Abstract
Patients with schizophrenia face high mortality from physical comorbidities; nonetheless, the gradual physiological decline preceding death is not well characterized. This retrospective study investigated temporal changes in key biomarkers among 64 inpatients with schizophrenia who died between 2014 and 2022. We analyzed data on body mass index (BMI), serum albumin (ALB), blood urea nitrogen/creatinine ratio (BCR), blood urea nitrogen/ALB ratio (BAR), and estimated glomerular filtration rate (eGFR) collected at five time points: 3, 2, and 1 year, 6 months before death, and prior to death. We hypothesized that these markers would exhibit significant changes during the last 3 years of life. BMI and ALB significantly decreased, while BCR and BAR increased (all p < 0.001). This pattern was also noted in the pneumonia subgroup, the leading cause of death (47%). A high BCR concomitant with low eGFR was attributable to chronic kidney failure in only 6% of patients, suggesting the elevated ratio was mostly driven by non-renal factors such as dehydration or sarcopenia. Therefore, the concurrent decline in BMI and ALB and rise in BCR and BAR represent a pattern of terminal physiological decline among patients with schizophrenia, supporting the need for timely risk assessment.
1. Introduction
Patients with schizophrenia face high rates of premature mortality, with estimates suggesting a 10–20-year reduction in life expectancy compared to that of the general population [1,2,3]. Although suicide is a contributing factor, most deaths are attributable to medical conditions, including cardiovascular disease, diabetes, and infections [4,5]. In Japan, where life expectancy remains among the highest worldwide (81.1 years for males and 87.1 years for females in 2024), and with a rapidly increasing older adult proportion, this survival gap is particularly striking [6]. Furthermore, a trend analysis from 2017 data demonstrated that nearly one-third of all long-term hospitalized psychiatric patients in Japan are aged above 75 years, while two-thirds are diagnosed with schizophrenia [7]. Despite this, the early detection of physiological decline remains limited in psychiatric settings, where medical symptoms are often overshadowed by psychiatric concerns and systemic barriers to integrated care persist [1,8].
This population is particularly vulnerable to cardiometabolic diseases, partly due to long-term use of antipsychotic medication, sedentary lifestyle, smoking, and dietary factors [4,9]. The complex burden of comorbidities often progresses silently, particularly in patients who have difficulty communicating physical symptoms. Consequently, physical health monitoring remains inconsistent in psychiatric inpatient settings, contributing to the under recognition of emerging medical complications [1], thereby warranting simple, objective markers to detect medical deterioration before it becomes critical.
Clinically available biomarkers, such as body mass index (BMI), serum albumin (ALB) [10], blood urea nitrogen (BUN)-to-creatinine ratio (BCR) [11,12], and BUN-to-albumin ratio (BAR), have demonstrated predictive value for mortality across a variety of general medical conditions. In a complex healthcare environment where decisions are often made quickly based on limited information, identifying predictive markers for in-hospital mortality may significantly improve patient outcomes.
A previous study [13] investigated predictors of general hospital transfer among psychiatric inpatients with conditions such as schizophrenia, schizoaffective disorder, bipolar disorder, and dementia. Elevated BUN (odds ratio [OR], 63.2), low hemoglobin (OR, 35.3), hypoalbuminemia (OR, 7.3), and age ≥ 65 years (OR, 5.73) were independently associated with an increased risk of transfer. Acute medical deterioration was observed in 46.2%, 37.5%, and 37.4% of patients with BUN > 24 mg/dL, ALB < 3.7 g/dL, and aged 65 years and older, respectively. Nevertheless, few studies have examined the relationship between these biomarkers and transfers to general hospitals among Japanese patients with schizophrenia.
The BCR is a widely used laboratory parameter that reflects renal function, hydration status, and catabolic stress [14]. While a normal BCR indicates adequate kidney function, elevated levels may indicate dehydration, increased protein catabolism, or acute renal impairment [11]. An elevated BCR of ≥20 has been associated with adverse outcomes across a range of medical conditions [11]. For example, BCR predicts poor prognosis in patients with respiratory disease [15], ischemic stroke [16], and chronic heart failure, representing an independent predictor of all-cause mortality [12,17,18]. In patients with acute myocardial infarction, in-hospital mortality is highest when the BCR exceeds 18.34 [19]. Additionally, BCR has been reported as a marker for cardiogenic shock [20] and dehydration [21,22], and values above 30 have been associated with increased mortality in upper and lower gastrointestinal bleeding [23,24]. Nonetheless, because an elevated BCR may not exclusively reflect impaired renal function, it is critical to also consider its relationship with estimated glomerular filtration rate (eGFR) for a more accurate assessment [25,26].
BAR is another clinically valuable index for monitoring the nutritional status, renal function, and systemic inflammation of patients [27]. Elevated BAR values have consistently been associated with increased risk of in-hospital mortality across a range of conditions [10], outperforming BUN or albumin alone in predicting mortality, with hypoalbuminemia often demonstrating the strongest effect [28,29]. Similarly, in acute respiratory failure, a BAR > 11.76 is correlated with significantly higher 30-day mortality [30].
Among older adults, a BAR > 6.25, in combination with BUN > 23 mg/dL and ALB < 3.5 g/dL, has been associated with increased in-hospital mortality risk [31]. Moreover, other studies have demonstrated that BAR predicts mortality risk in patients with acute type A aortic dissection [32], acute ischemic stroke [33], pneumonia [34,35], and sepsis [36].
Despite the high burden of physical comorbidities and the underrecognized medical deterioration risk in schizophrenia, little is known in the field of psychiatry about biomarkers that can predict patterns observed in deceased patients. BMI, ALB, BCR, BAR, and eGFR may offer psychiatrists accessible tools to identify high-risk patients likely to develop serious complications that could lead to intensive care admission or death.
This preliminary study aims to examine the longitudinal changes in BMI, ALB, BCR, BAR, and eGFR during the final 3 years of life in hospitalized patients with schizophrenia. We hypothesized that these markers would show significant longitudinal changes, reflecting the physiological decline preceding death.
