Abstract
Asthma is a persistent ailment that impacts the respiratory system and stands as a formidable public health challenge globally. Inhaled corticosteroids and bronchodilators, while effective in asthma management, are accompanied by side effects and high costs. Recently, nutraceuticals have gained significant attention as adjuvant therapy due to their promising outcomes. Given the antioxidant properties, nutrient richness, and an array of health benefits, beetroot and its bioactive compounds have been tested as an adjuvant therapy for asthma management. Although its main bioactive compound, betalains (betanin), has demonstrated promising results in mouse studies, beetroot juice has been found to worsen asthma. This review investigated the full spectrum of active compounds associated with beetroots to understand the underlying factors contributing to the conflicting findings. The finding suggests that individual bioactive compounds, such as phenolic compounds, flavonoids, nitrates, betalains, saponins, vitamins, fiber, and carotenoids, possess asthma-managing properties. However, the consumption of juice may exacerbate the condition. This discrepancy may be attributed to the presence of sugars and oxalates in the juice, which could counteract the beneficial effects of the bioactive compounds.
1. Introduction
Asthma is a chronic respiratory condition marked by airway inflammation and breathing difficulties [1]. In 2019, the Global Burden of Disease study reported an asthma prevalence of 3416 cases per 100,000 population, ranking it 34th among major disease burdens [2]. Asthma imposes a significant economic burden, costing $1.1 billion in low-income countries and $130.3 billion in high-income countries in 2023, with projections rising to $1.3 billion and $133.4 billion, respectively, by 2050, and it contributes to 461,000 deaths each year [3,4]. The disease is heterogeneous, with environmental triggers such as smoke, mold, and pollutants playing a role in oxidative stress and chronic inflammation through reactive oxygen species (ROS) production [5,6,7,8,9]. Despite the availability of treatments such as corticosteroids, leukotriene receptor antagonists, and beta-agonists, a substantial number of asthma cases remain poorly controlled, and side effects, including vomiting, infections, and weight gain, have also been reported in some cases [10,11,12,13,14,15,16,17,18,19,20,21,22]. Inhaled corticosteroids (ICS), in particular, may lead to side effects such as dysphonia, candidiasis, and in some cases, an increased risk of tuberculosis and other mycobacterial infections [23,24,25]. As a result, there is a growing need for adjuvant therapies to complement existing treatments, aiming to improve outcomes while reducing costs and side effects.
Dietary interventions, especially increased fruit and vegetable intake, have shown potential to improve asthma control due to their anti-inflammatory and antioxidant properties [26,27,28,29,30,31]. This has spurred interest in herbal and nutraceutical remedies as adjuncts or standalone therapies, including Curcuma longa L., nigella, and licorice root [32,33,34,35]. Roots of Curcuma longa L., nigella, and licorice root are some of the examples [36,37,38]. Among these, beetroot (Beta vulgaris), a rich source of phytochemicals, has gained attention for its potential role in asthma management [39].
Beetroot, belonging to the Chenopodiaceae family, is widely cultivated for its nutritionally rich roots [40,41,42]. Known as a garden or spinach beet, it ranks among the top plants with high antioxidant potential [43,44]. Its pharmacological properties stem from bioactive compounds, particularly betalains (e.g., betanin) and polyphenols, which enhance its antioxidant activity [45,46,47]. Betanin’s phenolic and cyclic amine groups boost its reducing capacity, offering antioxidant potential comparable to L-ascorbic acid [48].
Beetroot has been extensively studied for its potential health effects, such as anticancer, antiviral, neuroprotective, antibacterial, and anti-inflammatory potential [49,50,51]. Its high nitrate content contributes to ergogenic effects and blood pressure reduction [52,53]. Beetroot juice and chips have demonstrated reductions in obesity-related oxidative stress and inflammation [54]. However, the relationship between beetroot intake and asthma outcomes remains controversial. Betalains have shown anti-inflammatory effects in murine asthma models, reducing inflammatory markers, oxidative stress, and nitric oxide levels [55,56]. Conversely, another murine study found that beetroot juice exacerbated asthma symptoms [39]. Currently, no human studies have directly explored beetroot’s effects on asthma management. However, a study involving 76 participants reported that 70 mL of beetroot juice daily for seven days reduced cold symptoms associated with psychological stress, with the most notable benefits observed in the subgroup with asthma (n = 16) [57]. As per our knowledge, no review has been undertaken to analyze the evidence on the contradictory effects of beetroot and asthma.
The primary aim of this review is to summarize the key compounds commonly found in beetroot and to explore their potential role in asthma, including both benefits and adverse effects. By examining existing studies, the review seeks to determine whether the effects of these individual compounds, regardless of their food source, could provide insights into the potential of beetroot supplementation in alleviating asthma symptoms.
2. Bioactive Analysis of Beetroot
Bioactive compounds present in beetroot in high levels include betalains, coumarins, carotenoids, ascorbic acid, vitamin A, vitamin E, vitamin K, thiamine, riboflavin, niacin, pyridoxine, cobalamin, folate, pantothenic acid, cyanocobalamin, sesquiterpenoids, triterpenes, polyphenols, flavonoids (including astragalin, tiliroside, rhamnetin, rhamnocitrin, and kaempferol), saponins, fiber, and nitrate (Figure 1). Beetroot also contains lower levels of betaine and glycine [45,58,59,60,61,62,63]. A significant amount of oxalic acid (400 to 600 mg/100 gm fresh weight) is also reportedly present in beetroot [64,65,66]. Table 1 lists the bioactive compounds present in beetroot and their approximate concentrations
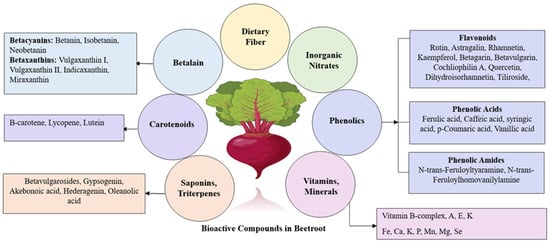
Figure 1.
Bioactive components of beetroot: a schematic presentation.

Table 1.
Concentration of bioactive compounds in beetroot.
3. Comparison of Bioactive Compounds in Beetroot Formulations
Beetroot gel has been reported to contain the highest total phenolic content (SD 1.98 ± 0.03 mg·g−1) compared to beetroot juice (SD 1.01 ± 0.03 mg·g−1), cooked beetroot (SD 2.79 ± 0.23 mg·g−1), chips (SD 0.75 ± 0.06 GAE mg·g−1), and beetroot powder (SD 0.51 ± 0.07 GAE mg·g−1) [69,70]. A comparative analysis of total polyphenols and antioxidant activity in beetroot pulp waste extracts using methanol, ethanol, and aqueous solvents revealed that the methanol extract of beetroot pulp waste had the highest polyphenol content, measuring 220 mg TAE/100 gm, surpassing the levels found in ethanol and aqueous extracts. However, the aqueous extract showed higher total antioxidant activity (10,735 to 91,225 μmoles of ascorbic acid/100 gm) compared to methanol and ethanol extracts [71]. Composition analysis of beetroot juice indicated the presence of gallic acid in the highest concentration, followed by caffeic, syringic, and ferulic acids, accounting for approximately 3% of the total phenolic content [64]. Results of another study explained that the fermentation process enhances the free phenolic acid content after extraction [72]. In contrast, reduced phenolic content was reported after freeze-drying and spray-drying, possibly due to the water-soluble nature of phenols [69].
