar] [CrossRef] [PubMed]
- Zarei, S.; Schwenter, F.; Luy, P.; Aurrand-Lions, M.; Morel, P.; Kopf, M.; Dranoff, G.; Mach, N. Role of GM-CSF Signaling in Cell-Based Tumor Immunization. Blood 2009, 113, 6658–6668. [Google Scholar] [CrossRef]
- Robb, L.; Drinkwater, C.C.; Metcalf, D.; Li, R.; Köntgen, F.; Nicola, N.A.; Begley, C.G. Hematopoietic and Lung Abnormalities in Mice with a Null Mutation of the Common Beta Subunit of the Receptors for Granulocyte-Macrophage Colony-Stimulating Factor and Interleukins 3 and 5. Proc. Natl. Acad. Sci. USA 1995, 92, 9565–9569. [Google Scholar] [CrossRef]
- Nicola, N.A.; Robb, L.; Metcalf, D.; Cary, D.; Drinkwater, C.C.; Begley, C.G. Functional Inactivation in Mice of the Gene for the Interleukin-3 (IL- 3)-Specific Receptor Beta-Chain: Implications for IL-3 Function and the Mechanism of Receptor Transmodulation in Hematopoietic Cells. Blood 1996, 87, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Gorski, S.A.; Lawrence, M.G.; Hinkelman, A.; Spano, M.M.; Steinke, J.W.; Borish, L.; Teague, W.G.; Braciale, T.J. Expression of IL-5 Receptor Alpha by Murine and Human Lung Neutrophils. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Gillessen, S.; Wilson, S.B.; Sheehan, C.; Mihm, M.; Dranoff, G. Differences in Dendritic Cells Stimulated in Vivo by Tumors Engineered to Secrete Granulocyte-Macrophage Colony-Stimulating Factor or Flt3-Ligand. Cancer Res. 2000, 60, 3239–3246. [Google Scholar]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of Large Numbers of Dendritic Cells from Mouse Bone Marrow Cultures Supplemented with Granulocyte/Macrophage Colony-Stimulating Factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef]
- Bogunovic, M.; Ginhoux, F.; Helft, J.; Shang, L.; Hashimoto, D.; Greter, M.; Liu, K.; Jakubzick, C.; Ingersoll, M.A.; Leboeuf, M.; et al. Origin of the Lamina Propria Dendritic Cell Network. Immunity 2009, 31, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Greter, M.; Helft, J.; Chow, A.; Hashimoto, D.; Mortha, A.; Agudo-Cantero, J.; Bogunovic, M.; Gautier, E.L.; Miller, J.; Leboeuf, M.; et al. GM-CSF Controls Nonlymphoid Tissue Dendritic Cell Homeostasis but Is Dispensable for the Differentiation of Inflammatory Dendritic Cells. Immunity 2012, 36, 1031–1046. [Google Scholar] [CrossRef]
- King, I.L.; Kroenke, M.A.; Segal, B.M. GM-CSF-Dependent, CD103+ Dermal Dendritic Cells Play a Critical Role in Th Effector Cell Differentiation after Subcutaneous Immunization. J. Exp. Med. 2010, 207, 953–961. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- De Vries, I.J.M.; Krooshoop, D.J.E.B.; Scharenborg, N.M.; Lesterhuis, W.J.; Diepstra, J.H.S.; Van Muijen, G.N.P.; Strijk, S.P.; Ruers, T.J.; Boerman, O.C.; Oyen, W.J.G.; et al. Effective Migration of Antigen-Pulsed Dendritic Cells to Lymph Nodes in Melanoma Patients Is Determined by Their Maturation State. Cancer Res. 2003, 63, 12–17. [Google Scholar] [PubMed]
- Dhodapkar, M.V.; Steinman, R.M.; Krasovsky, J.; Munz, C.; Bhardwaj, N. Antigen-Specific Inhibition of Effector T Cell Function in Humans after Injection of Immature Dendritic Cells. J. Exp. Med. 2001, 193, 233–238. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Steinman, R.M. Antigen-Bearing Immature Dendritic Cells Induce Peptide-Specific CD8(+) Regulatory T Cells in Vivo in Humans. Blood 2002, 100, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Puig, P.E.; Roux, S.; Parcellier, A.; Schmitt, E.; Solary, E.; Kroemer, G.; Martin, F.; Chauffert, B.; Zitvogel, L. Tumor Cells Convert Immature Myeloid Dendritic Cells into TGF-Beta-Secreting Cells Inducing CD4+CD25+ Regulatory T Cell Proliferation. J. Exp. Med. 2005, 202, 919–929. [Google Scholar] [CrossRef]
- Kanada, S.; Nishiyama, C.; Nakano, N.; Suzuki, R.; Maeda, K.; Hara, M.; Kitamura, N.; Ogawa, H.; Okumura, K. Critical Role of Transcription Factor PU.1 in the Expression of CD80 and CD86 on Dendritic Cells. Blood 2011, 117, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, K.S.; Laszlo, G.; Pucillo, C.; Linsley, P.; Hodes, R.J. Comparative Analysis of B7-1 and B7-2 Costimulatory Ligands: Expression and Function. J. Exp. Med. 1994, 180, 631–640. [Google Scholar] [CrossRef]
- Nabavi, N.; Freeman, G.J.; Gault, A.; Godfrey, D.; Nadler, L.M.; Glimcher, L.H. Signalling through the MHC Class II Cytoplasmic Domain Is Required for Antigen Presentation and Induces B7 Expression. Nature 1992, 360, 266–268. [Google Scholar] [CrossRef]
- Tan, J.K.H.; O’Neill, H.C. Maturation Requirements for Dendritic Cells in T Cell Stimulation Leading to Tolerance versus Immunity. J. Leukoc. Biol. 2005, 78, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Sathe, P.; Pooley, J.; Vremec, D.; Mintern, J.; Jin, J.-O.; Wu, L.; Kwak, J.-Y.; Villadangos, J.A.; Shortman, K. The Acquisition of Antigen Cross-Presentation Function by Newly Formed Dendritic Cells. J. Immunol. 2011, 186, 5184–5192. [Google Scholar] [CrossRef] [PubMed]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 Deficiency Reveals a Critical Role for CD8alpha+ Dendritic Cells in Cytotoxic T Cell Immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Labeur, M.S.; Roters, B.; Pers, B.; Mehling, A.; Luger, T.A.; Schwarz, T.; Grabbe, S. Generation of Tumor Immunity by Bone Marrow-Derived Dendritic Cells Correlates with Dendritic Cell Maturation Stage. J. Immunol. 1999, 162, 168–175. [Google Scholar]
- Baldin, A.V.; Savvateeva, L.V.; Bazhin, A.V.; Zamyatnin, A.A. Dendritic Cells in Anticancer Vaccination: Rationale for Ex Vivo Loading or In Vivo Targeting. Cancers 2020, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Wu, S.; Ciernik, I.F.; Chen, H.; Nadaf-Rahrov, S.; Gabrilovich, D.; Carbone, D.P. Genetic Immunotherapy of Established Tumors with Adenovirus-Murine Granulocyte-Macrophage Colony-Stimulating Factor. Hum. Gene Ther. 1997, 8, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Hodi, F.S.; Dranoff, G. Enhancing the Clinical Activity of Granulocyte-Macrophage Colony-Stimulating Factor-Secreting Tumor Cell Vaccines. Immunol. Rev. 2008, 222, 287–298. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).