Dysregulated Neuroimmune and Anhedonia-like Behavioral Response Following Peripheral Immune Challenge in Mice Carrying the Val66Met Brain-Derived Neurotrophic Factor Polymorphism
Abstract
1. Introduction
2. Methods and Materials
2.1. Animals
2.2. Genotyping
2.3. Experimental Timeline and Treatments
2.4. Behavior Testing
2.5. Tissue Preparation and RT-qPCR
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Statistical Analysis
3. Results
3.1. BDNF Val66Met Expression Results in Susceptibility to the Anhedonia-like Behavioral Effects of Immune Challenge
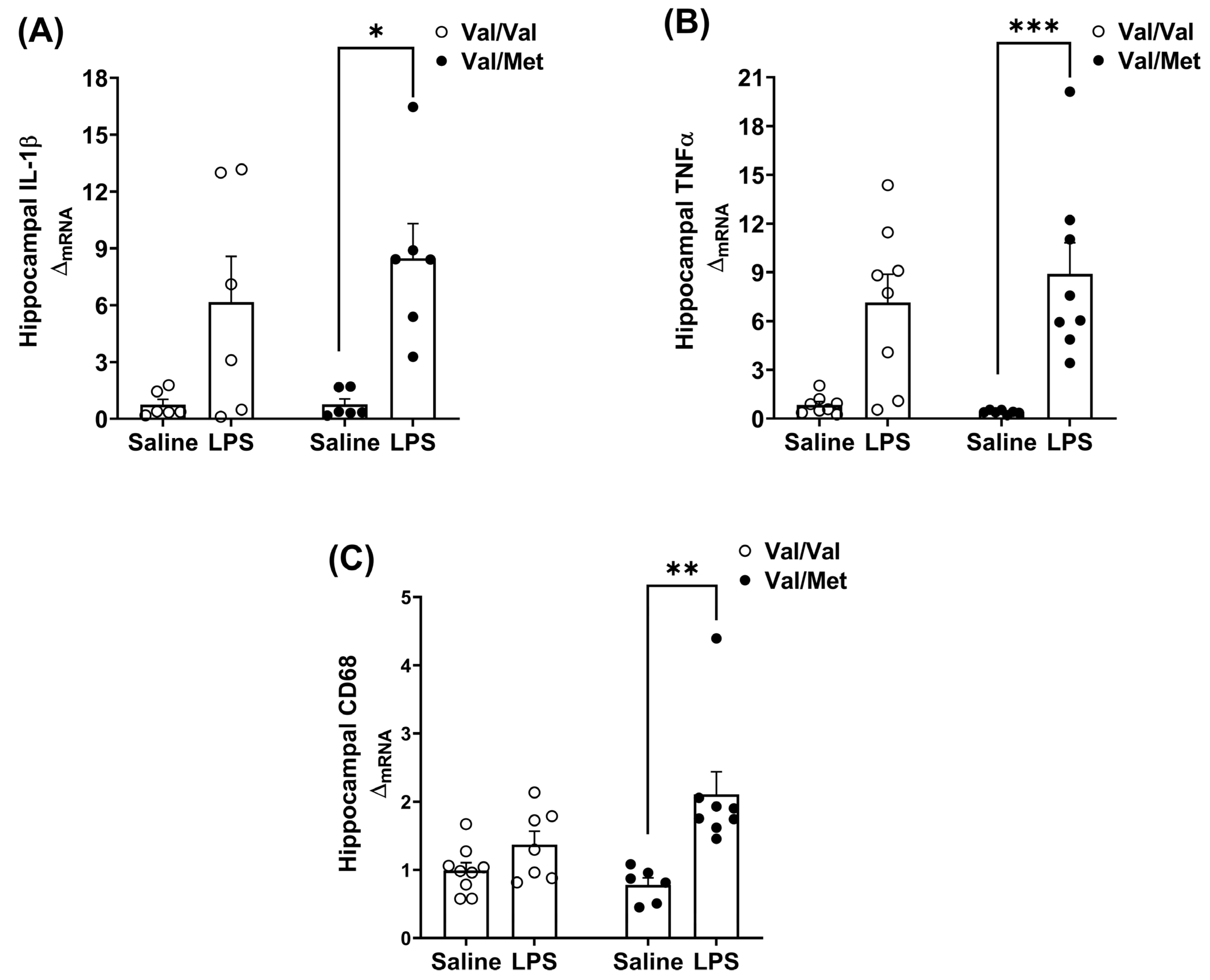
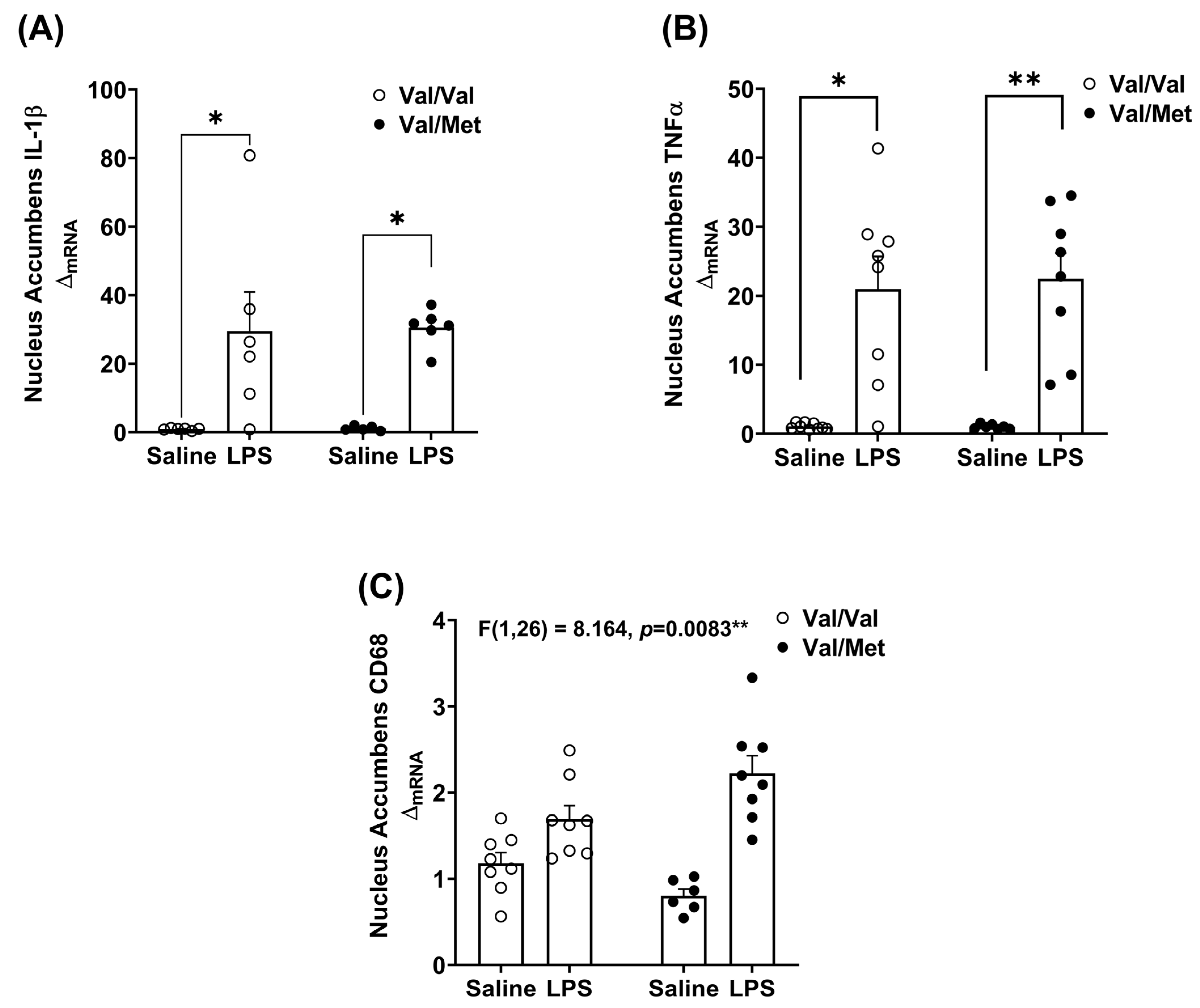
3.2. Immune Challenge Induces Dysregulated Whole-Brain Pro-Inflammatory Cytokine Expression in BDNF Val66Met Mice
3.3. BDNF Val66Met Mutation Differentially Affects Brain Regions in Response to LPS
4. Discussion
- Main Point
- The Val66Met genotype did not affect peripheral inflammatory response, acute neuroinflammation, or acute sickness behavior response to lipopolysaccharide.