On average, beetroot contains 0.41 to 1.16 mg/g of flavonoids [73]. The nutritional composition of most commercial beetroot juices is reported to include total flavonoid 2.02–2.36 mg/100 gm [64]. A comparative study of flavonoid content among beetroot juice, gel, and chips indicated that the highest levels were found in beetroot gel (mean 1.37 ± SD 0.03), followed by beetroot juice (mean 0.42 ± SD 0.01) and beetroot chips (mean 0.31 ± 0.02) [73]. Fermentation processing reportedly has negative effects on free flavonoids (such as kaempferol) and positive effects on the conjugated flavonoid (such as rutin) content of beetroot [72].
Betalains are found in the Caryophyllales family of plants. These pigments are nitrogenous compounds derived from the amino acid tyrosine and classified as betacyanin and betaxanthin [46]. The mean concentration of betacyanin and betaxanthins in red beetroots ranges from 400 to 2100 mg/kg and 200 to 1400 mg/kg, respectively. Betalains are reported in the range of 0.8–1.3 g/L in fresh juice of seven varieties of beetroot. The betacyanin to betaxanthin ratio was similar in all seven varieties of beetroot (juice), i.e., 1.75 to 1. Among all components of betalains, the vibrant red color of beetroot is mainly due to the presence of betanin, which makes up between 75 and 95 percent of the total betalain content [42]. A study investigating betalain components in beetroot juice found betanin in the highest concentration among other components [44,64,74]. Silva et al. compared the betanin content in different formulations of beetroot and demonstrated that freeze-dried beetroot chips had the highest betanin content (mean 1274 ± SD 0.01), followed by beetroot juice (mean 298.5 ± SD 0.03), gel, and cereal bar [42,69]. Spray-dried beetroot powder was reported to contain betacyanin around 283.3–302.7 mg/100 gm [75]. Whilst comparing the effect of processing on betanin losses, Sawicki et al. reported that crunchy beetroot slice production using microwave-assisted vacuum drying resulted in the retention of 78.9% betanin content, followed by boiled beetroot (62.5%) [76].
Total betalains, betacyanin, and flavonoid contents are significantly reduced when beetroot is heat dried. However, there is a positive association between betaxanthins, phenolic content, and heat treatment for drying [77]. Beetroot waste dried at 70 °C was found to contain 81.31 ± 0.69 betalain in 9 mg/gm DM [78]. The pH and water, along with exposure to heat, light, oxygen, enzymatic activities, and metal ions, can highly influence the extraction of betalain [79]. Spray drying has a negative impact on the violet pigment concentration of beetroot juice [75].
Beetroot gel was found to contain the highest levels of NO3 (390 mg/100 gm) compared to beetroot chips (279 mg/100 gm) and juice (217 mg/100 gm) [69,70,73]. Furthermore, beetroot juice reportedly contains significantly higher levels of NO3 than beetroot powder and the cooked form [69]. Saponins are mainly plant-derived triterpene glycosides [80] and are documented to exhibit bioactive properties (e.g., antiviral and antihyperglycemic). Beetroot gel has been reported to contain three times higher amounts of saponins, 22 mg/gm, than beetroot juice [73]. However, Baiao et al. reported saponin content in beetroot chips (mean 6371.00 ± SD 1.2) was higher than that in Beetroot gel (mean 2200.00 ± SD 0.17) and juice (mean 2599.00 ± SD 1.27) [42]. Studies suggest that saponin content in beetroot may be influenced by harvest conditions [81] and beetroot processing methods [70].
Yashwant et al. reported the presence of micronutrient vitamins in beetroot, such as 2 μg of vitamin A, 80 μg of folate, and 0.031, 0.027, 0.331, 0.145, and 0.067 mg of thiamine, riboflavin, niacin, pantothenic acid, vitamin B6, respectively, and 3.6 mg of ascorbic acid in each 100 g of fresh beetroot [43]. The vitamin C content of beetroot is highly influenced by cultivation, weather, and spatial distribution [82,83]. According to Pavlovic et al., vitamin C is reportedly present in the range of 10.05 to 11.65 mg/100 gm of beetroot [84]. Beetroot juice was found to contain 256, 3054, 413, 218, and 912 (mg/100 gm) of phosphorus, potassium, calcium, magnesium, and iron, respectively, exceeding the amount found in spray-dried beetroot juice [85].
Beetroot cereal bars reportedly contain the highest level of dietary fiber, potentially due to the presence of other cereals (mean 4.07 ± SD 0.14), which is followed by beetroot gel (mean 3.71 ± SD 0.10), chips (mean 3.22 ± SD 0.63), and juice (mean 0.91 ± SD 0.31) [70,86].
Beetroot is also a good source of oxalic acid, containing around 400 to 600 mg oxalic acid/100 gm fresh weight. Wruss et al. examined the difference in oxalic acid content among seven varieties of beetroot juice and reported 412 mg/L as the average oxalic acid content with a coefficient of variation of 21 percent [64]. The oxalic acid content in the powder of two beetroot varieties was reported as 9583.33 mg and 9166.67 mg/100 g for DetR and CrimG, respectively [87]. Beetroot pulp waste (after separation of juice) was found to have an insignificant amount of oxalic acid, i.e., 0.24 mg/100 g [71]. Heat treatment is considered an efficient approach to reduce oxalate content [88]. Lisiewska et al. reported that freeze-dried beetroot had the highest level of oxalate (soluble) compared to initial levels recorded before freezing [89]. On the other hand, boiling followed by sun drying resulted in reduced oxalic acid content in beetroot [87]. Moreover, microwaving and then hot air drying led to total oxalate loss in beans [90,91]. Ultrasonication is a non-conventional method for significantly eliminating oxalate content [90], and a combination of ultrasonication and temperature treatment successfully retains the bioactive compounds in beetroot juice [92]. Moreover, a study reported that finely sliced rye seedlings naturally contain oxalate oxidase, and beetroot juice treated with finely sliced rye seedlings had significantly reduced oxalate levels [93]. A study examined the effects of deoxilation with calcium ions on the antioxidant activity of beetroot juice. The results indicated a slight decrease in the total antioxidant capacity of deoxalated beetroot juice, 32.42 mg/gm, compared to whole beetroot juice, 48.38 mg/gm ascorbic acid equivalent (AAE). However, there were no differences in flavonoid and phenolic content. Also, an increased percentage of hydroxyl radical scavenging activity was recorded for deoxalated beetroot juice than that for beetroot juice. Also, lipid peroxidation inhibition activity was higher in concentrated deoxalated beetroot juice than in ascorbic acid (standard control) [94].
Beetroot is considered a valuable crop due to its high sucrose content [95], representing more than 98 percent of the total sugars in beet [96]. Baião et al. reported the sugar content of raw beetroot as 6.76 gm/100 gm. Additionally, the study finding suggests the sugar content of cooked/boiled, canned, and fresh juice of beetroot as 7.96 gm/100 gm, 5.51 gm/100 gm, and 6.6 gm/L, respectively [73]. Kazimierczak et al. determined the sugar levels in different commercially available beetroot juices produced from fermented, pure pressed, and concentrates of beetroot, either pure or in combination with the juice of apple or lemon. The total sugar content was in the range of 1.73 to 7.85 gm per 100 gm [68]. Sucrose content in beetroot juice has been reported as (SD 8.8 ± 0.03 g/100 gm). However, chips were analyzed to contain (SD 14.6 ± 0.01 mg/100 gm), followed by gel (SD 8.1 ± 0.05 g/100 gm) [97]. Beetroot juice prepared using a centrifuge blender reportedly contained (SD 963.41 ± 13.98 mg·g−1) total sugars, of which (SD 930.40 ± 13.65 mg·g−1) was sucrose. The total sugar and sucrose content of freeze-dried beetroot chips were (SD 627.96 ± 11.39 mg·g−1) and (SD 603.27 ± 10.30 mg·g−1), respectively. Beetroot powder and cooked beetroots had (SD 444.05 ± 26.08 mg·g−1) and (SD 249.51 ± 0.22 mg·g−1) total sugars, respectively, while sucrose content in beetroot powder and cooked beets was (SD 429.48 ± 25.96 mg·g−1) and (SD 241.37 ± 0.25 mg·g−1 ) [69].
