1. Introduction
Depression is a widespread mental disorder that can cause sadness, loss of interest, guilt, sleep and appetite disturbances, fatigue, and difficulty concentrating. It can impair one’s ability to function and can lead to suicide. Mild cases can be treated without medication, but moderate to severe cases require medication and therapy. Depression can be diagnosed and treated by non-specialists as part of primary healthcare [
1].
In addition to being a significant contributor to disability and early death, depression is a significant global public health issue [
2]. The Global Burden of Disease report found that depression is more prevalent in women than in men, with rates expected to rise significantly by 2030, and becoming the second leading cause of disability-adjusted life years (DALYs) after ischemic heart disease. Currently, depression affects 1.9% of men and 3.2% of women, with one-year prevalence rates of 5.8% for males and 9.5% for females [
3]. In Nepal, a study reported a crude prevalence of depression at 11.7% [
4]. Meanwhile, the incidence rate of adverse drug reactions (ADR) caused by antidepressant medications was found to be 4.54% [
5]. However, the prevalence of ADRs associated with antidepressants in Nepal has not been assessed yet. These findings highlight the significant burden of depression in the country and the potential risks associated with antidepressant use.
Antidepressants are prescribed to treat various mental health conditions, including major depressive disorder, dysthymia, anxiety disorders, obsessive compulsive disorder, eating disorders, ADHD, addiction, dependence, and sleep disorders, as well as physical conditions such as chronic pain, neuropathic pain, and migraines. These medications can be prescribed alone or in combination with other drugs [
6].
Adverse drug reactions (ADRs) are defined by the World Health Organization (WHO) as unexpected and harmful responses that occur at normal therapeutic doses of drugs used for prophylaxis, diagnosis, or treatment of disease, or for the modification of physiological function [
7]. To address adverse drug reactions (ADRs), preventive measures, targeted therapies, adjustments to dosage schedules, and potentially discontinuing the medication may be necessary. It is important to note that nearly all medications have some degree of side effects or negative consequences in addition to their intended therapeutic effects [
8]. Patients with depression are known to have a higher incidence of adverse drug reactions (ADRs) related to antidepressant medications. These ADRs can significantly impact medication adherence and contribute to treatment discontinuation [
9]. Adverse drug reactions (ADRs) can significantly decrease people’s quality of life, leading to poor adherence to antidepressant medications. This can result in longer hospital stays, increased healthcare costs, reduced treatment outcomes, physical morbidity, social stigma, and, in severe cases, even death [
10]. The incidence of adverse drug reactions (ADRs) leading to hospitalization is reported to range from 0.2% to 41.3%. As a result, healthcare costs have risen by 5–10% [
11].
Adverse effects of antidepressant drugs based on mechanism of action [12] Various literature and real world data have provided insights into the adverse effects of antidepressant drugs based on their mechanisms of action. These adverse effects can vary depending on the specific pharmacological actions of the medications. Here, we summarize some of these effects categorized by their respective mechanisms of action.
| Antidepressant Drug | Adverse Effect |
| Norepinephrine transporter blockade: | Anxiety, diaphoresis, tachycardia, tremor. |
| Serotonin reuptake inhibition: | Anorexia early in the treatment and weight gain later, anxiety, ejaculatory disturbances, decreased libido, nausea and vomiting, diarrhea, sedation, insomnia, serotonin syndrome. |
| Dopamine reuptake inhibition: | Psychosis, Parkinsonism, psychomotor activation. |
| Alpha-1 adrenergic receptor blockade: | Postural hypotension, dizziness, antihypertensive effect, reflex tachycardia. |
| Dopamine D2 receptor blockade: | Dystonia, Parkinsonism, prolactin elevation. |
| Histamine H1 receptor blockade: | Drowsiness, orthostatic hypotension, sedation, weight gain. |
| Muscarinic acetylcholine receptor blockade | Blurred vision, memory impairment, delirium, dry mouth, constipation, urinary retention. |
Monitoring for adverse drug reactions (ADRs) is critical in a hospital setting as it helps to identify the nature and types of ADRs and pinpoint patients who are at a higher risk of experiencing ADRs [
13]. Adverse drug reaction (ADR) monitoring is less prevalent in developing countries, such as India, where the rate is reported to be below 1%, compared to 5% in developed nations. This highlights the need for improved pharmacovigilance systems and increased awareness of ADRs in developing countries [
14]. There is a research gap in Nepal regarding the adverse drug profiles of adult patients using antidepressants, with a particular focus on adverse effects and depressed patients. This study aims to fill this gap and provide a better understanding of the factors associated with adverse drug profiles. It will also serve as a baseline for future studies and aid in developing appropriate strategies, interventions, and health education regarding depression and reducing adverse effects in adult Nepalese patients. Additionally, this study will highlight the importance of clinical pharmacists in ADR monitoring and reporting, as well as strengthening Nepal’s pharmacovigilance system.
Research Questions
- (i)
What is the existing profile among adult patients using antidepressant drugs?
- (ii)
Which is the most frequently used antidepressant drug?
- (iii)
How does the adverse profile differ in monotherapy and multiple therapy?
- (iv)
Is there any association between adverse effects and the mechanism of action of the drug?
- (v)
How common is the prescription of other drugs along with antidepressants in the management of depression and other mental illnesses?
2. Objectives
The main objective of this study is to investigate the adverse drug profile in adult patients using antidepressants in the outpatient department (OPD) of Chitwan Medical College Teaching Hospital in Chitwan, Nepal. Additionally, the study aims to determine the demographics of patients who visit the OPD and whether they receive single or multiple therapies. The study also seeks to identify the factors contributing to depression and the most frequently prescribed antidepressants at the hospital. By accomplishing these objectives, the study will contribute to a better understanding of the use of antidepressants in this setting and inform the development of appropriate strategies to improve patient outcomes.
3. Patients and Methods
3.1. Study Design
A retro-prospective observational study design was used to determine the adverse drug profile and its associated factors among adult patients using antidepressants.
3.2. Study Site/Study Population/Study Unit
This study was conducted at the Chitwan Medical College teaching hospital, Chitwan, Nepal, where various ethnic groups of depressive patients reside. The research sample consisted of 117 adult patients who had been taking antidepressant medication for at least 2 weeks and were above the age of 20.
3.3. Sampling Technique
The Chitwan Medical College Teaching Hospital’s OPD in Chitwan District was purposefully chosen for the study. The required number of samples was chosen using simple random sampling.
3.4. Data Collection Procedure
The study specifics were explained to every patient who arrived at the OPD, as well as any caregivers, and their verbal agreement was collected.
The patient’s OPD card was used to collect the data, together with communications with the doctor, the patient, and lab results.
Age, gender, prior medical history, prescribed medications (single or multiple), height, weight, sleep habits, state of hunger, headaches, and other details were all collected; information was consistently captured, facilitating data analysis and comparisons across different patients or cases.