Specifically, we sought to:
- Assess temporal changes in BMI, ALB, BCR, BAR, and eGFR at five time points: 3 years, 2 years, 1 year, and 6 months before death and prior to death.
- Conduct a subgroup analysis between patients who died of pneumonia and those who died from other diseases.
- Determine the association between eGFR and BCR to better interpret the clinical significance of a high BCR.
- Investigate the relationship between the chlorpromazine (CPZ) equivalent dose of antipsychotic medications and the BMI, ALB, BCR, BAR, and eGFR.
2. Materials and Methods
2.1. Study Design
This was a longitudinal study that retrospectively collected clinical data from 2014 to 2022. The manuscript was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [37].
2.2. Setting
Mifune Hospital, located in Japan, was founded in 1953 and is a 328-bed psychiatric hospital. It includes departments of psychiatry, internal medicine, dentistry, and oral surgery.
2.3. Participants
2.3.1. Inclusion Criteria
Eligible participants included all inpatients diagnosed with schizophrenia according to the criteria in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders [38], who were hospitalized at Mifune Hospital between January 2014 and December 2022 and died due to a physical illness, as reported in their death certificates and clinical and radiological reports, during the hospitalization period.
2.3.2. Exclusion Criteria
Patients were excluded if they had a primary diagnosis other than schizophrenia, had disseminated intravascular coagulation, or had missing values for key clinical variables (Figure 1).
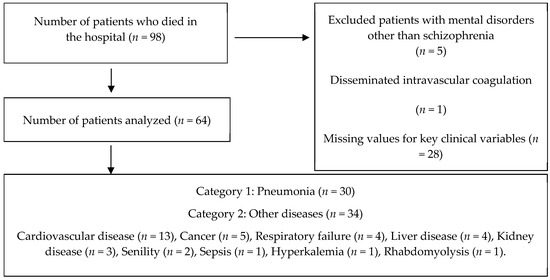
Figure 1.
Patient selection flow.
2.4. Variables
Demographic and anthropometric variables, including date and cause of death, health status, weight, BMI, and CPZ equivalent dose of antipsychotic medications, were collected from electronic medical records, covering the period up to three years before death. Supporting data to confirm the cause of death were obtained from the death certificates and clinical and associated radiological reports of the patients. Additionally, information on BUN, creatinine (Cr), ALB, and eGFR levels was collected as biomarkers to assess renal function.
2.5. Statistical Analysis
We assessed the normality of the data using the Shapiro–Wilk test. Because all variables significantly deviated from a normal distribution (p < 0.05), non-parametric tests were used for all subsequent analyses. For group comparisons, the Mann–Whitney U test was used for continuous variables (e.g., age, BMI, lab results), while the Chi-squared test or Fisher’s exact test was implemented for categorical variables (e.g., gender, symptoms).
To analyze longitudinal changes in biomarker values over five time points, we used the non-parametric Friedman test. For statistically significant results, we performed a post hoc analysis using the Durbin–Conover test. To mitigate the increased false positive risk from multiple comparisons, p-values from the post hoc tests were adjusted using the Benjamini–Hochberg false discovery rate method.
In addition to reporting p-values, the effect size for the Friedman test was calculated using Kendall’s W to quantify the magnitude of the observed changes, which is independent of sample size. The effect sizes were interpreted as very small (w < 0.10), small (w = 0.1–0.3), medium (w = 0.3–0.5), or large (w > 0.5) [39].
Finally, we used Spearman’s rank correlation coefficient to investigate the relationship between the CPZ equivalent dose of antipsychotic medications and the biomarkers (BMI, ALB, BCR, BAR, and eGFR).
All statistical analyses were performed using Jamovi statistical software version 2.4.11.0 (The Jamovi Project, Sydney, Australia) [40]. Statistical significance was set at p < 0.05.
3. Results
3.1. Patient Selection Flow
Data from 64 eligible participants were used for secondary data analysis. The cause of death among the study sample was divided into two main subgroups: those who died from the effects of pneumonia (n = 30) and those who died from other diseases (n = 34). The consolidated group of diseases the study sample died from included cardiovascular disease (n = 13), cancer (n = 5), respiratory failure (n = 4), liver disease (n = 4), kidney disease (n = 3), senility (n = 2), sepsis (n = 1), hyperkalemia (n = 1), and rhabdomyolysis (n = 1) (Figure 1).
3.2. Sample Characteristics
The study sample consisted of 64 participants, with 37 males (58%) and 27 females (42%). The median age was 70.9 years (interquartile range [IQR], 13) for males and 79.0 years (IQR, 15) for females.
There was no significant difference in gender distribution between patients who died from pneumonia and those who died from other diseases. Similarly, descriptive analysis of blood tests performed at death exhibited no significant differences in BMI, ALB, BAR, eGFR, or Cr between these two groups of patients. The sample characteristics summary is provided in Table 1.

Table 1.
Characteristics of Patients Prior to Death (n = 64).
3.3. Objective #1: To Analyze Changes in the Biomarkers of Eligible Participants at Each of the Five Time Points
Overall Cohort (n = 64)
As displayed in Table 2, both BMI and ALB levels demonstrated a significant decline as patients approached death. BMI was significantly lower at 6 months before death (median [IQR]: 17.4 [4.4] kg/m2, p < 0.05) and at death (16.7 [4.6] kg/m2, p < 0.001) than at 3 years prior to it (20.2 [5.6] kg/m2). Similarly, ALB levels were significantly lower at 6 months (3.4 g/dL, p < 0.05) and at death (2.9 g/dL, p < 0.001) than at 3 years prior to it (3.7 g/dL).

Table 2.
Longitudinal Changes in BMI, Serum Albumin, and Renal Biomarkers in the Overall Cohort (n = 64).