Overall, beetroot is a good source of various bioactive compounds, oxalates, and sugars, particularly sucrose. However, the composition of these compounds is highly influenced by cultivators and beetroot processing methods.
4. Bioavailability of Beetroot’s Bioactive Compounds
Phenolic compounds in beetroot exhibit varied bioavailability influenced by their chemical structure and gastrointestinal digestion. In vitro studies report a 72.93% loss in total phenolic content post-digestion, with gastric digestion reducing phenolic levels to 1/3–1/8 of baseline values [98,99,100] Specific compounds like caffeic and p-coumaric acids were undetectable post-stimulated gastrointestinal digestion (SGD), while chlorogenic acid levels surprisingly increased 2.5-fold [99]. Similarly, flavonoid bioavailability is affected by hydroxyl groups, with in vitro digestion resulting in a 63.63% loss in total flavonoids [99,101].
Dietary nitrate undergoes bacterial conversion to nitrite in the oral cavity, forming nitric oxide (NO) in the stomach under favorable conditions. NO promotes smooth muscle relaxation, but impaired bioavailability has been linked to pulmonary hypertension [102].
Betalains have low oral bioavailability and lack hepatic metabolism. Betanin shows 35% reduced absorption, while indicaxanthin is highly bioavailable, retaining activity through paracellular absorption [103,104]. In vitro digestion studies reveal significant losses of betacyanin (96.07%) and partial retention of betaxanthins (27%) [99]. Heat treatment minimally affects betalains, with beetroot juice retaining 80% of its antioxidant activity [48,70,105].
Oxalate, an antinutrient absorbed at 10–15%, binds minerals, reducing their bioavailability. An in vitro study observed a 43–65% reduction in mineral content in beetroot juice post-digestion [106,107,108].
5. Bioactive Compounds in Asthma Management: Implications for Beetroot Use
Phenolic compounds, such as gallic acid, ferulic acid, sinapic acid, caffeic acid, and chlorogenic acid, are known for their protective effects against chronic inflammation, including airway inflammation and asthma [109,110,111,112,113,114,115,116,117,118]. Flavonoids in beetroot, particularly kaempferol, have shown promise in reducing airway inflammation by lowering type 2 inflammatory cytokines (IL-13 and IL-5) and eosinophils in in vitro models of allergic airway inflammation [119]. Kaempferol also helps reduce mucus secretion, airway inflammation, oxidative stress, and airway remodeling [120,121,122,123,124,125,126]. Other flavonoids, such as rhamnetin 3-rhamnoside and quercetin, exhibit antioxidant and anti-inflammatory properties, with quercetin also promoting vasodilation [127,128,129,130]. Astragalin has been reported to reduce inflammatory cytokines and oxidative stress, while rutin decreases nitric oxide (NO) and reactive oxygen species (ROS) levels, offering protection from bronchial inflammation [125,131,132,133]. Tiliroside has demonstrated anti-inflammatory, anti-allergy, and antioxidant activities [134]. However, novel flavonoids like betagarin, betavulgarin, and cochliophilin found in beetroot remain understudied.
The role of nitric oxide (NO) in inflammatory respiratory diseases, including asthma, is complex. During stable phases of disease, NO can act as an anti-inflammatory, but in acute or severe stages, it may exacerbate inflammation [135]. In asthma, NO has a dual role: at normal levels, it is anti-inflammatory, but higher concentrations of inducible NO can worsen symptoms [136].
Fractional exhaled nitric oxide (FeNO) is a sensitive marker for diagnosing eosinophilic airway inflammation. However, dietary nitrates can increase FeNO levels, potentially leading to misinterpretation of inflammation severity. Studies show that sodium nitrite nebulization may have protective effects for asthmatic patients [136]. Additionally, supplementation with beetroot juice (400 mg nitrate) has reduced global sickness among young asthmatics, though FeNO levels increased post-intervention [57]. Beetroot juice has also shown inhibitory and anti-inflammatory effects on lipopolysaccharide-induced NO production [48].
Supplementation with betalains-rich beetroot juice has shown promising antioxidant and anti-inflammatory effects in preclinical asthma models. For instance, daily doses of 500–600 mL of freshly made juice containing 159.6 mg betacyanin and 79.3 mg betaxanthins improved antioxidant profiles in animals [137]. High doses of betanin reduced NO, reactive oxygen species, myeloperoxidase activity, and TNF-α in zebrafish with cigarette smoke-induced respiratory inflammation [138]. In OVA-induced asthma mouse models, purified betanin (60–180 mg/kg/day) significantly reduced IL-4, IL-5, IL-13, IL-17A, and eotaxin [56]. Betalain supplementation decreased eosinophils, IgE, IL-4, and TNF-α while increasing INF-γ [55]. However, studies reveal mixed results. An oral dose of 8 mL/kg beetroot juice with 45.07 mg betanin/100 mL failed to reduce inflammatory cytokines but increased catalase and total inflammatory cell counts in an asthma model [139].
In contrast to the studies mentioned above, an oral dose of 8 mL/kg beetroot juice containing betanin (mean 45.07 ± 1.53 mg/100 mL) and vulgaxanthin (mean 20.30 ± 0.69 mg/100 mL) did not reduce inflammatory cytokine levels but significantly increased catalase activity and total inflammatory cell count in an OVA-sensitized and challenged mouse model of asthma [39]. However, a mixed-population study including physician-diagnosed subjects with well-controlled asthma examined the effects of beetroot juice (commercially prepared) on cold symptoms. This study documented reduced cold symptoms, global sickness, and FeNO levels. Interestingly, the beneficial effects were more pronounced among asthmatic subjects [57]. These discrepancies warrant further exploration of experimental designs and asthma endotypes.
Other bioactive compounds in beetroot, such as saponins and vitamins, have been explored for their potential role in asthma management. For instance, asparagus cochinchinensis extract enriched with saponins demonstrated anti-inflammatory effects in OVA-induced asthma models [140]. Similarly, specific types of saponins and ginseng saponins have shown promise in managing asthma by mitigating oxygen deprivation and histamine effects, which are significant in allergic asthma [141,142,143,144].
Vitamin C is widely recognized for its antioxidant and anti-inflammatory properties, with studies demonstrating its efficacy in high doses for alleviating asthma symptoms [145,146]. When combined with calcitriol, vitamin C has been shown to reduce inflammation, oxidative stress, and airway remodeling in asthma-induced mouse models [147].
Beetroot contains all eight B-complex vitamins, though their link to asthma is underexplored [63,148]. Evidence from studies indicates varied associations: riboflavin intake shows a negative correlation with allergy [149], and long-term supplementation with vitamin B-6 (200 mg/day for 5 months) improved asthma symptoms in children [150]. Similarly, carotenoids such as alpha and beta-carotene, lutein, and lycopene are inversely correlated with asthma symptoms [151].