- The Val66Met genotype exacerbates LPS-induced anhedonia-like behavior and the neuroinflammatory response.
- BDNF Val66Met polymorphism may disrupt negative regulation of inflammation following peripheral immune challenge.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, M.J. Depression is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Bauer, M.; Carvalho, A.F.; Eyre, H.; Fava, M.; Kasper, S.; Kennedy, S.H.; Khoo, J.P.; Lopez Jaramillo, C.; Malhi, G.S.; et al. A clinical approach to treatment resistance in depressed patients: What to do when the usual treatments don’t work well enough? World J. Biol. Psychiatry 2021, 22, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Haroon, E.; Miller, A.H. Risk and Resilience: Animal Models Shed Light on the Pivotal Role of Inflammation in Individual Differences in Stress-Induced Depression. Biol. Psychiatry 2015, 78, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, D.; Kivimäki, M.; Brunner, E.J.; Elovainio, M.; De Vogli, R.; Steptoe, A.; Kumari, M.; Lowe, G.D.O.; Rumley, A.; Marmot, M.G.; et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009, 39, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.A.; Beurel, E.; Loewenstein, D.A.; Lowell, J.A.; Craighead, W.E.; Dunlop, B.W.; Mayberg, H.S.; Dhabhar, F.; Dietrich, W.D.; Keane, R.W.; et al. Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron 2018, 99, 914–924.e913. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.; Jokela, M.; Batty, G.D.; Cadar, D.; Steptoe, A.; Kivimaki, M. Association Between Systemic Inflammation and Individual Symptoms of Depression: A Pooled Analysis of 15 Population-Based Cohort Studies. Am. J. Psychiatry 2021, 178, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Kim, Y.-K. The Roles of BDNF in the Pathophysiology of Major Depression and in Antidepressant Treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; Bus, B.A.A.; Spinhoven, P.; Penninx, B.W.J.H.; Kenis, G.; Prickaerts, J.; Voshaar, R.C.O.; Elzinga, B.M. Serum levels of brain-derived neurotrophic factor in major depressive disorder: State–trait issues, clinical features and pharmacological treatment. Mol. Psychiatry 2011, 16, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.N.; Ren, X.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Dwivedi, Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int. J. Neuropsychopharmacol. 2008, 11, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Shelton, R.C.; Dwivedi, Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J. Psychiatr. Res. 2017, 89, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bus, B.A.; Molendijk, M.L.; Tendolkar, I.; Penninx, B.W.; Prickaerts, J.; Elzinga, B.M.; Voshaar, R.C. Chronic depression is associated with a pronounced decrease in serum brain-derived neurotrophic factor over time. Mol. Psychiatry 2015, 20, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Castren, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.Y.; Ruan, C.S.; Yang, C.R.; Li, J.Y.; Kang, Z.L.; Zhou, L.; Liu, D.; Zeng, Y.Q.; Wang, T.H.; Tian, C.F.; et al. ProBDNF Signaling Regulates Depression-Like Behaviors in Rodents under Chronic Stress. Neuropsychopharmacology 2016, 41, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Alboni, S.; van Dijk, R.M.; Poggini, S.; Milior, G.; Perrotta, M.; Drenth, T.; Brunello, N.; Wolfer, D.P.; Limatola, C.; Amrein, I.; et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol. Psychiatry 2017, 22, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.H.; Schlesinger, L.; Kodama, M.; Russell, D.S.; Duman, R.S. A Role for MAP Kinase Signaling in Behavioral Models of Depression and Antidepressant Treatment. Biol. Psychiatry 2007, 61, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Dugan, A.M.; Parrott, J.M.; Redus, L.; Hensler, J.G.; O’Connor, J.C. Low-Level Stress Induces Production of Neuroprotective Factors in Wild-Type but Not BDNF+/−Mice: Interleukin-10 and Kynurenic Acid. Int. J. Neuropsychopharmacol. 