3.5. Data Management and Analysis
Data compiling, checking, and editing were performed manually. Any necessary editions immediately after data collection were performed. Data entry and analysis were carried out using IBM SPSS version 20 (IBM corporation, Armonk, NY, USA) and Microsoft Office Excel 2007.
3.6. Operational Definitions of Variables
Educational Status: Educational status will be categorized as shown below.
- (i)
Illiterate: Those who cannot read and write their name;
- (ii)
Literate: Those who can only read and write their name;
- (iii)
Primary level: Those who have completed grade 8;
- (iv)
Secondary level: Those who have completed SLC;
- (v)
Higher secondary level: Those who have completed high school or a +2 level, or equivalent;
- (vi)
Graduated: Those who have completed an undergraduate or postgraduate degree, or equivalent.
3.7. Variables of Interests
Dependent variables
Associated factors of antidepressants.
A patient under treatment with an antidepressant drug.
Independent variables
Age, sex, family income, family history related to depression, psychiatric illness, duration of drug use, duration of illness, blood pressure, ethnicity, religion, type of family, and education of respondent.
3.8. Inclusion and Exclusion Inclusion
Inclusion Criteria:
Exclusion Criteria:
Adult patients aged below 20 years;
The patient has a condition other than depression;
The patient uses an antipsychotic drug.
3.9. Ethical Consideration
This study was conducted after obtaining ethical clearance from the institutional review committee (IRC) of Chitwan Medical College (reference number: CMC-IRC-2073/074:96). Permission from CMC Hospital was obtained before starting the data collection.
The information obtained from the respondents was kept confidential and privacy was maintained.
4. Results
4.1. Socio-Demographic Characteristics of the Respondents: Age, Education Level, Sex, Occupation, Religion, and Ethnic Group
The socio-demographic characteristics of the respondents are shown in
Table 1. The table shows that out of 117 respondents, most of the respondents, 43 (36.8%), were from the age group 26–40, followed by 42 (35.9%) from the age group 41–55, and 19 (16.2%) from age group less than or equal to 25.
Most respondents, 25 (21.4%), were literate followed by 24 (20.5%) literate, 22 (18.8%) with a secondary level, and 20 (17.1%) with a primary level. Regarding sex, 43 (36.7%) were male and 74 (63.2%) were female. Regarding occupation, most were housewives, 54 (46.2%), followed by those with a job, agricultural workers, and students. Related to religion, most of them were Hindus, 85 (72.7%), followed by Christians and Buddhists. Regarding ethnicity, the most common were Chhetris/Brahmins, 82 (72.6%), followed by Dalits, and Janajatis.
4.2. Socio-Demographic Characteristics of the Respondents: Family Income, Type of Family, and Family History Related to Depression
The socio-demographic characteristics of the respondents are shown in
Table 2. Most of the families had an income of more than Rs.100000 or 762.12 USD/years, 64 (54.7%), followed by 53 (45.29%) with less. Regarding family type, most of them were from a nuclear family, 56 (47.9%), followed by 47 (40.2%) from extended and 14 (12%) from joint family. Regarding family history, 86 (73.5%) had no history of depression and 31 (26.5%) had a history of depression.
4.3. Characteristics of the Population: Weight, Height, BMI, BPS, and BPD
The characteristics of the population are shown in
Table 3.
The mean ± SD of weight was 62.1 ± 11.924, height was 156.3 ± 9.047, BMI was 25.4 ± 4.316, BPS was 118.8 ± 15.874, and BPD was 80.9 ± 10.815.
4.4. Duration of Drug Use and Duration of Illness
The median duration of illness was 24 months (range: 1–228 months) and the median duration of drug use was 12 months (range: 0.5–144 months) (
Table 4).
4.5. Respondent Characteristics Regarding Disease Distribution, Drug Use Other than Antidepressants, and Type of Drug Therapy
The
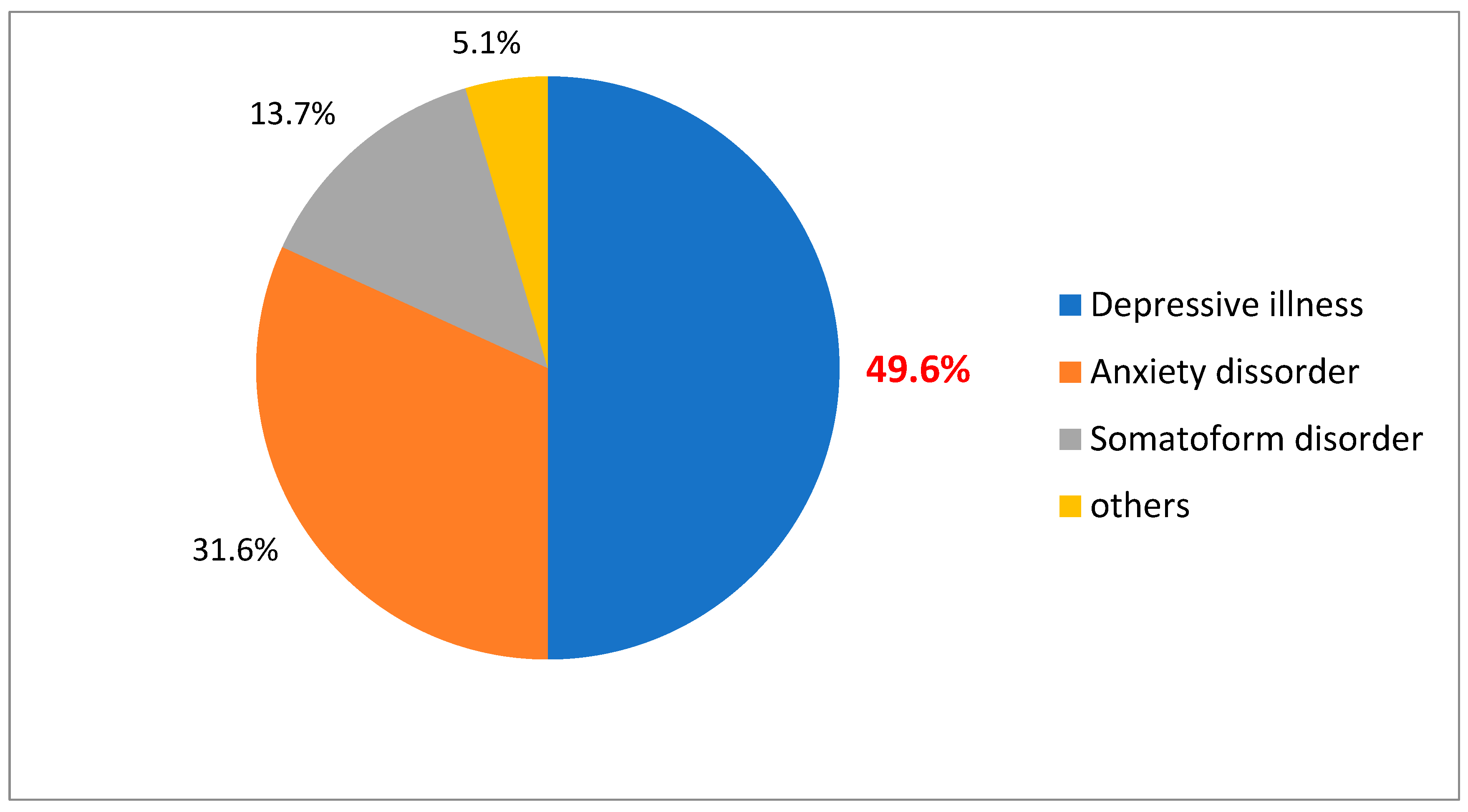
Table 5 shows that out of 117 respondents, most of them had depressive illness. 58 (49.6%), followed by anxiety disorder, somatoform disorder, and other categories of mental illness (
Figure 1).