Cross-sectional studies highlight strong negative associations between asthma and high vitamin C intake [152]. and between folate, B-6, and B-12 intake and asthma and atopy in children [153]. The role of vitamin E is mixed, with some studies suggesting benefits in alleviating symptoms while others show no effect [154]. Low vitamin K intake is linked to an increased asthma risk [155]. Deficiencies in selenium, iron, and manganese have also been associated with exacerbated asthma symptoms, though evidence on the benefits of mineral supplementation remains inconclusive [156,157,158,159].
Fiber intake is negatively associated with asthma symptoms, with higher fiber consumption linked to improved lung function and reduced inflammation [160,161,162,163]. In a randomized trial, inulin supplementation (12 g/day) improved asthma control and reduced sputum eosinophils [164]. Conversely, oxalates are positively associated with bronchial obstruction, inflammation, and epithelial degeneration, contributing to respiratory issues [165,166,167].
High-carbohydrate diets, particularly starch and sucrose, are linked to asthma exacerbation in children and murine models [168,169,170].
To sum up, beetroot’s bioactive compounds benefit asthma management, but oxalates and sucrose may exacerbate it. Figure 2 summarizes these effects, while Table 2 summarizes factors influencing their availability.
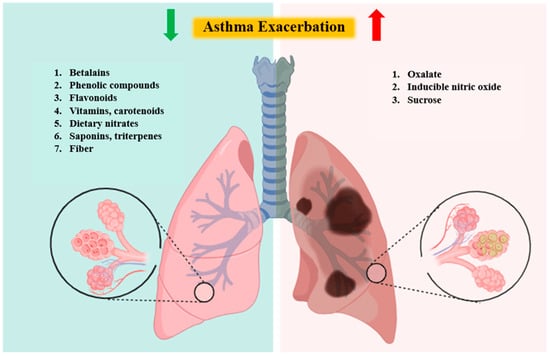
Figure 2.
Schematic presentation of beetroot-related compounds’ impact on asthma exacerbation.

Table 2.
Comparison of factors affecting the availability of betalains, oxalates, and sugars.
6. Conclusions
In conclusion, the evidence presented highlights the potential benefits of beetroot for asthma management due to its bioactive compounds, including polyphenols, flavonoids, saponins, vitamins, nitrate, fiber, and betalains. However, the antinutrient oxalate and sucrose may pose risks in asthma management. An ideal beetroot-based supplement could involve formulations with reduced oxalic acid and sucrose and increased betanin concentration. Processing techniques like microwave treatment, boiling, sun-drying, ultrasonication, and calcium salts could lower oxalate and sucrose levels. Assessing the impact of these techniques on betalain composition, particularly betanin, is critical. Comprehensive clinical studies are necessary to confirm the therapeutic potential, mechanisms, and safety of deoxalated, reduced-sucrose beetroot formulations or purified betalains for asthma treatment.
Author Contributions
Paper conception by M.A., N.S., I.S., L.V. and R.J.; M.A. drafted the manuscript, with all authors contributing to its review and edits. N.S., I.S., L.V., R.J. and S.D. provided guidance and support during the final drafting process. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Becker, A.B.; Abrams, E.M. Asthma guidelines: The Global Initiative for Asthma in relation to national guidelines. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 99–103. [Google Scholar] [CrossRef]
- Rutter, C.; Silverwood, R.; Pérez Fernández, V.; Pearce, N.; Strachan, D.; Mortimer, K.; Lesosky, M.; Asher, I.; Ellwood, P.; Chiang, C.-Y. The global burden of asthma. Int. J. Tuberc. Lung Dis. 2022, 26, 20–23. [Google Scholar]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Buendia, J.A.; Zuluaga, A.F.; Martínez-Rodríguez, C.E. Global and regional projections of the economic burden of Asthma: A value of statistical life approach. F1000Research 2025, 14, 146. [Google Scholar] [CrossRef]
- Ishmael, F.T. The inflammatory response in the pathogenesis of asthma. J. Osteopath. Med. 2011, 111, 11–17. [Google Scholar]
- Cho, Y.S.; Moon, H.-B. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 2010, 2, 183–187. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; Erzurum, S.C. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef]
- Albano, G.D.; Gagliardo, R.P.; Montalbano, A.M.; Profita, M. Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases. Antioxidants 2022, 11, 2237. [Google Scholar] [CrossRef]
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma exacerbations: Pathogenesis, prevention, and treatment. J. Allergy Clin. Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef]
- Mohammed, S.; Goodacre, S. Intravenous and nebulised magnesium sulphate for acute asthma: Systematic review and meta-analysis. Emerg. Med. J. 2007, 24, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.E.; Hurd, S.S.; Lemanske, R.F.; Becker, A.; Zar, H.J.; Sly, P.D.; Soto-Quiroz, M.; Wong, G.; Bateman, E.D. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr. Pulmonol. 2011, 46, 1–17. [Google Scholar] [CrossRef]
- Barnes, P.J. Drugs for asthma. Br. J. Pharmacol. 2006, 147, S297–S303. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Crockett, S.; Bedir, E.; Khan, I.A. The role of chemical fingerprinting: Application to Ephedra. Phytochemistry 2003, 62, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.K.; Cydulka, R.K. Asthma evaluation and management. Emerg. Med. Clin. 2003, 21, 315–330. [Google Scholar] [CrossRef]
- DiMartino, S.J. Idiopathic inflammatory myopathy: Treatment options. Curr. Rheumatol. Rep. 2008, 10, 321–327. [Google Scholar] [CrossRef]
- Kesler, S.M.; Sprenkle, M.D.; David, W.S.; Leatherman, J.W. Severe weakness complicating status asthmaticus despite minimal duration of neuromuscular paralysis. Intensive Care Med. 2009, 35, 157–160. [Google Scholar] [CrossRef]
- Persaud, P.N.; Tran, A.P.; Messner, D.; Thornton, J.D.; Williams, D.; Harper, L.J.; Tejwani, V. Perception of burden of oral and inhaled corticosteroid adverse effects on asthma-specific quality of life. Ann. Allergy Asthma Immunol. 2023, 131, 745–751.e11. [Google Scholar] [CrossRef]
- Khan, M.; Hirsch, C.; Jones, A.M. Suspected Adverse Drug Reactions Associated With Leukotriene Receptor Antagonists Versus First Line Asthma Medications: A National Registry-Pharmacology Approach. medRxiv 2024, in press. [Google Scholar] [CrossRef]
- Song, W.-J.; Lee, J.-H.; Kang, Y.; Joung, W.J.; Chung, K.F. Future risks in patients with severe asthma. Allergy Asthma Immunol. Res. 2019, 11, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.W.; Ghushchyan, V.H.; Globe, G.; Schatz, M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J. Allergy Clin. Immunol. 2018, 141, 110–116.e7. [Google Scholar] [CrossRef]
- Davis, S.R.; Ampon, R.D.; Poulos, L.M.; Lee, T.; Marks, G.B.; Toelle, B.G.; Reddel, H.K. Prevalence and burden of difficult-to-treat and severe asthma in Australia: A national population survey. Respirology 2024, 29, 685–693. [Google Scholar] [CrossRef]
- Miravitlles, M.; Auladell-Rispau, A.; Monteagudo, M.; Vázquez-Niebla, J.C.; Mohammed, J.; Nuñez, A.; Urrútia, G. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur. Respir. Rev. 2021, 30, 210075. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kim, K.; Hyun, M.K.; Jang, E.J.; Lee, N.R.; Yim, J.-J. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 2013, 68, 1105–1113. [Google Scholar] [CrossRef]