Conversely, BCR and BAR ratios significantly increased as the years approached death. BCR was significantly higher at death (30.6 [32.2]; p < 0.001) than at 3 years prior to it (20.1). Likewise, BAR was significantly higher at death (6.6 [9.3]; p < 0.001) than at 3 years prior to it (3.6 [1.9]). Cr and eGFR levels exhibited no significant changes across all time points. The CPZ equivalent dose was also significantly higher at 6 months before death (p < 0.01) and at 1 to 3 years before death than at prior to death (p < 0.001).
BMI and ALB level changes demonstrated a moderate effect (w = 0.3), while BCR and BAR exhibited a small effect (w = 0.2) when tested for effect size. Cr and eGFR showed a very small effect (w = 0.03).
3.4. Objective #2: To Determine Subgroup Analysis of Patients Who Died of Pneumonia and Other Diseases
Table 3 shows the longitudinal changes in BMI and the laboratory indices among the subgroups according to cause of death.

Table 3.
Longitudinal Changes in BMI, Serum Albumin, and Renal Biomarkers in Subgroups by Cause of Death.
3.4.1. Pneumonia Subgroup (n = 30)
The longitudinal trends in patients who died from pneumonia were more pronounced. BMI progressively declined, becoming significantly lower at 6 months before death (17.0 [4.1] kg/m2, p < 0.01) and at death (16.4 [2.9] kg/m2, p < 0.01) than at 1, 2, and 3 years prior to it. ALB levels remained similar until 1 year before death, but then significantly declined at 6 months before (3.3 [0.6] g/dL; p < 0.01) and at death (2.8 [0.9] g/dL; p < 0.001).
BCR was significantly higher at death (39.0 [26.3]) than at 3 years prior to it (22.4 [11.3]; p < 0.001). BAR was also significantly higher at death (8.6 [9.2], p < 0.01) than at 3 years prior to it (4.0 [1.9]). No significant changes were observed in Cr, eGFR, or the CPZ equivalent dose. For this subgroup, BMI (w = 0.32), ALB (w = 0.39), and CPZ (w = 0.3) exhibited a moderate effect, while BAR (w = 0.18) and BCR (w = 0.2) demonstrated a small effect. Cr and eGFR showed a very small effect (w = 0.05).
3.4.2. Other Causes Subgroup (n = 34)
In patients who died from causes other than pneumonia, similar trends were observed. BMI declined from a median of 20.6 kg/m2 at 3 years before death to 17.0 kg/m2 at death (p < 0.001). Pairwise analysis demonstrated that earlier BMI values were significantly higher than those at death. Similarly, ALB decreased progressively, from 3.85 g/dL at 3 years before to 3.0 g/dL at death (p < 0.001).
Conversely, both BCR and BAR ratios increased significantly toward the end of life. BCR rose from a median of 16.7 at 3 years before to 29.1 at death (p < 0.001), and BAR rose from 3.1 to 4.1 (p < 0.001). Pairwise comparisons confirmed that values at earlier years were significantly lower than those prior to death. No significant changes were observed in Cr or eGFR. The CPZ equivalent dose was significantly higher at 6 months before death (p < 0.01) and at 2 years prior to it (p < 0.001). For this subgroup, BMI (w = 0.23), ALB (w = 0.18), BCR (w = 0.2), BAR (w = 0.2), and CPZ (w = 0.1) exhibited a small effect. Nevertheless, Cr (w = 0.02) and eGFR (w = 0.02) demonstrated very small effect (w = 0.05).
3.5. Objective #3: To Determine the Association Between eGFR and BCR to Better Interpret the Clinical Significance of a High BCR
Table 4 describes the relationship between eGFR and BCR among the overall cohort and the subgroups. In the overall cohort (N = 64), there was no significant association between eGFR and BCR at any of the five time points (all p > 0.05). Subgroup analysis by cause of death (pneumonia, n = 30; other causes, n = 34) also yielded no significant association. Although a higher proportion of patients with elevated BCR (≥20) tended to have a reduced eGFR (<60) at certain time points, these differences were not statistically significant.

Table 4.
Relationship between eGFR and BCR.
3.6. Objective #4: To Investigate the Relationship Between the CPZ Equivalent Dose of Antipsychotic Medications and the BMI, ALB, BCR, BAR, and eGFR
As seen in Table 5, we found a consistent negative correlation between the CPZ equivalent dose and the BCR, particularly in the overall cohort and the “Others” subgroup. This relationship was statistically significant at multiple time points (e.g., at death, 6 months, 1 year, 2 years, and 3 years before death). A positive correlation was observed between CPZ equivalent dose and ALB levels at several time points, particularly in the pneumonia group (e.g., 1, 2, and 3 years before death). The relationship between CPZ equivalent dose and BMI was generally weak and not statistically significant in the overall cohort.

Table 5.
Relationship between CPZ Equivalent Dose of Antipsychotic Medications and Biomarkers.
4. Discussion
4.1. Objective #1: To Assess Temporal Changes in BMI, ALB, BCR, BAR, and eGFR at Five Time Points: 3 Years, 2 Years, 1 Year, and 6 Months Before Death and at Death
Our findings reveal a clear pattern of physiological decline among deceased patients with schizophrenia. For all subjects, BMI and ALB levels exhibited a significant decline in the final 6 months of life, a trend that was particularly pronounced in the pneumonia subgroup, where the decline began as early as 2 years before death. This progressive deterioration in nutritional status highlights the importance of regular assessments and early intervention, including nutritional support and physical rehabilitation, especially once BMI falls below 18. This aligns with the findings of prior research indicating that low body weight and frailty are associated with increased mortality in individuals with schizophrenia [45,46,47,48].
BMI and ALB changes were statistically significant and demonstrated a moderate effect size (w = 0.3), suggesting a clinically important deterioration in the physiological status of patients, thereby strongly indicating that the progressive deterioration of nutritional status in the terminal phase of schizophrenia is a key mechanism leading to death.
Conversely, BCR and BAR ratios were significantly higher immediately before death, reflecting a chronic catabolic stress and dehydration state. The median BCR value for all subjects was 20.1 at 3 years before death, suggesting that these patients were already in a hypercatabolic state, which is consistent with the findings from a previous study by Song Shen et al. [17]. Although the increases in BCR and BAR were statistically significant, the small effect size (w = 0.2) suggests that these changes vary among individuals based on specific pathophysiological factors such as dehydration or catabolic stress. Therefore, these markers may serve as particularly useful early warning indicators in certain patient populations.