Concerning drug use other than antidepressants, 21/117 (17.9%) were only using antidepressants; the remaining 96 (82.2%) had other drugs in their treatment plan in addition to antidepressants.
Regarding to type of drug therapy, most of the respondents were using monotherapy, 75 (64.1%), 35 (29.9%) were under multiple therapies, and 7 (6%) were taking no antidepressant drugs.
4.6. Distribution of Patients Based on Drug Use (Monotherapy Only)
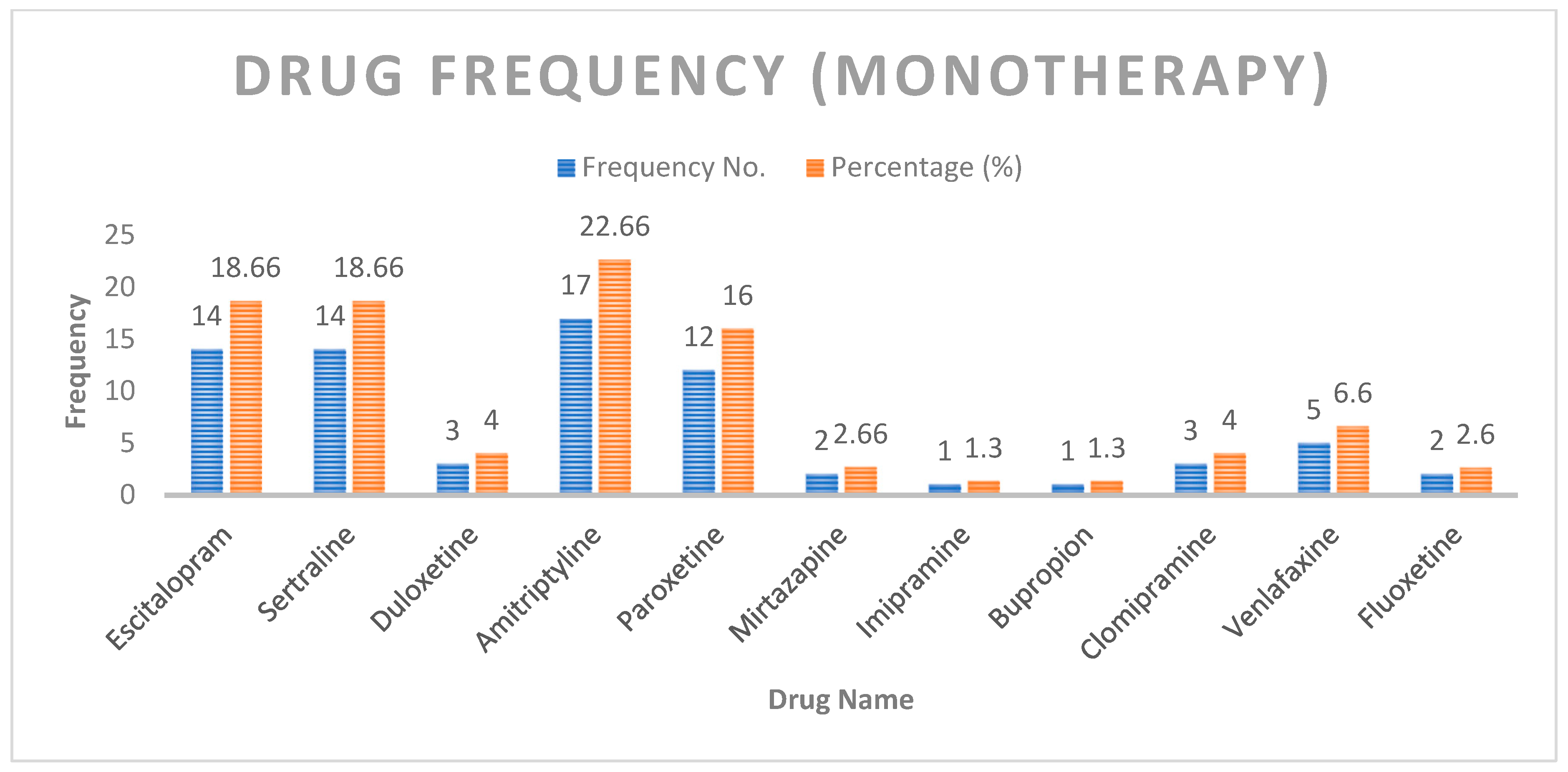
The most common drug used in monotherapy was amitriptyline (22.6%), followed by escitalopram, sertraline, and paroxetine (
Figure 2).