- Güner Zırıh, N.M.; Yılmaz Kara, B.; Özyurt, S.; Okçu, O.; İlgar, T.; Şahin, Ü. Giant lung cavity due to three different pathogens in a patient receiving inhaled salmeterol plus fluticasone propionate for asthma. J. Asthma 2024, 61, 643–648. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; García-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G.; Group, I.P.I.S. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Yi, S.; Ichimura, Y.; Hori, A.; Izumi, S.; Sugiyama, H.; Kudo, K.; Mizoue, T.; Kobayashi, N. Effect of lifestyle on asthma control in Japanese patients: Importance of periodical exercise and raw vegetable diet. PLoS ONE 2013, 8, e68290. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Wijga, A.H.; Brunekreef, B.; Scholtens, S.; Postma, D.S.; Kerkhof, M.; de Jongste, J.C.; Smit, H.A. Childhood diet and asthma and atopy at 8 years of age: The PIAMA birth cohort study. Eur. Respir. J. 2011, 37, 1060–1067. [Google Scholar] [CrossRef]
- Butland, B.; Strachan, D.; Anderson, H. Fresh fruit intake and asthma symptoms in young British adults: Confounding or effect modification by smoking? Eur. Respir. J. 1999, 13, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Uddenfeldt, M.; Janson, C.; Lampa, E.; Leander, M.; Norbäck, D.; Larsson, L.; Rask-Andersen, A. High BMI is related to higher incidence of asthma, while a fish and fruit diet is related to a lower–: Results from a long-term follow-up study of three age groups in Sweden. Respir. Med. 2010, 104, 972–980. [Google Scholar] [CrossRef]
- La Vecchia, C.; Decarli, A.; Pagano, R. Vegetable consumption and risk of chronic disease. Epidemiology 1998, 9, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Shergis, J.L.; Wu, L.; Zhang, A.L.; Guo, X.; Lu, C.; Xue, C.C. Herbal medicine for adults with asthma: A systematic review. J. Asthma 2016, 53, 650–659. [Google Scholar] [CrossRef]
- Clarke, R.; Lundy, F.; McGarvey, L. Herbal treatment in asthma and COPD–current evidence. Clin. Phytosci. 2015, 1, 4. [Google Scholar] [CrossRef]
- Ajaz, M.; Singh, I.; Vugic, L.; Jani, R.; Rathnayake, H.; Diyapaththugama, S.; Mulaw, G.F.; Colson, N.J. The Interplay of Plant-Based Antioxidants, Inflammation, and Clinical Outcomes in Asthma: A Systematic Review. Respir. Med. 2024, 236, 107918. [Google Scholar] [CrossRef] [PubMed]
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Qutub, M.; Pushparaj, P.N.; Heinrich, M. Nigella sativa supplementation improves asthma control and biomarkers: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2017, 31, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Manarin, G.; Anderson, D.; Silva, J.M.e.; Coppede, J.d.S.; Roxo-Junior, P.; Pereira, A.M.S.; Carmona, F. Curcuma longa L. ameliorates asthma control in children and adolescents: A randomized, double-blind, controlled trial. J. Ethnopharmacol. 2019, 238, 111882. [Google Scholar] [CrossRef]
- Fouladi, S.; Masjedi, M.; Hakemi, M.G.; Eskandari, N. The review of in vitro and in vivo studies over the glycyrrhizic acid as natural remedy option for treatment of allergic asthma. Iran. J. Allergy Asthma Immunol. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- El-Elimat, T.; Al-khawlani, A.R.; Al-Sawalha, N.A.; Sa’ed, M.M.; Al-Qiam, R.; Sharie, A.H.A.; Qinna, N.A. The effect of beetroot juice on airway inflammation in a murine model of asthma. J. Food Biochem. 2022, 46, e14381. [Google Scholar] [CrossRef]
- Chawla, H.; Parle, M.; Sharma, K.; Yadav, M. Beetroot: A health promoting functional food. Inven. Rapid Nutraceuticals 2016, 1, 0976–3872. [Google Scholar]
- Mikołajczyk-Bator, K.; Błaszczyk, A.; Czyżniejewski, M.; Kachlicki, P. Characterisation and identification of triterpene saponins in the roots of red beets (Beta vulgaris L.) using two HPLC–MS systems. Food Chem. 2016, 192, 979–990. [Google Scholar] [CrossRef] [PubMed]
- dos S. Baião, D.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, A Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Yashwant, K. Beetroot: A super food. Int. J. Eng. Stud. Tech. Approach 2015, 1, 20–26. [Google Scholar]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef]
- Dhiman, A.; Suhag, R.; Chauhan, D.S.; Thakur, D.; Chhikara, S.; Prabhakar, P.K. Status of beetroot processing and processed products: Thermal and emerging technologies intervention. Trends Food Sci. Technol. 2021, 114, 443–458. [Google Scholar] [CrossRef]
- Carrillo, C.; Wilches-Pérez, D.; Hallmann, E.; Kazimierczak, R.; Rembiałkowska, E. Organic versus conventional beetroot. Bioactive compounds and antioxidant properties. LWT 2019, 116, 108552. [Google Scholar] [CrossRef]
- Czapski, J.; Mikołajczyk, K.; Kaczmarek, M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Pol. J. Food Nutr. Sci. 2009, 59, 119–122. [Google Scholar]
- Muramatsu, D.; Uchiyama, H.; Higashi, H.; Kida, H.; Iwai, A. Effects of heat degradation of betanin in red beetroot (Beta vulgaris L.) on biological activity and antioxidant capacity. PLoS ONE 2023, 18, e0286255. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 2014, 983952. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S. Chemistry and pharmacological effect of Beta vulgaris: A systematic review. Der Pharm. Lett. 2016, 8, 404–409. [Google Scholar]
- Ormsbee, M.J.; Lox, J.; Arciero, P.J. Beetroot juice and exercise performance. Nutr. Diet. Suppl. 2013, 5, 27–35. [Google Scholar] [CrossRef]
- Coles, L.T.; Clifton, P.M. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: A randomized, placebo-controlled trial. Nutr. J. 2012, 11, 106. [Google Scholar] [CrossRef]
- Zielińska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In Vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, Y.; Wang, N. Betalain alleviates airway inflammation in an ovalbumin-induced-asthma mouse model via the TGF-β1/Smad signaling pathway. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, Y.; Guo, X.; Xu, Y.; Mao, Y.; Wu, Y.; He, F.; Wang, C.; Chen, Y.; Yang, Y. Betanin Dose-Dependently Ameliorates Allergic Airway Inflammation by Attenuating Th2 Response and Upregulating cAMP–PKA–CREB Pathway in Asthmatic Mice. J. Agric. Food Chem. 2022, 70, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Werchan, C.A.; Kroll, J.L.; Rosenfield, D. Beetroot juice supplementation for the prevention of cold symptoms associated with stress: A proof-of-concept study. Physiol. Behav. 2019, 202, 45–51. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Sharma, N.; Sanwal, N.; Lorenzo, J.M.; Sahu, J.K. Bioactive potential of beetroot (Beta vulgaris). Food Res. Int. 2022, 158, 111556. [Google Scholar] [CrossRef]
- Deshmukh, G.; Inka, P.; Sindhav, R.; Jose, N. Application of beetroot as natural coloring pigment and functional ingredient in dairy and food products. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2010–2016. [Google Scholar] [CrossRef]
- Kale, R.; Sawate, A.; Kshirsagar, R.; Patil, B.; Mane, R. Studies on evaluation of physical and chemical composition of beetroot (Beta vulgaris L.). Int. J. Chem. Stud. 2018, 6, 2977–2979. [Google Scholar]
- Sentkowska, A.; Pyrzyńska, K. Old-Fashioned, but Still a Superfood—Red Beets as a Rich Source of Bioactive Compounds. Appl. Sci. 2023, 13, 7445. [Google Scholar] [CrossRef]
- Ceclu, L.; Oana-Viorela, N. Red Beetroot: Composition and Health Effects—A Review. J. Nutr. Med. Diet Care 2020, 6, 43. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Salovaara, S.; Sandberg, A.-S.; Andlid, T. Organic acids influence iron uptake in the human epithelial cell line Caco-2. J. Agric. Food Chem. 2002, 50, 6233–6238. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Phytochemical Constituent Grass, Herbs and Other Economic Plants: Herbal Reference Library; Routledge: Oxfordshire, UK, 2017. [Google Scholar]
- Food Standards Australia New Zealand. Australian Food Composition Database: Beetroot, Fresh, Purple, Peeled, Boiled, Drained (F001014). Available online: https://afcd.foodstandards.gov.au/fooddetails.aspx?PFKID=F001014 (accessed on 5 July 2025).