2016, 19, pyv089. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.M.; Porter, G.A.; Redus, L.; O’Connor, J.C. Brain derived neurotrophic factor deficiency exacerbates inflammation-induced anhedonia in mice. Psychoneuroendocrinology 2021, 134, 105404. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. The Correlation between Plasma Brain-derived Neurotrophic Factor and Cognitive Function in Bipolar Disorder is Modulated by the BDNF Val66Met Polymorphism. Eur. Psychiatry 2017, 41, S76. [Google Scholar] [CrossRef][Green Version]
- Soliman, F.; Glatt, C.E.; Bath, K.G.; Levita, L.; Jones, R.M.; Pattwell, S.S.; Jing, D.; Tottenham, N.; Amso, D.; Somerville, L.H.; et al. A Genetic Variant BDNF Polymorphism Alters Extinction Learning in Both Mouse and Human. Science 2010, 327, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J. Biol. Chem. 2014, 5, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.-J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic Variant BDNF (Val66Met) Polymorphism Alters Anxiety-Related Behavior. Science 2006, 314, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Hallmayer, J.; Wang, P.W.; Hill, S.J.; Johnson, S.L.; Ketter, T.A. Brain-derived neurotrophic factor val66met genotype and early life stress effects upon bipolar course. J. Psychiatr. Res. 2013, 47, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; De Kloet, E.R.; Yehuda, R.; Malaspina, D.; Kranz, T.M. Early Life Stress Effects on Glucocorticoid-BDNF Interplay in the Hippocampus. Front. Mol. Neurosci. 2015, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, L.; Yang, J.; Han, D.; Fang, D.; Qiu, X.; Yang, X.; Qiao, Z.; Ma, J.; Wang, L.; et al. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J. Affect. Disord. 2018, 227, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Caldieraro, M.A.; McKee, M.; Leistner-Segal, S.; Vares, E.A.; Kubaski, F.; Spanemberg, L.; Brusius-Facchin, A.C.; Fleck, M.P.; Mischoulon, D. Val66Met polymorphism association with serum BDNF and inflammatory biomarkers in major depression. World J. Biol. Psychiatry 2018, 19, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Lotrich, F.E.; Albusaysi, S.; Ferrell, R.E. Brain-Derived Neurotrophic Factor Serum Levels and Genotype: Association with Depression during Interferon-α Treatment. Neuropsychopharmacology 2013, 38, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Dooley, L.N.; Ganz, P.A.; Cole, S.W.; Crespi, C.M.; Bower, J.E. Val66Met BDNF polymorphism as a vulnerability factor for inflammation-associated depressive symptoms in women with breast cancer. J. Affect. Disord. 2016, 197, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, J.S.; Castren, E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front. Behav. Neurosci. 2014, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.M.; Redus, L.; Santana-Coelho, D.; Morales, J.; Gao, X.; O’Connor, J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 2016, 6, e918. [Google Scholar] [CrossRef] [PubMed]