The lack of a significant effect size for Cr and eGFR (w = 0.03) is notable, providing important clinical evidence that the elevated BCR observed here is not primarily driven by declining renal function but rather reflects a deteriorating nutritional and metabolic status, such as dehydration and sarcopenia. Proactive monitoring and timely hydration support are therefore warranted when BCR exceeds standard thresholds.
4.2. Objective #2: To Assess Subgroup Analysis by Cause of Death
Patients with schizophrenia have a higher burden of physical comorbidities, including a greater prevalence of respiratory symptoms and pulmonary dysfunction compared to the general population [49]. Here, patients who died from pneumonia demonstrated a particularly pronounced physiological decline pattern.
Specifically, persistently elevated BCR values (≥20) were present as early as 3 years before death, with a further increase prior to it, suggesting that prolonged dehydration and catabolic stress may be long-standing issues preceding pneumonia-related mortality in this vulnerable patient population. Additionally, BAR values increased significantly over time, with the pneumonia group consistently having levels above 4, which rose to over 8 before death. These sustained abnormalities in both renal and nutritional biomarkers highlight a chronic physiological vulnerability state. This is consistent with previous research indicating that BAR indicates respiratory disease progression [50]. Similarly, to BCR, routine monitoring of BAR over several years may help reduce pneumonia-associated mortality risk.
Nutritional status deterioration, as indicated by BMI and ALB levels, was particularly pronounced in the pneumonia group. The moderate effect size (BMI: w = 0.32, ALB: w = 0.39) observed for these markers suggests that the decline is both statistically significant and clinically important, strongly indicating that the specific pathophysiology of pneumonia may have accelerated the rapid nutritional status decline, making it a critical factor directly linked to mortality. While the effect sizes for BAR (w = 0.18) and BCR (w = 0.2) were smaller, the notable BAR increase within the pneumonia group likely reflects the severity of their respiratory condition, as it may represent respiratory disease progression [30,34,35]. Moreover, the moderate effect size for CPZ (w = 0.3) suggests that medication dose adjustments were made as death approached, indicating that clinicians may have attempted aggressive interventions in response to the deteriorating physical condition of the patient.
Conversely, the physiological deterioration in the “other causes” subgroup progressed more gradually, potentially reflecting a higher proportion of chronic conditions, such as cardiovascular disease, as causes of death in this group. The small effect sizes observed for all biomarkers, including CPZ (w = 0.1), suggest that drug dose adjustments were limited even in the terminal phase. In contrast to the pneumonia group, this may be interpreted as a prioritization of medication maintenance due to fewer instances of rapid physical deterioration.
Furthermore, ALB levels were consistently low (<3.5 g/dL) across all deceased patients and subgroups [51]. While the decline was modest in later stages, these persistently low levels point to early malnutrition, chronic disease, and inflammation—all of which are associated with poorer clinical outcomes [52]. Because hypoalbuminemia is independently associated with all-cause mortality across inpatient populations [53,54], routine ALB monitoring in psychiatric settings is crucial [54,55]. Based on public health screening standards, nutritional support may be warranted when ALB falls below 3.9 g/dL.
These findings align with those of prior research identifying schizophrenia as an independent risk factor for both pneumonia development and related mortality [56]. The elevated risk has been attributed to behavioral factors, impaired immunity, and adverse effects of antipsychotic medications [56]. Our results, which demonstrate early and sustained abnormalities in nutritional and renal biomarkers, reinforce the need for proactive, biomarker-guided monitoring in psychiatric inpatients to help reduce pneumonia-associated mortality.
4.3. Objective #3: To Discuss the Association Between eGFR and BCR to Better Interpret the Clinical Significance of a High BCR
Although the association between eGFR and BCR was not statistically significant at any time point, the persistent concurrent presence of reduced eGFR and elevated BCR warrants discussion, suggesting underlying physiological processes such as progressive chronic kidney disease, malnutrition, or acute stress responses [57]. Elevated BCR, particularly when coupled with low eGFR, is often indicative of dehydration, increased protein catabolism, or prerenal azotemia due to conditions such as hypovolemia or heart failure [20,58,59].
While a high BCR can signal renal impairment, our data suggest this was not the main driver for the majority of our cohort. From our findings in Table 4, a closer examination revealed that while 20.3% of patients presented with the combination of high BCR (≥20) and reduced kidney function (eGFR < 60) prior to death, only a small fraction of them (n = 4, representing just 6% of the total cohort) exhibited an eGFR below 30, the threshold for severe chronic kidney disease [25,57]. Therefore, in this vulnerable population, an elevated BCR may be a biomarker for non-renal factors such as dehydration or a high catabolism state driven by sarcopenia and terminal illness [25,26]. In the pneumonia subgroup, this relationship may reflect the combined impact of infection-related catabolic stress and compromised renal reserve, corroborating evidence that high BCR is associated with worse outcomes in acute respiratory infections [60]. In the subgroup with other causes of death, the elevated BCR alongside renal decline may reflect systemic metabolic changes occurring during terminal deterioration, consistent with the findings of studies linking BCR to frailty and poor nutritional status in older adult populations [57,61].
From a clinical standpoint, while eGFR remains the gold standard for assessing renal function, BCR offers an additional dimension that captures metabolic and nutritional status [11,20]. Combined monitoring of these markers may provide valuable insights into end-of-life physiological changes and support individualized care planning. Nevertheless, given the small subgroup sizes in this study, statistical power may have been insufficient to detect meaningful associations. Future studies with larger cohorts are warranted to clarify whether the combined assessment of eGFR and BCR has prognostic utility in predicting mortality risk or disease progression among these patients.
4.4. Objective #4: To Interpret the Relationship Between CPZ Equivalent Dose and the BMI, ALB, BCR, BAR, and eGFR
Although correlations were not consistent across all biomarkers, the significant associations with BCR [20,62] and ALB [63] suggest that antipsychotic medications should be considered.