- Kazimierczak, R.; Siłakiewicz, A.; Hallmann, E.; Srednicka-Tober, D.; Rembiałkowska, E. Chemical composition of selected beetroot juices in relation to beetroot production system and processing technology. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 491–498. [Google Scholar] [CrossRef]
- Vasconcellos, J.; Conte-Junior, C.; Silva, D.; Pierucci, A.P.; Paschoalin, V.; Alvares, T.S. Comparison of total antioxidant potential, and total phenolic, nitrate, sugar, and organic acid contents in beetroot juice, chips, powder, and cooked beetroot. Food Sci. Biotechnol. 2016, 25, 79–84. [Google Scholar] [CrossRef]
- Silva, D.V.T.d.; Silva, F.d.O.; Perrone, D.; Pierucci, A.P.T.R.; Conte-Junior, C.A.; Alvares, T.d.S.; Aguila, E.M.D.; Paschoalin, V.M.F. Physicochemical, nutritional, and sensory analyses of a nitrate-enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food Nutr. Res. 2016, 60, 29909. [Google Scholar] [CrossRef] [PubMed]
- Shyamala, B.; Jamuna, P. Nutritional content and antioxidant properties of pulp waste from Daucus carota and Beta vulgaris. Malays. J. Nutr. 2010, 16, 397–408. [Google Scholar] [PubMed]
- Platosz, N.; Sawicki, T.; Wiczkowski, W. Profile of phenolic acids and flavonoids of red beet and its fermentation products. Does long-term consumption of fermented beetroot juice affect phenolics profile in human blood plasma and urine? Pol. J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]
- Baião, D.d.S.; da Silva, D.V.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. Food Addit. 2017, 6, 21–43. [Google Scholar]
- Fu, Y.; Shi, J.; Xie, S.-Y.; Zhang, T.-Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef]
- Gawałek, J. Effect of Spray Dryer Scale Size on the Properties of Dried Beetroot Juice. Molecules 2021, 26, 6700. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Martinez-Villaluenga, C.; Frias, J.; Wiczkowski, W.; Peñas, E.; Bączek, N.; Zieliński, H. The effect of processing and in vitro digestion on the betalain profile and ACE inhibition activity of red beetroot products. J. Funct. Foods 2019, 55, 229–237. [Google Scholar] [CrossRef]
- Hamid, M.G.; Mohamed Nour, A.A.A. Effect of different drying methods on quality attributes of beetroot (Beta vulgaris) slices. World J. Sci. Technol. Sustain. Dev. 2018, 15, 287–298. [Google Scholar] [CrossRef]
- Costa, A.P.D.; Hermes, V.S.; Rios, A.d.O.; Flôres, S.H. Minimally processed beetroot waste as an alternative source to obtain functional ingredients. J. Food Sci. Technol. 2017, 54, 2050–2058. [Google Scholar] [CrossRef]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Chemistry and Pharmacology of Natural Products; Cambridge University Press: Cambridge, UK, 1995; Volume 548. [Google Scholar]
- Murakami, T.; Matsuda, H.; Inadzuki, M.; Hirano, K.; Yoshikawa, M. Medicinal Foodstuffs. XVI. Sugar beet. (3): Absolute stereostructures of betavulgarosides II and IV, hypoglycemic saponins having a unique substituent, from the roots of Beta vulgaris L. Chem. Pharm. Bull. 1999, 47, 1717–1724. [Google Scholar] [CrossRef][Green Version]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Szopińska, A.A.; Gawęda, M. Comparison of Yield and Quality of Red Beet Roots Cultivated Using Conventional, Integrated and Organic Method. J. Hortic. Res. 2013, 21, 107–114. [Google Scholar] [CrossRef]
- Pavlović, N.V.; Mladenović, J.; Stevović, V.; Bošković-Rakočević, L.; Moravčević, Đ.; Poštić, D.; Zdravković, J. Effect of processing on vitamin C content, total phenols and antioxidative activity of organically grown red beetroot (Beta vulgaris ssp. Rubra). Food Feed Res. 2021, 48, 131–139. [Google Scholar] [CrossRef]
- Abdo, E.; El-Sohaimy, S.; Shaltout, O.; Abdalla, A.; Zeitoun, A. Nutritional Evaluation of Beetroots (Beta vulgaris L.) and Its Potential Application in a Functional Beverage. Plants 2020, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Baião, D.; Silva, F.; d’El-Rei, J.; Neves, M.; Perrone, D.; Del Aguila, E.; Paschoalin, V. A new functional beetroot formulation enhances adherence to nitrate supplementation and health outcomes in clinical practice. SDRP J. Food Sci. Technol 2018, 3, 484–498. [Google Scholar]
- Mubajje, M.S. The Potential Use of Beetroot (Beta vulgaris L.) Powder to Complement Dietary Iron Intake of Adolescent School Girls (10–19 Years Old). Master’s Thesis, Makerere University, Kampala, Uganda, 2021. [Google Scholar]
- Natesh, H.; Abbey, L.; Asiedu, S. An overview of nutritional and antinutritional factors in green leafy vegetables. Hortic. Int. J. 2017, 1, 00011. [Google Scholar]
- Lisiewska, Z.; Gebczynski, P.; Slupski, J. Effect of processing and cooking on total and soluble oxalate content in frozen root vegetables prepared for consumption. Agric. Food Sci. 2011, 20, 305–314. [Google Scholar] [CrossRef]
- Srivastava, S.; Pandey, V.K.; Singh, P.; Bhagya Raj, G.V.S.; Dash, K.K.; Singh, R. Effects of microwave, ultrasound, and various treatments on the reduction of antinutritional factors in elephant foot yam: A review. eFood 2022, 3, e40. [Google Scholar] [CrossRef]
- Kala, B.; Mohan, V. Effect of microwave treatment on the antinutritional factors of two accessions of velvet bean, Mucuna pruriens (L.) DC. var. utilis (Wall. ex Wight) Bak. ex Burck. Int. Food Res. J. 2012, 19, 961–969. [Google Scholar]
- Jat, K.; Jayachandran, L.E.; Rao, P.S. Impact of temperature assisted ultrasonication on the quality attributes of beetroot (Beta vulgaris L.) juice. J. Food Process Eng. 2023, 46, e14329. [Google Scholar] [CrossRef]
- Enkhtuya, N.; Baatar, D.; Odontuya, L. Possible ways of decreasing oxalate content in red beet foods. Proc. Mong. Acad. Sci. 2017, 52, 55. [Google Scholar] [CrossRef]
- Babatunde, O.M.; Ibukun, O.E. Effect of deoxalation on in-vitro antioxidant activity and inhibition of ferric induced lipid peroxidation of beetroot juice. Coast J. Sch. Sci. Oaustech Okitipupa 2020, 2, 356–367. [Google Scholar]
- Zhen, G.M. Study on the correlation between root weight and sugar content in sugar beet. China Sugarbeet. 1995, 1, 16–19. [Google Scholar]
- Hoffmann, C.M.; Kenter, C. Yield potential of sugar beet–have we hit the ceiling? Front. Plant Sci. 2018, 9, 289. [Google Scholar] [CrossRef]