- Laumet, G.; Zhou, W.; Dantzer, R.; Edralin, J.D.; Huo, X.; Budac, D.P.; O’Connor, J.C.; Lee, A.W.; Heijnen, C.J.; Kavelaars, A. Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain Behav. Immun. 2017, 66, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Connor, J.C.; Hartman, M.E.; Tapping, R.I.; Freund, G.G. Acute Hypoxia Activates the Neuroimmune System, Which Diabetes Exacerbates. J. Neurosci. 2007, 27, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Sarnyai, Z.; Sibille, E.L.; Pavlides, C.; Fenster, R.J.; McEwen, B.S.; Tóth, M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin 1A receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 14731–14736. [Google Scholar] [CrossRef] [PubMed]
- Bussey, T.J.; Padain, T.L.; Skillings, E.A.; Winters, B.D.; Morton, A.J.; Saksida, L.M. The touchscreen cognitive testing method for rodents: How to get the best out of your rat. Learn. Mem. 2008, 15, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.M.; O’Connor, J.C. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav. Immun. 2015, 50, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Depression, antidepressants, and the shrinking hippocampus. Proc. Natl. Acad. Sci. USA 2001, 98, 12320–12322. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, M.; Russo, S.J. Anhedonia and the Brain Reward Circuitry in Depression. Curr. Behav. Neurosci. Rep. 2015, 2, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, Y.; Chaki, S. Neurochemistry of the Nucleus Accumbens and its Relevance to Depression and Antidepressant Action in Rodents. Curr. Neuropharmacol. 2006, 4, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Kiraly, D.D.; Howell, J.L.; Olausson, P.; Taylor, J.R. Acute Hippocampal Brain-Derived Neurotrophic Factor Restores Motivational and Forced Swim Performance After Corticosterone. Biol. Psychiatry 2008, 64, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Gyekis, J.P.; Yu, W.; Dong, S.; Wang, H.; Qian, J.; Kota, P.; Yang, J. No association of genetic variants in BDNF with major depression: A meta- and gene-based analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162B, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chang, H.; Xiao, X. BDNF Val66Met polymorphism and bipolar disorder in European populations: A risk association in case-control, family-based and GWAS studies. Neurosci. Biobehav. Rev. 2016, 68, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, D.D.; Wang, Y.; Liu, T.; Lee, F.S.; Chen, Z.Y. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J. Neurosci. 2012, 32, 4092–4101. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; McGue, M. Interacting effect of BDNF Val66Met polymorphism and stressful life events on adolescent depression. Genes. Brain Behav. 2012, 11, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Notaras, M.; Du, X.; Gogos, J.; van den Buuse, M.; Hill, R.A. The BDNF Val66Met polymorphism regulates glucocorticoid-induced corticohippocampal remodeling and behavioral despair. Transl. Psychiatry 2017, 7, e1233. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Miller, A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Miller, A.H. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Front. Neuroendocr. 2012, 33, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Bekhbat, M.; Treadway, M.T.; Felger, J.C. Inflammation as a Pathophysiologic Pathway to Anhedonia: Mechanisms and Therapeutic Implications. Curr. Top. Behav. Neurosci. 2022, 58, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, T.; Cai, J.; Fu, X.; Li, H.; Wu, J. Systemic inflammatory regulators and 7 major psychiatric disorders: A two-sample Mendelian randomization study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110534. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Gutierrez, E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994, 179, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Relton, J.K.; Dripps, D.; Kiechle, R.; Tartaglia, N.; Maier, S.F.; Watkins, L.R. Vagal Paraganglia Bind Biotinylated Interleukin-1 Receptor Antagonist: A Possible Mechanism for Immune-to-Brain Communication. Brain Res. Bull. 1997, 43, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2014, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Reader, B.F.; Jarrett, B.L.; McKim, D.B.; Wohleb, E.S.; Godbout, J.P.; Sheridan, J.F. Peripheral and central effects of repeated social defeat stress: Monocyte trafficking, microglial activation, and anxiety. Neuroscience 2015, 289, 429–442. [Google Scholar] [CrossRef] [PubMed]
- McKim, D.B.; Yin, W.; Wang, Y.; Cole, S.W.; Godbout, J.P.; Sheridan, J.F. Social Stress Mobilizes Hematopoietic Stem Cells to Establish Persistent Splenic Myelopoiesis. Cell Rep. 2018, 25, 2552–2562.e3. [Google Scholar] [CrossRef] [PubMed]
- McKim, D.B.; Weber, M.D.; Niraula, A.; Sawicki, C.M.; Liu, X.; Jarrett, B.L.; Ramirez-Chan, K.; Wang, Y.; Roeth, R.M.; Sucaldito, A.D.; et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 2018, 23, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Gaire, S.; An, J.; Yang, H.; Lee, K.A.; Dumre, M.; Lee, E.J.; Park, S.M.; Joe, E.H. Systemic inflammation attenuates the repair of damaged brains through reduced phagocytic activity of monocytes infiltrating the brain. Mol. Brain 2024, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Cazareth, J.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: Importance of CCR2/CCL2 signaling. J. Neuroinflamm. 2014, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Bodea, L.G.; Wang, Y.; Linnartz-Gerlach, B.; Kopatz, J.; Sinkkonen, L.; Musgrove, R.; Kaoma, T.; Muller, A.; Vallar, L.; Di Monte, D.A.; et al. Neurodegeneration by activation of the microglial complement-phagosome pathway. J. Neurosci. 2014, 34, 8546–8556. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.G.; Hong, J.J.; Lee, Y.; Yi, K.S.; Jeon, C.Y.; Park, J.; Won, J.; Seo, J.; Ahn, Y.J.; Kim, K.; et al. Increased CD68/TGFbeta Co-expressing Microglia/Macrophages after Transient Middle Cerebral Artery Occlusion in Rhesus Monkeys. Exp. Neurobiol. 2019, 28, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Zhang, Y.; Tan, S.; Sun, J.; Ye, M.; Gao, H.; Pu, K.; Wu, M.; Wang, Q.; Zhai, Q. Therapeutic targeting of STING-TBK1-IRF3 signalling ameliorates chronic stress induced depression-like behaviours by modulating neuroinflammation and microglia phagocytosis. Neurobiol. Dis. 2022, 169, 105739. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Fan, Y.; Chung, C.Y. Mefenamic acid can attenuate depressive symptoms by suppressing microglia activation induced upon chronic stress. Brain Res. 2020, 1740, 146846. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Haddad, Y.; Yun, H.J.; Geng, X.; Ding, Y. Induced Inflammatory and Oxidative Markers in Cerebral Microvasculature by Mentally Depressive Stress. Mediat. Inflamm. 2023, 2023, 4206316. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, L.; Castiglioni, L.; Amadio, P.; Werba, J.P.; Eligini, S.; Fiorelli, S.; Zara, M.; Castiglioni, S.; Bellosta, S.; Lee, F.S.; et al. Impact of BDNF Val66Met Polymorphism on Myocardial Infarction: Exploring the Macrophage Phenotype. Cells 2020, 9, 1084. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.D.; Rubin, T.G.; Kogan, J.F.; Marrocco, J.; Weidmann, J.; Lindkvist, S.; Lee, F.S.; Schmidt, E.F.; McEwen, B.S. Translational profiling of stress-induced neuroplasticity in the CA3 pyramidal neurons of BDNF Val66Met mice. Mol. Psychiatry 2018, 23, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Lee, F.S.; Ninan, I. The BDNF Val66Met polymorphism enhances glutamatergic transmission but diminishes activity-dependent synaptic plasticity in the dorsolateral striatum. Neuropharmacology 2017, 112, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Huwart, S.J.P.; Fayt, C.; Gangarossa, G.; Luquet, S.; Cani, P.D.; Everard, A. TLR4-dependent neuroinflammation mediates LPS-driven food-reward alterations during high-fat exposure. J. Neuroinflamm. 2024, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lai, S.; Zhou, T.; Xia, Z.; Li, W.; Sha, W.; Liu, J.; Chen, Y. Progranulin from different gliocytes in the nucleus accumbens exerts distinct roles in FTD- and neuroinflammation-induced depression-like behaviors. J. Neuroinflamm. 2022, 19, 318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lai, S.; Wang, R.; Zhou, T.; Dong, N.; Zhu, L.; Chen, T.; Zhang, X.; Chen, Y. Dopamine D3 receptor in the nucleus accumbens alleviates neuroinflammation in a mouse model of depressive-like behavior. Brain Behav. Immun. 2022, 101, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.F.; Seligman, M.E. Learned helplessness at fifty: Insights from neuroscience. Psychol. Rev. 2016, 123, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Dincheva, I.; Yang, J.; Li, A.; Marinic, T.; Freilingsdorf, H.; Huang, C.; Casey, B.J.; Hempstead, B.; Glatt, C.E.; Lee, F.S.; et al. Effect of Early-Life Fluoxetine on Anxiety-Like Behaviors in BDNF Val66Met Mice. Am. J. Psychiatry 2017, 174, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Jing, D.Q.; Dincheva, I.; Neeb, C.C.; Pattwell, S.S.; Chao, M.V.; Lee, F.S.; Ninan, I. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology 2012, 37, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Qi, Y.; Hou, D.N.; Ji, Y.Y.; Zheng, C.Y.; Li, C.Y.; Yung, W.H.; Lu, B.; Huang, Y. BDNF val66met Polymorphism Impairs Hippocampal Long-Term Depression by Down-Regulation of 5-HT3 Receptors. Front. Cell Neurosci. 2017, 11, 306. [Google Scholar] [CrossRef]
- Mallei, A.; Ieraci, A.; Corna, S.; Tardito, D.; Lee, F.S.; Popoli, M. Global epigenetic analysis of BDNF Val66Met mice hippocampus reveals changes in dendrite and spine remodeling genes. Hippocampus 2018, 28, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Ninan, I.; Bath, K.G.; Dagar, K.; Perez-Castro, R.; Plummer, M.R.; Lee, F.S.; Chao, M.V. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci. 2010, 30, 8866–8870. [Google Scholar] [CrossRef] [PubMed]
- Reichenberg, A.; Yirmiya, R.; Schuld, A.; Kraus, T.; Haack, M.; Morag, A.; Pollmächer, T. Cytokine-Associated Emotional and Cognitive Disturbances in Humans. Arch. Gen. Psychiatry 2001, 58, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Mac Giollabhui, N.; Ng, T.H.; Ellman, L.M.; Alloy, L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Mol. Psychiatry 2021, 26, 3302–3314. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, W.A.; van Hemert, A.M.; Carlier, I.V.E.; Penninx, B.; Lamers, F.; Fried, E.I.; Schoevers, R.; Giltay, E.J. Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Transl. Psychiatry 2020, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 59, e3638. [Google Scholar] [CrossRef]