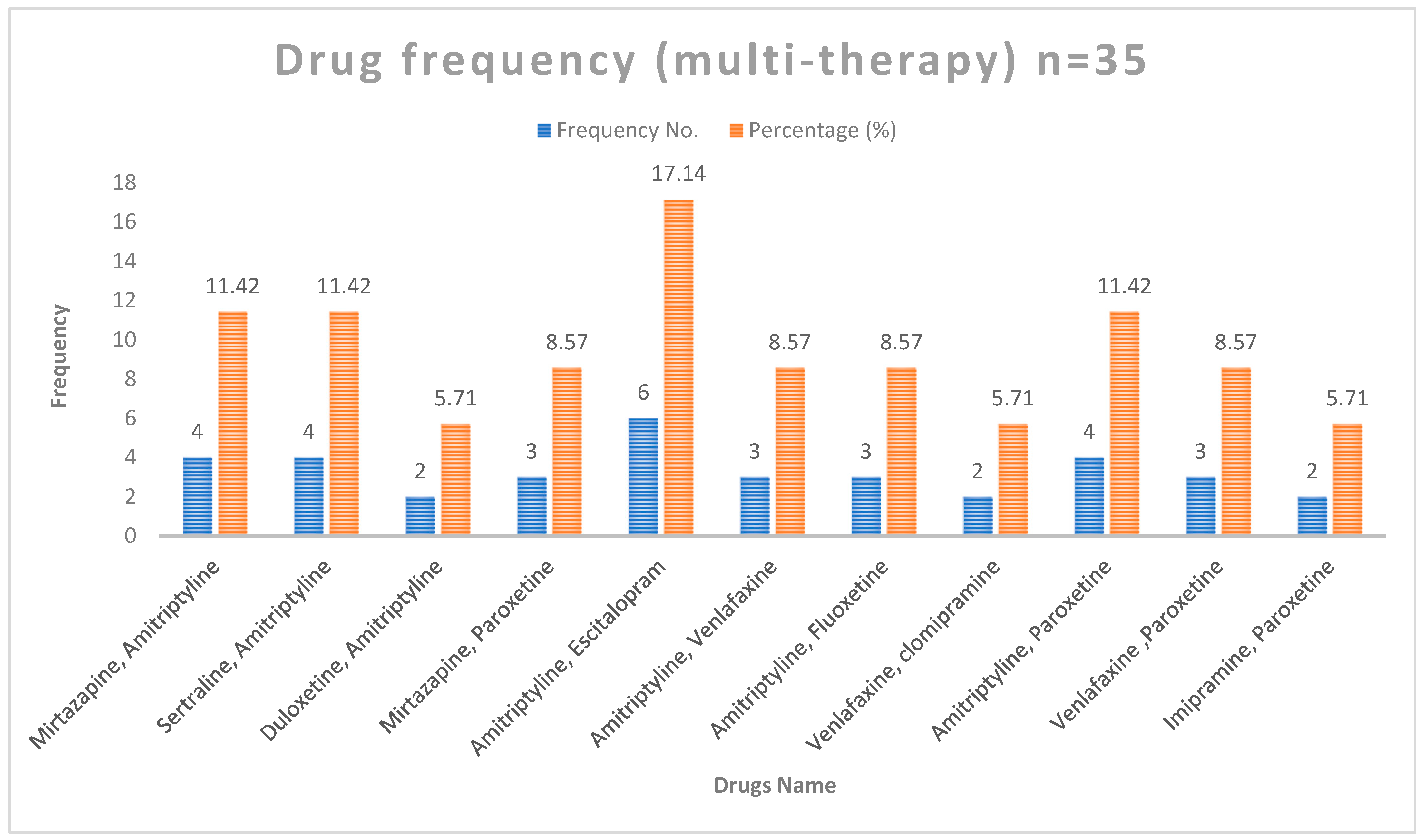
The different drug combinations and their frequency are shown in
Figure 3. The most common combination used in multiple therapy was amitriptyline and escitalopram (17.1%).
4.7. Adverse Drug Profile of Total Respondents under Treatment of Antidepressants
Based on the side effect check list develop by the NHS foundation trust used for the adverse drug profile in the patients using antidepressants, dry mouth was the most commonly reported adverse effect (60.9%), followed by weight gain (58.1%), drowsiness (56.4%), and blurred vision (46.4%). The incidence of adverse effects, such as increased appetite, problems with sexual function, palpitation, sweating, and light headedness, was almost similar (43.6–40.9%). The least commonly reported adverse effect was disorientation (3.6%) (
Table 6).
4.8. Comparison of ADRs in Monotherapy (n = 75) and Multiple Therapy (n = 35)
The comparison of monotherapy and multiple therapy ADRs are shown in
Table 7. The incidence of certain adverse effects (diarrhea, problems with sexual function, palpitations, and tremor) was higher (
p < 0.05) in the multiple therapy group compared to monotherapy.
4.9. Percentage of Moderate/Severe Symptoms: Comparison between Monotherapy and Multiple Therapy
The incidence of many of the adverse effects (16/20) was not significantly different between the monotherapy and multiple therapy groups. The incidence of diarrhea, problems with sexual function, palpitation, and tremor were significantly higher in the multiple therapy group (
p < 0.05) (
Table 8).
4.10. Respondent Responses on Adverse Effects among Different Antidepressant Drugs
The respondent responses on adverse effects among different antidepressant drugs are shown in
Table 9. ADRs were more common in patients treated with escitalopram, sertraline, amitriptyline, and paroxetin, followed by venlafaxine, mirtazapine, duloxetine, clomipramine, imipramine, bupropion, and fluoxetine.
4.11. Patient Side Effects with Most Commonly Used Drugs
The most commonly used drugs with side effects are shown in
Table 10. The most common adverse effects are dry mouth, drowsiness, headache, diarrhea, problems with urination, problems with sexual function, palpitation, feeling light-headed on standing, and feeling like the room is spinning with escitalopram use. Blurred vision, decreased appetite, sweating, increase body temperature, and tremor were found with sertraline use. Constipation, increased appetite, disorientation, and weight gain were found with amitriptyline, and insomnia, nausea, and vomiting with paroxetine use.
5. Discussion of the Study
The study found that in the cohort of depressed patients, 63.2% were female, which is consistent with previous research by Bhawesh Koirala in 2015 where 63% of the cohort was female [
15], suggesting that females may be more susceptible to depression or more likely to seek help for depressive symptoms compared to males. In terms of age, the mean was 40.9 ± 12.807 years, with 54.5% male and 65.5% female. These findings indicate that depression can affect individuals across a wide age range, with the highest incidence observed in middle-aged and older adults. Regarding education, 20.5% were illiterate and 79.5% were literate. The majority of the patients were housewives (46.2%), with other occupations including a job (16.2%), agriculture (11.1%), business (7.7%), labor (5.1%), student (10.3%), and other (3.4%). These findings highlight the diverse socioeconomic backgrounds and occupations of individuals affected by depression. The most commonly reported adverse drug reactions were problems with sexual function (60%), dryness of the mouth (66.66%), weight gain (66.6%), and insomnia (60%). It is important to note that the prevalence of ADRs can vary depending on the specific antidepressant medication used. Escitalopram and paroxetine were mentioned as commonly prescribed antidepressants associated with sexual dysfunction, while escitalopram was linked to dry mouth and weight gain. A similar study by Vijay Kaul and Shaktibala Dutta, 2015, using the Hamilton depression rating scale, found that the mean age was 46.8 ± 1.10 years, with 41.41% male and 58.59% female. Regarding education, 47.4% were illiterate and 52.5% were literate. The majority of patients were farmers (65.6%), with other occupations including employed (23.2%) and other (11.1%). The most commonly reported adverse drug reactions were dryness of the mouth (10.5%), weight gain (5.2%), sexual dysfunction (10.5%), and insomnia (5.2%) [
16]. Escitalopram is the most often prescribed antidepressant in much of Europe [
17], although amitriptyline was the most commonly used antidepressant in this research, followed by escitalopram. This suggests that the choice of antidepressant may vary across different regions and healthcare settings. In a study performed in the UAE, escitalopram was reported as the primary culprit for ADRs [
13].