As shown in Table 5, a significant negative correlation was found between the CPZ equivalent dose and both BCR and BAR in the overall cohort, suggesting that patients on higher antipsychotic doses may have been in a better physiological state (i.e., lower BCR) [64]. Conversely, the significant correlations from earlier years disappeared in the pneumonia subgroup at 6 months prior to death, which may be due to the tapering or discontinuation of antipsychotic medication in their clinical reports during this critical period of physiological deterioration, potentially obscuring any potential association.
In the “other diseases” subgroup (Table 5), which included a large proportion of patients who died from cardiovascular disease, a more sustained negative correlation was observed between CPZ dose and BCR. For these patients, antipsychotic doses were typically maintained according to their data. The lack of a consistent correlation between medication dose and BMI further suggests that in this terminal phase, BMI changes were not solely driven by medication dose, despite the known metabolic side effects of some antipsychotics [9,65].
The positive correlation observed between the CPZ equivalent dose and ALB levels at several time points, particularly in the pneumonia group, may indicate that patients on higher doses exhibited higher ALB levels, potentially due to variations in nutritional status. The disappearance of this significant correlation at 6 months prior to death is notable and may suggest that clinicians reduced antipsychotic medication dosages as the physical health of the patients declined.
5. Limitations and Future Study
These findings underscore the importance of routine blood tests and biomarker-guided interventions in psychiatric inpatient settings. Nonetheless, our study has some limitations. The small sample size may have limited the statistical power to detect meaningful associations, necessitating a larger cohort in future studies to identify reliable risk factors. Additionally, our cohort may be subject to selection bias, as the most severely ill patients were likely overrepresented in the bedridden group, and some causes of death, such as pneumonia, may have been underdiagnosed in others. These biases could potentially lead to either an underestimation or an overestimation of biomarker trends. Lastly, the absence of a control group from the general population prevents a direct statistical comparison of causes of death with age-matched controls.
Cr may not accurately reflect hydration status in cases of prerenal failure, and muscle volume can affect its level. The eGFR formula only considers Cr, age, and sex, assuming a normal muscle volume. This method is particularly limited for older people with chronic illnesses who are more likely to have sarcopenia.
Nonetheless, our study provides valuable insights by identifying specific causes of death and associated clinical factors within a cohort of prematurely deceased patients. By focusing on this high-risk population, our findings contribute to a better understanding of the mechanisms underlying the mortality gap. Future research should address these limitations by conducting a case–control study with a larger cohort of patients with schizophrenia to clarify these associations.
6. Conclusions
Declining BMI and ALB, coinciding with rising BCR and BAR, are significant patterns observed in deceased patients with schizophrenia and reflect the terminal physiological decline from a state of chronic undernutrition, dehydration, and catabolic stress. These biomarkers should be incorporated into clinical assessments to improve the accuracy of identifying patients at terminal physical deterioration risk.
Understanding these biomarker trajectories enables more informed clinical decisions and can signify the need for early intervention. Because patients with chronic schizophrenia may struggle to report changes in diet, hydration, or physical symptoms, biomarker surveillance is even more essential. Therefore, routine monitoring of these parameters should become standard clinical practice in psychiatric inpatient care. Regular physical assessments, comprehensive nursing education, and the incorporation of physical rehabilitation and occupational therapy are also warranted to improve overall quality of life and care, and ultimately, mitigate risks and improve patient outcomes.
Author Contributions
Conceptualization, Y.M., K.M. and T.T.; methodology, K.M., Y.M. and T.T.; validation, I.E. and H.U.; formal analysis, H.I. and T.T.; investigation, Y.M., K.M. and T.T.; writing—original draft preparation, Y.M., K.M., H.I., T.A. and T.T.; writing—review and editing, K.S., K.O., L.A.B., H.U., R.Y.C.K., A.C.Y.T. and T.T.; project administration, K.M. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Mifune Hospital Clinical Research Ethics Review Committee (protocol code #20250312-1 and date of approval 12 March 2025).
Informed Consent Statement
Informed consent was obtained from the families and relatives of all subjects involved in the study.
Data Availability Statement
Data available on request due to restrictions (privacy and ethical). The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors express gratitude and appreciation to the participants. The authors thank the Mifune Hospital staff for their assistance while conducting the research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body mass index |
| ALB | Serum albumin |
| BUN | Blood urea nitrogen |
| BCR | BUN/creatinine ratio |
| BAR | Blood urea nitrogen-to-albumin ratio |
| CPZ | Chlorpromazine equivalent dose |
| Cr | Serum creatinine |
| eGFR | Estimated glomerular filtration rate |
| IQR | Interquartile range |
References
- Bui, T.N.T.; Au, R.T.; Janetzki, J.L.; McMillan, S.S.; Hotham, E.; Suppiah, V. Metabolic Monitoring for Adults Living with a Serious Mental Illness on a Second-Generation Antipsychotic Agent: A Scoping Review. Adm. Policy Ment. Health 2025, 52, 289–317. [Google Scholar] [CrossRef]
- Yi, W.; Wu, H.; Fu, W.; Feng, H.; Huang, J.; Li, H.; Song, Z.; Chen, Y.; Zheng, Y.; She, S. Prevalence and Risk Factors of Non-Alcoholic Fatty Liver Disease (NAFLD) in Non-Obese Patients with Schizophrenia: A Retrospective Study. Diabetes Metab. Syndr. Obes. 2024, 17, 841–849. [Google Scholar] [CrossRef]
- Drevinskaite, M.; Kaceniene, A.; Germanavicius, A.; Smailyte, G. Increased Mortality Risk in People with Schizophrenia in Lithuania 2001–2020. Schizophrenia 2025, 11, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, L.; Chen, L.; Zhou, X.; Huo, L.; Wang, T.; Zhou, Y.; Zhang, X. The Prevalence and Clinical Characteristics of Diabetes Mellitus in Chinese Inpatients with Chronic Schizophrenia: A Multicenter Cross-Sectional Study. PeerJ 2021, 9, e12553. [Google Scholar] [CrossRef]
- Nishi, M.; Shikuma, A.; Seki, T.; Horiguchi, G.; Matoba, S. In-Hospital Mortality and Cardiovascular Treatment during Hospitalization for Heart Failure among Patients with Schizophrenia: A Nationwide Cohort Study. Epidemiol. Psychiatr. Sci. 2023, 32, e62. [Google Scholar] [CrossRef]
- Director-General for Statistics, Information; System Management and Industrial Relations; Ministry of Health, Labour and Welfare; Government of Japan Life Expectancies at Birth in Some Countries: Abridged Life Tables for Japan 2024. Available online: https://www.mhlw.go.jp/english/database/db-hw/lifetb24/index.html (accessed on 1 September 2025).