- Diego dos, S.B.; Davi, V.T.d.S.; Eduardo, M.D.A.; Vânia, M.F.P. Nutritional, Bioactive and Physicochemical Characteristics of Different Beetroot Formulations. In Food Additives; Desiree Nedra, K., Geethi, P., Eds.; IntechOpen: Rijeka, Croatia, 2017; p. Ch.2. [Google Scholar]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T. In Vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Desseva, I.; Stoyanova, M.; Petkova, N.; Mihaylova, D. Red beetroot juice phytochemicals bioaccessibility: An In Vitro approach. Pol. J. Food Nutr. Sci. 2020, 70, 45–53. [Google Scholar] [CrossRef]
- Guldiken, B.; Toydemir, G.; Nur Memis, K.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Volino-Souza, M.; Oliveira, G.V.d.; Conte-Junior, C.A.; Alvares, T.S. COVID-19 Quarantine: Impact of Lifestyle Behaviors Changes on Endothelial Function and Possible Protective Effect of Beetroot Juice. Front. Nutr. 2020, 7, 582210. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Martínez, J.A.; Portillo, M.P. Current Knowledge on Beetroot Bioactive Compounds: Role of Nitrate and Betalains in Health and Disease. Foods 2021, 10, 1314. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef]
- Davi Vieira Teixeira da, S.; Diego dos Santos, B.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Eduardo Mere Del, A. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- Bargagli, M.; Tio, M.C.; Waikar, S.S.; Ferraro, P.M. Dietary Oxalate Intake and Kidney Outcomes. Nutrients 2020, 12, 2673. [Google Scholar] [CrossRef]
- Ramírez-Ojeda, A.; Moreno-Rojas, R.; Cámara-Martos, F. Mineral and trace element content in legumes (lentils, chickpeas and beans): Bioaccesibility and probabilistic assessment of the dietary intake. J. Food Compos. Anal. 2018, 73, 17–28. [Google Scholar] [CrossRef]
- Igual, M.; Fernandes, Â.; Dias, M.I.; Pinela, J.; García-Segovia, P.; Martínez-Monzó, J.; Barros, L. The In Vitro Simulated Gastrointestinal Digestion Affects the Bioaccessibility and Bioactivity of Beta vulgaris Constituents. Foods 2023, 12, 338. [Google Scholar] [CrossRef]
- Jelena, C.H.; Giorgio, R.; Justyna, G.; Neda, M.-D.; Natasa, S.; Artur, B.; Giuseppe, G. Beneficial effects of polyphenols on chronic diseases and ageing. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–102. [Google Scholar]
- Xiao, J.; Hogger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Ma, C.; Lv, L.; Feng, J.; Han, S. Gallic acid attenuates allergic airway inflammation via suppressed interleukin-33 and group 2 innate lymphoid cells in ovalbumin-induced asthma in mice. In International Forum of Allergy & Rhinology; Wiley Online Library: Hoboken, NJ, USA, 2018. [Google Scholar]
- Singla, E.; Dharwal, V.; Naura, A.S. Gallic acid protects against the COPD-linked lung inflammation and emphysema in mice. Inflamm. Res. 2020, 69, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, T.; Fu, Y.; Yu, T.; Ding, Y.; Nie, H. Ferulic Acid: A Review of Pharmacology, Toxicology, and Therapeutic Effects on Pulmonary Diseases. Int. J. Mol. Sci. 2023, 24, 8011. [Google Scholar] [CrossRef]
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 119958. [Google Scholar] [CrossRef]
- Saeedavi, M.; Goudarzi, M.; Mehrzadi, S.; Basir, Z.; Hasanvand, A.; Hosseinzadeh, A. Sinapic acid ameliorates airway inflammation in murine ovalbumin-induced allergic asthma by reducing Th2 cytokine production. Life Sci. 2022, 307, 120858. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, A.A.; Dianat, M.; Jalali, A. Evaluation of the Effect of Caffeic Acid Phenethyl Ester (CAPE) on Pharmacological Responses of Isolated Rat Trachea In Vitro. Tanaffos 2020, 19, 256. [Google Scholar] [PubMed]
- Lin, L.-J.; Huang, H.Y. DFSG, a novel herbal cocktail with anti-asthma activity, suppressed MUC5AC in A549 cells and alleviated allergic airway hypersensitivity and inflammatory cell infiltration in a chronic asthma mouse model. Biomed. Pharmacother. 2020, 121, 109584. [Google Scholar] [CrossRef]
- Molitorisova, M.; Sutovska, M.; Kazimierova, I.; Barborikova, J.; Joskova, M.; Novakova, E.; Franova, S. The anti-asthmatic potential of flavonol kaempferol in an experimental model of allergic airway inflammation. Eur. J. Pharmacol. 2021, 891, 173698. [Google Scholar] [CrossRef]
- Li, X.; Jin, F.; Lee, H.J.; Lee, C.J. Kaempferol regulates the expression of airway MUC5AC mucin gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 signaling pathways. Biomol. Ther. 2021, 29, 303. [Google Scholar] [CrossRef]
- Podder, B.; Song, K.S.; Song, H.-Y.; Kim, Y.-S. Cytoprotective effect of kaempferol on paraquat-exposed BEAS-2B cells via modulating expression of MUC5AC. Biol. Pharm. Bull. 2014, 37, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.K.; Costa, M.F.; Pitombeira, M.; Barroso, V.M.; Silveira, E.R.; Canuto, K.M.; Viana, G.S. Mechanisms underlying the relaxation induced by isokaempferide from Amburana cearensis in the guinea-pig isolated trachea. Life Sci. 2006, 79, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Nie, X. Afzelin attenuates asthma phenotypes by downregulation of GATA3 in a murine model of asthma. Mol. Med. Rep. 2015, 12, 71–76. [Google Scholar] [CrossRef]
- Chung, M.J.; Pandey, R.P.; Choi, J.W.; Sohng, J.K.; Choi, D.J.; Park, Y.I. Inhibitory effects of kaempferol-3-O-rhamnoside on ovalbumin-induced lung inflammation in a mouse model of allergic asthma. Int. Immunopharmacol. 2015, 25, 302–310. [Google Scholar] [CrossRef]