According to the Antidepressant Adverse-Effect Checklist and the UKU Side Effect Rating Scale, diarrhea (26.6%) is a frequent adverse effect of escitalopram medication, and insomnia (84.6%) is a common side effect of paroxetine treatment. In a 2009 study performed by Rudolf Uher and Anne Farmer, it was shown that diarrhea (9%) and insomnia (36%) were also prevalent with escitalopram administration [
18]. The 2014 meta-analysis, comprising 58 randomized controlled trials and five observational studies identified a higher risk of sexual dysfunction with escitalopram and paroxetine compared to other antidepressants [
19].
According to the study, the occurrence of sexual dysfunction as a side effect of antidepressants varied depending on the medicine used. Sexual dysfunction was reported in 60% of escitalopram patients, 20% of sertraline patients, 29.4% of amitriptyline patients, and 23.0% of paroxetine patients. This is congruent with the findings of a research published in 2001 by Montejo A and Llorca G, who discovered that the incidence of sexual dysfunction was high with numerous antidepressants, including fluoxetine (57.7%), sertraline (62.9%), fluvoxamine (62.3%), venlafaxine (67%), paroxetine (70.7%), and citalopram (72.7%) [
20].
The ADE tool was utilized to evaluate the adverse effects of commonly prescribed drugs, including escitalopram and sertraline, for the treatment of depression. The study findings were substantiated by a comparable investigation conducted by Bhawesh Koirala at a tertiary care center in Eastern Nepal. The reference article reinforces the study’s observations of a higher occurrence of dry mouth among patients receiving escitalopram (66.6% in the study vs. 34.9% in the reference article) and sertraline (53.3% in the study vs. 30% in the reference article). Additionally, the reference article supports the study’s outcomes regarding insomnia, demonstrating elevated rates for both escitalopram (60% in the study vs. 12.8% in the reference article) and sertraline (60% in the study vs. 6.7% in the reference article). Furthermore, the reference article aligns with the study’s findings on headaches, diarrhea, nausea and vomiting, sweating, tremor, and weight gain, indicating comparable or similar prevalence rates for escitalopram and sertraline [
15] (
Table 11).
6. Conclusions
This study on adverse medication responses to antidepressants in adult patients, carried out in Nepal, offers insightful information on the patient profile and frequency of side effects. According to the data, adverse medication responses are very common in adult antidepressant users.
The study included predominantly female patients within the age range of 26–40 years, many of whom were literate housewives. Most patients belonged to the Hindu religion and Chettri/Brahmin ethnicity, with a higher income level and nuclear family structure. The prevalence of family history related to depression was relatively low.
The patients exhibited average BMI, systolic blood pressure, and diastolic blood pressure values within the study’s measured ranges. The duration of drug use and illness varied, with most patients experiencing depressive illness and using monotherapy. Some patients also received additional medications such as clonazepam, propranolol, pantoprazole, amisulpride, amlodipine, and valproate.
When comparing adverse drug reactions between monotherapy and multiple therapy for antidepressants, this study found a higher incidence of certain adverse reactions, including diarrhea, problems with sexual function, palpitations, and tremors, in the multiple therapy group. The most commonly prescribed drugs in monotherapy were escitalopram, sertraline, amitriptyline, and paroxetine.
The adverse effects reported by patients using antidepressants included dry mouth, weight gain, drowsiness, blurred vision, increased appetite, problems with sexual function, palpitations, sweating, lightheadedness, and disorientation. Dry mouth was the most frequently reported adverse effect, while disorientation was the least commonly reported.
In conclusion, this prospective study emphasizes the importance of monitoring and managing adverse drug reactions in adult patients using antidepressants. The findings highlight the need for healthcare providers to be aware of the potential side effects associated with different antidepressant medications and to consider the impact of multiple therapy. Individualized patient care and regular evaluation of adverse reactions can contribute to optimizing treatment outcomes and improving patient well-being.
7. Recommendations
Multiple therapy for antidepressants was associated with a higher incidence of adverse effects compared to monotherapy. Therefore, monotherapy is preferred.
Selective serotonin reuptake inhibitors (SSRIs) are commonly associated with sexual dysfunction in adults. Therefore, they may be less preferred.
Weight gain is a major side effect of antidepressants, and patients should engage in regular exercise to manage it.
Patients should not discontinue or miss doses of antidepressants due to adverse effects without first consulting with their physician.
8. Limitation of the Study
The research study was conducted only on adult patients above 20 years of age. Therefore, the findings may not be generalizable to other age groups.
The data were collected from only one psychiatric department in the OPD of Chitwan Medical College. Therefore, the findings may not be representative of the larger population.
There was limited time for data collection and poor patient compliance, which may have affected the accuracy and completeness of the data collected.
Author Contributions
Conceptualization, U.B.S., A.A., S.T., A.G., K.C.S. and S.P.; Methodology, U.B.S., A.A., S.T., A.G., K.C.S. and S.P.; Software, A.G. and K.C.S.; Validation, U.B.S., S.T. and A.G.; Formal analysis, U.B.S., A.A., S.T., A.G. and K.C.S.; Investigation, U.B.S., S.T., A.G. and K.C.S.; Resources, A.A., S.T. and A.G.; Data curation, A.A., A.G. and K.C.S.; Writing—original draft, U.B.S., A.A., S.T., A.G., K.C.S. and S.P.; Writing—review and editing, A.A., S.T., A.G., K.C.S. and S.P.; Supervision, U.B.S., A.A., S.T. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted after obtaining ethical clearance from the institutional review committee (IRC) of Chitwan Medical College (reference number: CMC-IRC-2073/074:96). Permission from CMC Hospital was obtained before starting the data collection.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. The information obtained from the respondents was kept confidential and privacy was maintained.
Data Availability Statement
Data will be made available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO Regional Office of Europe. Depression. 2017. Available online: www.who.int/topics/depression/en (accessed on 14 February 2023).