- Okayama, T.; Usuda, K.; Okazaki, E.; Yamanouchi, Y. Number of Long-Term Inpatients in Japanese Psychiatric Care Beds: Trend Analysis from the Patient Survey and the 630 Survey. BMC Psychiatry 2020, 20, 522. [Google Scholar] [CrossRef]
- Couttas, T.A.; Jieu, B.; Rohleder, C.; Leweke, F.M. Current State of Fluid Lipid Biomarkers for Personalized Diagnostics and Therapeutics in Schizophrenia Spectrum Disorders and Related Psychoses: A Narrative Review. Front. Psychiatry 2022, 13, 885904. [Google Scholar] [CrossRef]
- Chang, S.-C.; Goh, K.K.; Lu, M.-L. Metabolic Disturbances Associated with Antipsychotic Drug Treatment in Patients with Schizophrenia: State-of-the-Art and Future Perspectives. World J. Psychiatry 2021, 11, 696–710. [Google Scholar] [CrossRef]
- Xia, H.; Lin, J.; Liu, M.; Lai, J.; Yang, Z.; Qiu, L. Association of Blood Urea Nitrogen to Albumin Ratio with Mortality in Acute Pancreatitis. Sci. Rep. 2025, 15, 13327. [Google Scholar] [CrossRef]
- Paulus, M.C.; Melchers, M.; Van Es, A.; Kouw, I.W.K.; Van Zanten, A.R.H. The Urea-to-Creatinine Ratio as an Emerging Biomarker in Critical Care: A Scoping Review and Meta-Analysis. Crit. Care 2025, 29, 175. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, L.; Cheng, Y.; Huang, D. Predictive Value of Blood Urea Nitrogen to Creatinine Ratio and Estimated Plasma Volume Status in Heart Failure. BMC Cardiovasc. Disord. 2025, 25, 282. [Google Scholar] [CrossRef]
- Manu, P.; Asif, M.; Khan, S.; Ashraf, H.; Mani, A.; Guvenek-Cokol, P.; Lee, H.; Kane, J.M.; Correll, C.U. Risk Factors for Medical Deterioration of Psychiatric Inpatients: Opportunities for Early Recognition and Prevention. Compr. Psychiatry 2012, 53, 968–974. [Google Scholar] [CrossRef]
- Hosten, A.O. BUN and Creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Ma, H.; Lin, S.; Xie, Y.; Mo, S.; Huang, Q.; Ge, H.; Shi, Z.; Li, S.; Zhou, D. Association between BUN/Creatinine Ratio and the Risk of in-Hospital Mortality in Patients with Trauma-Related Acute Respiratory Distress Syndrome: A Single-Centre Retrospective Cohort from the MIMIC Database. BMJ Open 2023, 13, e069345. [Google Scholar] [CrossRef]
- Schrock, J.W.; Glasenapp, M.; Drogell, K. Elevated Blood Urea Nitrogen/Creatinine Ratio Is Associated with Poor Outcome in Patients with Ischemic Stroke. Clin. Neurol. Neurosurg. 2012, 114, 881–884. [Google Scholar] [CrossRef]
- Shen, S.; Yan, X.; Xu, B. The Blood Urea Nitrogen/Creatinine (BUN/Cre) Ratio Was U-Shaped Associated with All-Cause Mortality in General Population. Ren. Fail. 2022, 44, 184–190. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Shi, S.; Gao, X.; Li, Y.; Wu, H.; Song, Q.; Zhang, B. Blood Urea Nitrogen to Creatinine Ratio and Long-Term Survival in Patients with Chronic Heart Failure. Eur. J. Med. Res. 2023, 28, 343. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, T.; Chen, M.; Lei, X.; Li, Q.; Tan, Y.; Chen, X. Association between the Severity of Sarcopenia and Pneumonia in Patients with Stable Schizophrenia: A Prospective Study. J. Nutr. Health Aging 2022, 26, 799–805. [Google Scholar] [CrossRef]
- Sun, D.; Wei, C.; Li, Z. Blood Urea Nitrogen to Creatinine Ratio Is Associated with In-Hospital Mortality among Critically Ill Patients with Cardiogenic Shock. BMC Cardiovasc. Disord. 2022, 22, 258. [Google Scholar] [CrossRef]
- Mohanty, S.; Tripathi, B.; Bhatia, K.; Gupta, B.; Mittal, M. Predictors of Early Neurological Deterioration in Patients with Acute Ischaemic Stroke with Special Reference to Blood Urea Nitrogen (BUN)/Creatinine Ratio & Urine Specific Gravity. Indian J. Med. Res. 2015, 141, 299. [Google Scholar] [CrossRef]
- Thomas, D.R.; Tariq, S.H.; Makhdomm, S.; Haddad, R.; Moinuddin, A. Physician Misdiagnosis of Dehydration in Older Adults. J. Am. Med. Dir. Assoc. 2004, 5, S31–S34. [Google Scholar] [CrossRef]
- Wu, K.-H.; Shih, H.-A.; Hung, M.-S.; Hsiao, C.-T.; Chen, Y.-C. The Association between Blood Urea Nitrogen to Creatinine Ratio and Mortality in Patients with Upper Gastrointestinal Bleeding. Arab. J. Gastroenterol. 2018, 19, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Calim, A. The Role of BUN/Creatinine Ratio in Determining the Severity of Gastrointestinal Bleeding and Bleeding Localization. North. Clin. Istanb. 2025, 12, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-M.; Chen, Y.-T.; Hung, S.-C.; Shih, C.-J.; Lin, C.-H.; Chiang, C.-K.; Tarng, D.-C. The Taiwan Geriatric Kidney Disease (TGKD) Research Group Association of Estimated Glomerular Filtration Rate with All-Cause and Cardiovascular Mortality: The Role of Malnutrition-Inflammation-Cachexia Syndrome: eGFR with Mortality and the Role of MICS. J. Cachexia Sarcopenia Muscle 2016, 7, 144–151. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Zou, X.; Zhang, K.; Zhou, J.; Chen, M. High Blood Urea Nitrogen to Creatinine Ratio Is Associated with Increased Risk of Sarcopenia in Patients with Chronic Obstructive Pulmonary Disease. Exp. Gerontol. 2022, 169, 111960. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Yang, P.; Chen, X.; Chen, S.; Deng, L.; Zeng, X.; Luo, H.; Zhang, D.; Cai, X.; et al. Exploring the Value of Blood Urea Nitrogen-to-Albumin Ratio in Patients with Acute Pancreatitis Admitted to the Intensive Care Unit: A Retrospective Cohort Study. Front. Nutr. 2025, 12, 1435356. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, S. Blood Urea Nitrogen/Albumin Ratio and Mortality Risk in Patients with COVID-19. Indian J. Crit. Care Med. 2022, 26, 626–631. [Google Scholar] [CrossRef]
- Okşul, M.; Bilge, Ö.; Taştan, E.; Işık, F.; İnci, Ü.; Akın, H.; Söner, S.; Cömert, A.D.; Tüzün, R.; Çap, M.; et al. Evaluation of the Effect of Bun/Albumin Ratio on in-Hospital Mortality in Hypertensive COVID-19 Patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2127–2131. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, Q.; Shen, W.; Dong, S.; Tan, Y.; Zhang, X.; Yao, L.; Li, Q.; Qin, Z.; Wang, T. Blood Urea Nitrogen-to-Albumin Ratio Independently Predicts 30-Day Mortality in Acute Respiratory Failure Patients: A Retrospective Cohort Study. J. Thorac. Dis. 2024, 16, 4892–4903. [Google Scholar] [CrossRef]
- Dundar, Z.D.; Kucukceran, K.; Ayranci, M.K. Blood Urea Nitrogen to Albumin Ratio Is a Predictor of In-Hospital Mortality in Older Emergency Department Patients. Am. J. Emerg. Med. 2021, 46, 349–354. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, J.; Lin, J.; Xie, L.; Tang, M.; Ke, M.; Chen, L. Preoperative Blood Urea Nitrogen-to-Serum Albumin Ratio for Prediction of in-Hospital Mortality in Patients Who Underwent Emergency Surgery for Acute Type A Aortic Dissection. Hypertens. Res. 2024, 47, 1934–1942. [Google Scholar] [CrossRef]
- Li, W.; Huang, Q.; Zhan, K. Association of Serum Blood Urea Nitrogen to Albumin Ratio with In-Hospital Mortality in Patients with Acute Ischemic Stroke: A Retrospective Cohort Study of the eICU Database. Balk. Med. J. 2024, 41, 458–468. [Google Scholar] [CrossRef]
- Milas, G.P.; Issaris, V.; Papavasileiou, V. Blood Urea Nitrogen to Albumin Ratio as a Predictive Factor for Pneumonia: A Meta-Analysis. Respir. Med. Res. 2022, 81, 100886. [Google Scholar] [CrossRef]
- Feng, D.-Y.; Zhou, Y.-Q.; Zou, X.-L.; Zhou, M.; Yang, H.-L.; Chen, X.-X.; Zhang, T.-T. Elevated Blood Urea Nitrogen-to-Serum Albumin Ratio as a Factor That Negatively Affects the Mortality of Patients with Hospital-Acquired Pneumonia. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 1547405. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Hong, L.; Hou, T.; Liu, H.; Li, M.; Yang, S.; Zhang, Y. Prognostic Impact of Blood Urea Nitrogen to Albumin Ratio on Patients with Sepsis: A Retrospective Cohort Study. Sci. Rep. 2023, 13, 10013. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- López-Martín, E.; Ardura, D. El Tamaño Del Efecto En La Publicación Científica. Educ. XX1 2023, 26, 9–17. [Google Scholar] [CrossRef]
- Jamovi, version 2.6.44. [Computer Software, jamovi]. The Jamovi Project: Sydney, NSW, Australia, 2025. Available online: https://www.jamovi.org (accessed on 30 May 2025).
- Brucato, A.; Ferrari, A.; Tiraboschi, M.; Zucchi, A.; Cogliati, C.; Torzillo, D.; Dentali, F.; Tavecchia, L.; Gessi, V.; Squizzato, A.; et al. Three-Month Mortality in Permanently Bedridden Medical Non-Oncologic Patients. The BECLAP Study (Permanently BEdridden, Creatinine CLearance, Albumin, Previous Hospital Admissions Study). Eur. J. Intern. Med. 2020, 72, 60–66. [Google Scholar] [CrossRef]
- ACP (American College of Physicians). Laboratory Values. Available online: https://annualmeeting.acponline.org/sites/default/files/shared/documents/for-meeting-attendees/normal-lab-values.pdf (accessed on 30 August 2025).
- ABIM (American Board of Internal Medicine). Laboratory Tests Reference Ranges. 2025. Available online: https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf (accessed on 30 August 2025).
- American College of Clinical Pharmacy. Reference Values for Common Laboratory Tests. PSAP (Pharmacotherapy Self-Assessment Program). 2017. Available online: https://www.accp.com/docs/sap/Lab_Values_Table_PSAP.pdf (accessed on 30 August 2025).