- Bogdanova, O.V.; Kanekar, S.; D’Anci, K.E.; Renshaw, P.F. Factors influencing behavior in the forced swim test. Physiol. Behav. 2013, 118, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Anyan, J.; Amir, S. Too Depressed to Swim or Too Afraid to Stop? A Reinterpretation of the Forced Swim Test as a Measure of Anxiety-Like Behavior. Neuropsychopharmacology 2018, 43, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Montag, C.; Basten, U.; Stelzel, C.; Fiebach, C.J.; Reuter, M. The BDNF Val66Met polymorphism and anxiety: Support for animal knock-in studies from a genetic association study in humans. Psychiatry Res. 2010, 179, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Negron, M.; Kristensen, J.; Nguyen, V.T.; Gansereit, L.E.; Raucci, F.J.; Chariker, J.L.; Heck, A.; Brula, I.; Kitchen, G.; Awgulewitsch, C.P.; et al. Sex-Based Differences in Cardiac Gene Expression and Function in BDNF Val66Met Mice. Int. J. Mol. Sci. 2021, 22, 7002. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Chuang, J.; Spencer-Segal, J.L.; Amso, D.; Altemus, M.; McEwen, B.S.; Lee, F.S. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol. Psychiatry 2012, 72, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]



| Val/Val | Val/Met | Main Effects | Effect Size | ||||
|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | ||||
| Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | Genotype p-Value | Treatment p-Value | η2p | |
| 2 h | |||||||
| IL-1β | 0.152 (0.152) | 7.596 (6.078) | 0.543 (0.543) | 4.381 (4.381) | 0.2413 | 0.7630 | na |
| IL-6 | 1.379 (0.939) | 195.172 (0.0) | 1.500 (0.815) | 152.397 (42.776) | 0.3461 | <0.0001 **** | 0.839 |
| TNFα | 0.783 (0.783) | 73.288 (19.506) | 11.628 (11.533) | 40.409 (23.830) | 0.5309 | 0.0111 * | 0.402 |
| 24 h | |||||||
| IL-1β | 2.841 (0.998) | 2.315 (1.037) | 3.460 (1.042) | 4.678 (1.903) | 0.8070 | 0.2981 | na |
| IL-6 | 0.539 (0.173) | 72.034 (27.041) | 0.956 (0.620) | 75.048 (17.211) | 0.9191 | 0.0001 *** | 0.350 |
| TNFα | 1.296 (0.746) | 3.292 (1.370) | 0.543 (0.543) | 4.211 (0.644) | 0.9292 | 0.0064 ** | 0.345 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mithaiwala, M.N.; Dugan, A.M.; de la Flor, M.A.; Subramanian, S.K.; Acheson, A.; O’Connor, J.C. Dysregulated Neuroimmune and Anhedonia-like Behavioral Response Following Peripheral Immune Challenge in Mice Carrying the Val66Met Brain-Derived Neurotrophic Factor Polymorphism. Psychiatry Int. 2025, 6, 87. https://doi.org/10.3390/psychiatryint6030087
Mithaiwala MN, Dugan AM, de la Flor MA, Subramanian SK, Acheson A, O’Connor JC. Dysregulated Neuroimmune and Anhedonia-like Behavioral Response Following Peripheral Immune Challenge in Mice Carrying the Val66Met Brain-Derived Neurotrophic Factor Polymorphism. Psychiatry International. 2025; 6(3):87. https://doi.org/10.3390/psychiatryint6030087
Chicago/Turabian StyleMithaiwala, Mustafa N., Allison M. Dugan, Miguel A. de la Flor, Sandeep K. Subramanian, Ashley Acheson, and Jason C. O’Connor. 2025. "Dysregulated Neuroimmune and Anhedonia-like Behavioral Response Following Peripheral Immune Challenge in Mice Carrying the Val66Met Brain-Derived Neurotrophic Factor Polymorphism" Psychiatry International 6, no. 3: 87. https://doi.org/10.3390/psychiatryint6030087
APA StyleMithaiwala, M. N., Dugan, A. M., de la Flor, M. A., Subramanian, S. K., Acheson, A., & O’Connor, J. C. (2025). Dysregulated Neuroimmune and Anhedonia-like Behavioral Response Following Peripheral Immune Challenge in Mice Carrying the Val66Met Brain-Derived Neurotrophic Factor Polymorphism. Psychiatry International, 6(3), 87. https://doi.org/10.3390/psychiatryint6030087