- Sadock. Mood Disorders: Historical Introduction and Conceptual Overview, Textbook of Psychiatry, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 559–575. [Google Scholar]
- Mathers, C.D. Projections of global mortality and burden of global disease from 2002 to 2030. PLoS Med. 2006, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Risal, A.; Manandhar, K.; Linde, M.; Steiner, T.J.; Holen, A. Anxiety and depression in Nepal: Prevalence, comorbidity and associations. BMC Psychiatry 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Sankhi, S.; Marasine, N.R.; Sankhi, S.; Lamichhane, R. Adverse Drug Reaction due to Antidepressants among Patients with Depression in a Private Psychiatric Hospital of Nepal. BioMed Res. Int. 2020, 2020, 6682928. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.K.; Pellmar, T.C.; Kleinman, A.M.; Bunney, W.E. Reducing Suicide, a National Imperative; National Academies Press: Washington, DC, USA, 2002; pp. 1–516. [Google Scholar]
- World Health Organization. International drug monitoring: The role of national centres. In Proceedings of the WHO Meeting, Geneva, Switzerland, 20–25 September 1971. [Google Scholar]
- Rauniar, G.; Panday, D. Adverse drug reaction (ADR) monitoring at the eastern regional pharmacovigilance centre, Nepal. Kathmandu Univ. Med. J. 2017, 15, 296–300. [Google Scholar]
- Ho, S.C.; Jacob, S.A.; Tangiisuran, B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: A qualitative study. PLoS ONE 2017, 12, e0179290. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.M.; Sharma, S.G. Adverse effects of atypical antipsychotics: Differential risk and clinical implications. CNS Drugs 2007, 21, 911–936. [Google Scholar] [CrossRef]
- Beijer, H.J.M.; De Blaey, C.J. Hospitalizations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm. World Sci. 2002, 24, 46–54. [Google Scholar] [CrossRef]
- Richelson, E. Interaction of antidepressants with neurotransmitter Transporters and receptors and their clinical relevance. J. Clin. Psychiatry 2003, 64 (Suppl. 13), 5–13. [Google Scholar] [PubMed]
- Sridhar, S.B.; Al-Thamer, S.; Jabbar, R. Monitoring of adverse drug reactions in psychiatry outpatient department of a Secondary Care Hospital of Ras Al Khaimah, UAE. J. Basic Clin. Pharm. 2016, 7, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, H.B.; Vora, M.; Nagar, J.; Patel, P.B. Knowledge, attitude and practices toward pharmacovigilance and adverse drug reactions in postgraduate students of tertiary Care Hospital in Gujarat. J. Adv. Pharm. Technol. Res. 2015, 6, 29. [Google Scholar] [PubMed]
- Koirala, B.; Rauniar, G.; Shakya, D. Adverse effects including sexual problems associated with the use of selective serotonin reuptake inhibitors in a tertiary care center of Eastern Nepal. Int. J. Basic Clin. Pharmacol. 2015, 4, 651. [Google Scholar] [CrossRef][Green Version]
- Kaul, V.; Dutta, S.; Beg, M.A.; Singh, N.K.; Bawa, S.; Anjoom, M.; Dutta, S. Comparative evaluation of amisulpride and escitalopram on Hamilton depression rating scale among depression patients in a tertiary care teaching hospital in Nepal. Int. J. Med. Sci. Public Health 2015, 4, 642–646. [Google Scholar] [CrossRef]
- Forns, J.; Pottegård, A.; Reinders, T.; Poblador-Plou, B.; Morros, R.; Brandt, L.; Cainzos-Achirica, M.; Hellfritzsch, M.; Schink, T.; Prados-Torres, A.; et al. Antidepressant use in Denmark, Germany, Spain, and Sweden between 2009 and 2014: Incidence and comorbidities of antidepressant initiators. J. Affect. Disord. 2019, 249, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Farmer, A.; Henigsberg, N.; Rietschel, M.; Mors, O.; Maier, W.; Kozel, D.; Hauser, J.; Souery, D.; Placentino, A.; et al. Adverse reactions to antidepressants. Br. J. Psychiatry 2009, 195, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Reichenpfader, U.; Gartlehner, G.; Morgan, L.C.; Greenblatt, A.; Nussbaumer, B.; Hansen, R.A.; Van Noord, M.; Lux, L.; Gaynes, B.N. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: Results from a systematic review with network meta-analysis. Drug Saf. 2014, 37, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Montejo, A.L.; Llorca, G.; Izquierdo, J.A.; Rico-Villademoros, F. Incidence of sexual dysfunction associated with antidepressant agents: A prospective multicenter study of 1022 outpatients. J. Clin. Psychiatry 2001, 62, 10–21. [Google Scholar] [PubMed]
Figure 1.
Classification of psychiatric illness.
Figure 1.
Classification of psychiatric illness.
Figure 2.
Distribution of patients based on drug use (monotherapy only).
Figure 2.
Distribution of patients based on drug use (monotherapy only).
Figure 3.
Distribution of patients based on drug use (multi-therapy only).
Figure 3.
Distribution of patients based on drug use (multi-therapy only).
Table 1.
Socio-demographic characteristics of the respondents: age, education level, sex, occupation, religion, and ethnic Group (n = 117).
Table 1.
Socio-demographic characteristics of the respondents: age, education level, sex, occupation, religion, and ethnic Group (n = 117).
| Variable | Frequency | Percentage% |
|---|
| Category of Age | | |
| <25 years | 19 | 16.2 |
| 26–40 | 43 | 36.8 |
| 41–55 | 42 | 35.9 |
| 56–70 | 11 | 9.4 |
| Above 70 years | 2 | 1.7 |
| Education level (n = 117) | | |
| Illiterate | 24 | 20.5 |
| Literate | 25 | 21.4 |
| Primary Level | 20 | 17.1 |
| Secondary Level | 22 | 18.8 |
| Higher Secondary Level | 14 | 12 |
| Graduated | 12 | 10.3 |
| Sex (n = 117) | | |
| Male | 43 | 36.7 |
| Female | 74 | 63.2 |
| Occupation (n = 117) | | |
| Job | 19 | 16.2 |
| Agriculture | 13 | 11.1 |
| Business | 9 | 7.7 |
| Labor | 6 | 5.1 |
| Housewife | 54 | 46.2 |
| Student | 12 | 10.3 |
| Others | 4 | 3.4 |
| Religion (n = 117) | | |
| Hindu | 85 | 72.7 |
| Buddhism | 13 | 10 |
| Christian | 17 | 15.5 |
| Islam | 0 | 0 |
| Others | 2 | 1.8 |
| Ethnic group (n = 117) | | |
| Chettri/Brahmin | 82 | 72.6 |
| Janajati | 24 | 11.1 |
| Dalits | 8 | 14.5 |
| Others | 3 | 1.7 |
Table 2.
Socio-demographic characteristics of the respondents: family income, type of family, and family history related to depression.
Table 2.
Socio-demographic characteristics of the respondents: family income, type of family, and family history related to depression.
| Variable | Frequency | Percentage% |
|---|
| Family income | | |
| less than Rs.100000 or 762.12 USD/yr. | 53 | 45.3 |
| more than Rs.100000 or 762.12 USD/yr. | 64 | 54.7 |
| Type of family | | |
| Nuclear | 56 | 47.9 |
| Extended | 47 | 40.2 |
| Joint | 14 | 12 |
| Family history related to depression | | |
| No | 86 | 73.5 |
| Yes | 31 | 26.5 |
Table 3.