- O’Brien, J.M.; Phillips, G.S.; Ali, N.A.; Lucarelli, M.; Marsh, C.B.; Lemeshow, S. Body Mass Index Is Independently Associated with Hospital Mortality in Mechanically Ventilated Adults with Acute Lung Injury. Crit. Care Med. 2006, 34, 738–744. [Google Scholar] [CrossRef]
- Cereda, E.; Klersy, C.; Hiesmayr, M.; Schindler, K.; Singer, P.; Laviano, A.; Caccialanza, R. Body Mass Index, Age and in-Hospital Mortality: The NutritionDay Multinational Survey. Clin. Nutr. 2017, 36, 839–847. [Google Scholar] [CrossRef]
- Yoshida, N.; Ogawa, M.; Nakai, M.; Kanaoka, K.; Sumita, Y.; Emoto, T.; Saito, Y.; Yamamoto, H.; Izawa, K.P.; Sakai, Y.; et al. Impact of Body Mass Index on In-Hospital Mortality for Six Acute Cardiovascular Diseases in Japan. Sci. Rep. 2022, 12, 18934. [Google Scholar] [CrossRef]
- Chen, J.; Perera, G.; Shetty, H.; Broadbent, M.; Xu, Y.; Stewart, R. Body Mass Index and Mortality in Patients with Schizophrenia Spectrum Disorders: A Cohort Study in a South London Catchment Area. Gen. Psychiatry 2022, 35, e100819. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Schreurs, V.; Vancampfort, D.; Van Winkel, R. Metabolic Syndrome in People with Schizophrenia: A Review. World Psychiatry 2009, 8, 15–22. [Google Scholar] [CrossRef]
- Huang, D.; Yang, H.; Yu, H.; Wang, T.; Chen, Z.; Liang, Z.; Yao, R. Blood Urea Nitrogen to Serum Albumin Ratio (BAR) Predicts Critical Illness in Patients with Coronavirus Disease 2019 (COVID-19). IJGM 2021, 14, 4711–4721. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Mandiga, P. Physiology, Plasma Osmolality and Oncotic Pressure; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gounden, V.; Vashisht, R.; Jialal, I. Hypoalbuminemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Akirov, A.; Masri-Iraqi, H.; Atamna, A.; Shimon, I. Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients. Am. J. Med. 2017, 130, 1465.e11–1465.e19. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. JCM 2019, 8, 775. [Google Scholar] [CrossRef]
- Gremese, E.; Bruno, D.; Varriano, V.; Perniola, S.; Petricca, L.; Ferraccioli, G. Serum Albumin Levels: A Biomarker to Be Repurposed in Different Disease Settings in Clinical Practice. JCM 2023, 12, 6017. [Google Scholar] [CrossRef]
- Chou, F.H.-C.; Tsai, K.-Y.; Chou, Y.-M. The Incidence and All-Cause Mortality of Pneumonia in Patients with Schizophrenia: A Nine-Year Follow-up Study. J. Psychiatr. Res. 2013, 47, 460–466. [Google Scholar] [CrossRef]
- Brookes, E.M.; Power, D.A. Elevated Serum Urea-to-Creatinine Ratio Is Associated with Adverse Inpatient Clinical Outcomes in Non-End Stage Chronic Kidney Disease. Sci. Rep. 2022, 12, 20827. [Google Scholar] [CrossRef]
- Feinfeld, D.A.; Bargouthi, H.; Niaz, Q.; Carvounis, C.P. Massive and Disproportionate Elevation of Blood Urea Nitrogen in Acute Azotemia. Int. Urol. Nephrol. 2002, 34, 143–145. [Google Scholar] [CrossRef]
- Nishioka, N.; Kobayashi, D.; Izawa, J.; Irisawa, T.; Yamada, T.; Yoshiya, K.; Park, C.; Nishimura, T.; Ishibe, T.; Kobata, H.; et al. Association between Blood Urea Nitrogen to Creatinine Ratio and Neurologically Favourable Outcomes in Out-of-Hospital Cardiac Arrest in Adults: A Multicentre Cohort Study. J. Cardiol. 2023, 81, 397–403. [Google Scholar] [CrossRef]
- Casas Aparicio, G.; Fernández Plata, R.; Higuera Iglesias, A.; Martínez Briseño, D.; Claure-Del Granado, R.; Castillejos Lopez, M.; Vázquez Pérez, J.; Alvarado Vásquez, N.; Velázquez Cruz, R.; Hernández Silva, G.; et al. Clinical Implications of Persistently Increased Blood Urea Nitrogen/Serum Creatinine Ratio (PI-BUN/Cr) in Severe COVID-19 Patients. Pneumonia 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative Stress and Frailty: A Systematic Review and Synthesis of the Best Evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kamoi, R.; Mifune, Y.; Soriano, K.; Tanioka, R.; Yamanaka, R.; Ito, H.; Osaka, K.; Umehara, H.; Shimomoto, R.; Bollos, L.A.; et al. Association Between Dynapenia/Sarcopenia, Extrapyramidal Symptoms, Negative Symptoms, Body Composition, and Nutritional Status in Patients with Chronic Schizophrenia. Healthcare 2024, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Y.; Cai, Y.; Zhu, Z.H.; Zhai, C.P.; Li, J.; Ji, C.F.; Chen, P.; Wang, J.; Wu, Y.M.; Chan, R.C.K.; et al. Associations of Decreased Serum Total Protein, Albumin, and Globulin with Depressive Severity of Schizophrenia. Front. Psychiatry 2022, 13, 957671. [Google Scholar] [CrossRef]
- Papatriantafyllou, E.; Efthymiou, D.; Markopoulou, M.; Sakellariou, E.-M.; Vassilopoulou, E. The Effects of Use of Long-Term Second-Generation Antipsychotics on Liver and Kidney Function: A Prospective Study. Diseases 2022, 10, 48. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, H.; Jiang, X.; Mai, Q. The Effect of Several Commonly Used Antipsychotic Drugs on the Renal Function of Patients with Mental Illness. Nat. Sci. 2022, 14, 19–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).