Characteristics of the population: weight, height, BMI, BPS, BPD.
Table 3.
Characteristics of the population: weight, height, BMI, BPS, BPD.
| Variable | Mean Values | ±SD |
|---|
| Weight-kg | 62.1 | ±11.924 |
| Height-cm | 156.3 | ±9.047 |
| BMI | 25.4 | ±4.316 |
| Systolic pressure | 118.8 | ±15.874 |
| Diastolic pressure | 80.9 | ±10.815 |
Table 4.
Duration of drug use and duration of illness.
Table 4.
Duration of drug use and duration of illness.
| Variable | Minimum | Maximum | Mean | Median |
|---|
| Duration of drug use (months) | 0.5 | 144 | 20.9 | 12 |
| Duration of illness (months) | 1 | 228 | 48.4 | 24 |
Table 5.
Respondent characteristics regarding disease distribution, drug use other than antidepressants, and type of drug therapy.
Table 5.
Respondent characteristics regarding disease distribution, drug use other than antidepressants, and type of drug therapy.
| Variable | Values | Percentage% |
|---|
| Disease distribution (n = 117) | | |
| Depressive illness | 58 | 49.6 |
| Anxiety disorder | 37 | 31.6 |
| Somatoform disorder | 16 | 13.7 |
| Others | 6 | 5.1 |
| Drug use other than antidepressants (n = 117) | | |
| No | 21 | 17.9 |
| Yes | 96 | 82.1 |
| Type of drug therapy (n = 117) | | |
| Monotherapy | 75 | 64.1 |
| Multiple therapy | 35 | 29.9 |
| Not antidepressant | 7 | 6 |
Table 6.
Adverse drug profile of total respondents under treatment of antidepressants.
Table 6.
Adverse drug profile of total respondents under treatment of antidepressants.
| Side Effect | No. (%) |
|---|
| Dry mouth | 67 (60.9) |
| Drowsiness | 62 (56.3) |
| Insomnia | 19 (17.2) |
| Blurred vision | 51 (46.3) |
| Headache | 24 (21.8) |
| Constipation | 34 (30.9) |
| Diarrhea | 35 (31.8) |
| Increase appetite | 48 (43.6) |
| Decrease appetite | 30 (27.2) |
| Nausea and vomiting | 30 (27.2) |
| Problem with urination | 23 (20.9) |
| Problem with sexual function | 49 (44.5) |
| Palpitation | 46 (41.8) |
| Feeling light-headed on standing | 45 (40.9) |
| Feeling like the room is spinning | 19 (17.2) |
| Sweating | 46 (41.8) |
| Increase body temperature | 35 (31.8) |
| Tremor | 37 (33.6) |
| Disorientation | 4 (3.6) |
| Weight gain | 64 (58.1) |
Table 7.
Comparison of ADRs in monotherapy (n = 75) and multiple therapy (n = 35).
Table 7.
Comparison of ADRs in monotherapy (n = 75) and multiple therapy (n = 35).
| Adverse Effect | Monotherapy | Multiple Therapy | p-Value |
|---|
| Variable | Variable |
|---|
| Dry mouth | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
0.123 |
| 35 | 12 | 24 | 4 | 8 | 8 | 14 | 4 |
| Drowsiness | 31 | 24 | 15 | 4 | 17 | 11 | 6 | 1 | 0.872 |
| Insomnia | 64 | 7 | 4 | 0 | 27 | 1 | 6 | 1 | 0.061 |
| Blurred vision | 42 | 26 | 7 | 0 | 17 | 14 | 4 | 0 | 0.765 |
| Headache | 61 | 8 | 5 | 1 | 25 | 6 | 3 | 1 | 0.680 |
| Constipation | 53 | 7 | 14 | 1 | 23 | 9 | 2 | 1 | 0.056 |
| Diarrhea | 60 | 14 | 1 | 0 | 25 | 5 | 5 | 0 | 0.020 |
| Increase appetite | 43 | 18 | 12 | 2 | 19 | 8 | 8 | 0 | 0.659 |
| Decrease appetite | 55 | 7 | 6 | 7 | 25 | 3 | 3 | 4 | 0.986 |
| Nausea and vomiting | 56 | 13 | 5 | 1 | 24 | 4 | 6 | 1 | 0.310 |
| Problem with urination | 58 | 11 | 6 | 0 | 29 | 5 | 1 | 0 | 0.581 |
| Problem with sexual function | 45 | 10 | 15 | 5 | 16 | 2 | 9 | 8 | 0.050 |
| Palpitation | 52 | 17 | 6 | 0 | 12 | 15 | 6 | 2 | 0.002 |
| Feeling light-headed on standing | 42 | 26 | 7 | 0 | 23 | 10 | 2 | 0 | 0.596 |
| Feeling like the room is spinning | 63 | 10 | 2 | 0 | 28 | 5 | 2 | 0 | 0.715 |
| Sweating | 45 | 17 | 9 | 4 | 19 | 10 | 3 | 3 | 0.771 |
| Increase body temperature | 49 | 18 | 7 | 1 | 26 | 5 | 4 | 0 | 0.587 |
| Tremor | 55 | 16 | 4 | 0 | 18 | 10 | 7 | 0 | 0.025 |
| Disorientation | 71 | 4 | 0 | 0 | 35 | 0 | 0 | 0 | 0.164 |
| Weight gain | 20 | 27 | 20 | 8 | 26 | 9 | 8 | 9 | 0.220 |
Table 8.
Percentage of moderate/severe symptoms compared between the monotherapy and multiple therapy groups.
Table 8.
Percentage of moderate/severe symptoms compared between the monotherapy and multiple therapy groups.
| Adverse Effect | Monotherapy
(n = 75) | Multiple Therapy (n = 35) | p Value |
|---|
| N (%) | N (%) |
|---|
| Dry mouth | 28 (37.3) | 18 (51.4) | 0.123 |
| Drowsiness | 19 (25.3) | 7 (20) | 0.872 |
| Insomnia | 4 (5.3) | 7 (20) | 0.061 |
| Blurred vision | 7 (9.3) | 4 (11.4) | 0.765 |
| Headache | 6 (8) | 4 (11.4) | 0.680 |
| Constipation | 15 (20) | 3 (8.5) | 0.056 |
| Diarrhea | 1 (1.3) | 5 (14.2) | 0.020 |
| Increase appetite | 14 (18.6) | 8 (22.8) | 0.659 |
| Decrease appetite | 13 (17.3) | 7 (20) | 0.986 |
| Nausea and vomiting | 6 (8) | 7 (20) | 0.310 |
| Problem with urination | 6 (8) | 1 (2.8) | 0.581 |
| Problem with sexual function | 20 (26.6) | 17 (48.5) | 0.050 |
| Palpitation | 6 (8) | 8 (22.8) | 0.002 |
| Feeling light-headed on standing | 7 (9.3) | 2 (5.7) | 0.596 |
| Feeling like the room is spinning | 2 (2.6) | 2 (5.7) | 0.715 |
| Sweating | 13 (17.3) | 6 (17.1) | 0.771 |
| Increase body temperature | 8 (10.6) | 4 (11.4) | 0.587 |
| Tremor | 4 (5.3) | 7 (20) | 0.025 |
| Disorientation | 0 (0) | 0 (0) | 0.164 |
| Weight gain | 28 (37.3) | 17 (48.5) | 0.220 |
Table 9.
Respondent responses on the adverse effects among different antidepressant drugs.
Table 9.
Respondent responses on the adverse effects among different antidepressant drugs.
| Adverse Effect | Different Drugs Name |
|---|
| Escitalopram (n = 15) | Sertraline (n = 15) | Duloxetine (n = 3) | Amitriptyline (n = 17) | Paroxetine (n = 13) | Mirtazapine (n = 4) | Clomipramine (n = 3) | Venlafaxine (n = 5) | Fluoxetine (n = 2) |
|---|
| Dry mouth | 10 | 8 | 1 | 11 | 6 | 3 | 3 | 2 | 0 |
| Drowsiness | 4 | 2 | 0 | 4 | 0 | 1 | 0 | 0 | 1 |
| Insomnia | 9 | 9 | 0 | 11 | 11 | 1 | 3 | 1 | 0 |
| Blurred vision | 7 | 8 | 0 | 7 | 4 | 3 | 2 | 2 | 2 |
| Headache | 6 | 3 | 0 | 3 | 1 | 1 | 0 | 1 | 0 |
| Constipation | 4 | 2 | 1 | 6 | 3 | 2 | 1 | 1 | 0 |
| Diarrhea | 4 | 3 | 0 | 1 | 2 | 1 | 2 | 0 | 0 |
| Increase appetite | 7 | 6 | 1 | 9 | 5 | 3 | 2 | 0 | 1 |
| Decrease appetite | 3 | 6 | 0 | 4 | 4 | 1 | 1 | 1 | 1 |
| Nausea and vomiting | 5 | 3 | 0 | 3 | 5 | 1 | 1 | 2 | 0 |
| Problem with urination | 4 | 3 | 0 | 3 | 3 | 3 | 0 | 1 | 0 |
| Problem with sexual function | 9 | 3 | 1 | 5 | 3 | 3 | 3 | 2 | 2 |
| Palpitation | 9 | 4 | 0 | 5 | 4 | 1 | 1 | 0 | 0 |
| Feeling light headed on standing | 9 | 6 | 1 | 7 | 4 | 2 | 1 | 2 | 1 |
| Feeling like the room is spinning | 6 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 0 |
| Sweating | 4 | 9 | 3 | 8 | 3 | 1 | 1 | 1 | 1 |
| Increase body temperature | 5 | 6 | 1 | 6 | 4 | 1 | 1 | 3 | 1 |
| Tremor | 2 | 6 | 0 | 5 | 1 | 3 | 1 | 1 | 2 |
| Disorientation | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Weight gain | 10 | 11 | 2 | 13 | 9 | 4 | 2 | 4 | 2 |
Table 10.
Patient side effects with the most commonly used drugs.
Table 10.
Patient side effects with the most commonly used drugs.
| Adverse Effect | Escitalopram | Sertraline | Amitriptyline | Paroxetin |
|---|
(n = 15)
(N, %) | (n = 15)
(N, %) | (n = 17)
(N, %) | (n = 13)
(N, %) |
|---|
| Dry mouth | 10 (66.6) | 8 (53.3) | 11 (64.7) | 6 (46.1) |
| Drowsiness | 4 (26.6) | 2 (13.3) | 4 (23.5) | 0 (0) |
| Insomnia | 9 (60) | 9 (60) | 11 (64.7) | 11 (84.6) |
| Blurred vision | 7 (46.6) | 8 (53.3) | 7 (41.1) | 4 (30.7) |
| Headache | 6 (40) | 3 (20) | 3 (17.6) | 1 (7.6) |
| Constipation | 4 (26.6) | 2 (13.3) | 6 (35.2) | 3 (23.0) |
| Diarrhea | 4 (26.6) | 3 (20) | 1 (5.8) | 2 (15.3) |
| Increase appetite | 7 (46.6) | 6 (40) | 9 (52.9) | 5 (38.4) |
| Decrease appetite | 3 (20) | 6 (40) | 4 (23.5) | 4 (30.7) |
| Nausea and vomiting | 5 (33.3) | 3 (20) | 3 (17.6) | 5 (38.4) |
| Problem with urination | 4 (26.6) | 3 (20) | 3 (17.6) | 3 (23.0) |
| Problem with sexual function | 9 (60) | 3 (20) | 5 (29.4) | 3 (23.0) |
| Palpitation | 9 (60) | 4 (26.6) | 5 (29.4) | 4 (30.7) |
| Feeling light-headed on standing | 9 (60) | 6 (40) | 7 (41.1) | 4 (30.7) |
| Feeling like the room is spinning | 6 (40) | 1 (6.6) | 2 (11.7) | 2 (15.3) |
| Sweating | 4 (26.6) | 9 (60) | 8 (47.0) | 3 (23.0) |
| Increase body temperature | 5 (33.3) | 6 (40) | 6 (35.2) | 4 (30.7) |
| Tremor | 2 (13.3) | 6 (40) | 5 (29.4) | 1 (7.6) |
| Disorientation | 0 (0) | 0 (0) | 1 (5.8) | 0 (0) |
| Weight gain | 10 (66.6) | 11 (73.3) | 13 (76.4) | 9 (69.2) |
Table 11.
Comparison of the ADRs of commonly used drugs with a reference article [
15].
Table 11.
Comparison of the ADRs of commonly used drugs with a reference article [
15].
| Adverse Effect | Escitalopram | Escitalopram | Sertraline | Sertraline |
|---|
| Study Shows | Reference Article Result | Study Shows | Reference Article Result |
|---|
| Dry mouth | 66.6% | 34.9% | 53.3% | 30% |
| Insomnia | 60% | 12.8% | 60% | 6.7% |
| Headache | 40% | 24.4% | 20% | 35% |
| Diarrhea | 26.6% | 4.7% | 20% | 1.7% |
| Nausea and vomiting | 33.3% | 15.1% | 20% | 13.3% |
| Sweating | 26.6% | 15.1% | 60% | 13.3% |
| Tremor | 13.3% | 17.4% | 40% | 25% |
| Weight gain | 66.6% | 62.8% | 73.3% | 55% |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).