- Cho, I.-H.; Gong, J.-H.; Kang, M.-K.; Lee, E.-J.; Park, J.H.Y.; Park, S.-J.; Kang, Y.-H. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulm. Med. 2014, 14, 122. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, Y.-J.; Kang, M.-K.; Park, S.-H.; Antika, L.D.; Lee, E.-J.; Kim, D.Y.; Kang, Y.-H. Astragalin inhibits allergic inflammation and airway thickening in ovalbumin-challenged mice. J. Agric. Food Chem. 2017, 65, 836–845. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, A.Y.; Lim, J.-O.; Lee, S.-J.; Kim, W.-I.; Yang, Y.-G.; Kim, B.; Kim, J.-S.; Chae, S.-W.; Na, K.; et al. Loranthus tanakae Franch. & Sav. Suppresses Inflammatory Response in Cigarette Smoke Condensate Exposed Bronchial Epithelial Cells and Mice. Antioxidants 2022, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez-Vizcaíno, F.; Zarzuelo, A.; Jiménez, J.; Tamargo, J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1993, 239, 1–7. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Korbut, R.; Robak, J.; Świȩs, J. On the mechanism of antithrombotic action of flavonoids. Biochem. Pharmacol. 1987, 36, 317–322. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Zhang, X.; Zhang, X.; Chen, S.; Hu, Z.; Zhou, C.; Zhang, E.; Ma, S. Astragalin attenuates allergic inflammation in a murine asthma model. Inflammation 2015, 38, 2007–2016. [Google Scholar] [CrossRef]
- Paudel, K.R.; Wadhwa, R.; Mehta, M.; Chellappan, D.K.; Hansbro, P.M.; Dua, K. Rutin loaded liquid crystalline nanoparticles inhibit lipopolysaccharide induced oxidative stress and apoptosis in bronchial epithelial cells in vitro. Toxicol. Vitr. 2020, 68, 104961. [Google Scholar] [CrossRef]
- Mehta, M.; Paudel, K.R.; Shukla, S.D.; Shastri, M.D.; Satija, S.; Singh, S.K.; Gulati, M.; Dureja, H.; Zacconi, F.C.; Hansbro, P.M. Rutin-loaded liquid crystalline nanoparticles attenuate oxidative stress in bronchial epithelial cells: A PCR validation. Future Med. Chem. 2021, 13, 543–549. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A review on the dietary flavonoid tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef] [PubMed]
- Soodaeva, S.; Klimanov, I.; Kubysheva, N.; Popova, N.; Batyrshin, I. The state of the nitric oxide cycle in respiratory tract diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4859260. [Google Scholar] [CrossRef]
- Sriboonyong, T.; Kawamatawong, T.; Sriwantana, T.; Srihirun, S.; Titapiwatanakun, V.; Vivithanaporn, P.; Pornsuriyasak, P.; Sibmooh, N.; Kamalaporn, H. Efficacy and safety of inhaled nebulized sodium nitrite in asthmatic patients. Pulm. Pharmacol. Ther. 2021, 66, 101984. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Krajka-Kuźniak, V.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Evaluation of the effect of beetroot juice on DMBA-induced damage in liver and mammary gland of female sprague–dawley rats. Phytother. Res. 2014, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Alqasmi, I. Ameliorative potential of betanin on cigarette smoke extract-induced respiratory mucosal inflammation and oxidative stress in the adult zebrafish model. Pharmacogn. Mag. 2023, 19, 244–253. [Google Scholar] [CrossRef]
- Tural, K.; Ozden, O.; Bilgi, Z.; Kubat, E.; Ermutlu, C.S.; Merhan, O.; Findik Guvendi, K.; Kucuker, S.A. The protective effect of betanin and copper on heart and lung in end-organ ischemia reperfusion injury. Bratisl. Med. J. Bratisl. Lek. Listy 2020, 121, 211–217. [Google Scholar] [CrossRef]
- Sung, J.-E.; Lee, H.-A.; Kim, J.-E.; Yun, W.-B.; An, B.-S.; Yang, S.-Y.; Kim, D.-S.; Lee, C.-Y.; Lee, H.-S.; Bae, C.-J. Saponin-enriched extract of Asparagus cochinchinensis alleviates airway inflammation and remodeling in ovalbumin-induced asthma model. Int. J. Mol. Med. 2017, 40, 1365–1376. [Google Scholar] [CrossRef]
- Xue, K.; Ruan, L.; Hu, J.; Fu, Z.; Tian, D.; Zou, W. Panax notoginseng saponin R1 modulates TNF-α/NF-κB signaling and attenuates allergic airway inflammation in asthma. Int. Immunopharmacol. 2020, 88, 106860. [Google Scholar] [CrossRef]
- Alternative Medicine: Expanding Medical Horizons: A Report to the National Institutes of Health on Alternative Medical Systems and Practices in the United States; Alternative Medicine: Chantilly, VA, USA, 1995; pp. 183–206.
- Bielory, L.; Lupoli, K. Herbal interventions in asthma and allergy. J. Asthma 1999, 36, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Chantilly, V.; Achterberg, J.; Ansher, I.; Potomac, M.; Arnold, L.E.; Expert, S.; Bahor, R.; Barbatsis, B.; Barlow, E.; Barnard, R.J. Alternative Medicine: Expanding Medical Horizons; University Press of the Pacific Publisher: Honolulu, HI, USA, 2002. [Google Scholar]
- Jeong, Y.-J.; Kim, J.-H.; Kang, J.S.; Lee, W.J.; Hwang, Y.-i. Mega-dose vitamin C attenuated lung inflammation in mouse asthma model. Anat. Cell Biol. 2010, 43, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Chen, C.-S.; Lin, J.-Y. High dose vitamin C supplementation increases the Th1/Th2 cytokine secretion ratio, but decreases eosinophilic infiltration in bronchoalveolar lavage fluid of ovalbumin-sensitized and challenged mice. J. Agric. Food Chem. 2009, 57, 10471–10476. [Google Scholar] [CrossRef] [PubMed]
- Kianian, F.; Karimian, S.M.; Kadkhodaee, M.; Takzaree, N.; Seifi, B.; Sadeghipour, H.R. Protective effects of ascorbic acid and calcitriol combination on airway remodelling in ovalbumin-induced chronic asthma. Pharm. Biol. 2020, 58, 107–115. [Google Scholar] [CrossRef]
- Zajac, D.; Wojciechowski, P. The Role of Vitamins in the Pathogenesis of Asthma. Int. J. Mol. Sci. 2023, 24, 8574. [Google Scholar] [CrossRef]
- Quyen, D.T.; Irei, A.V.; Sato, Y.; Ota, F.; Fujimaki, Y.; Sakai, T.; Kunii, D.; Khan, N.C.; Yamamoto, S. Nutritional factors, parasite infection and allergy in rural and suburban Vietnamese school children. J. Med. Investig. 2004, 51, 171–177. [Google Scholar] [CrossRef][Green Version]
- Collipp, P.; Goldzier, S.; Weiss, N.; Soleymani, Y.; Snyder, R. Pyridoxine treatment of childhood bronchial asthma. Ann. Allergy 1975, 35, 93–97. [Google Scholar] [PubMed]
- Zhang, W.; Li, W.; Du, J. Association between dietary carotenoid intakes and the risk of asthma in adults: A cross-sectional study of NHANES, 2007–2012. BMJ Open 2022, 12, e052320. [Google Scholar] [CrossRef]
- García-García, C.; Kim, M.; Baik, I. Associations of dietary vitamin A and C intake with asthma, allergic rhinitis, and allergic respiratory diseases. Nutr. Res. Pract. 2023, 17, 997–1006. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, S.-Y.; Kwon, S.-O.; Kang, M.-J.; Seo, J.-H.; Yoon, J.; Cho, H.-J.; Jung, S.; Hong, S.-J. The association between MTHFR polymorphism, dietary methyl donors, and childhood asthma and atopy. Asian Pac. J. Allergy Immunol. 2023, 43, 244–253. [Google Scholar]
- Shams, M.-H.; Jafari, R.; Eskandari, N.; Masjedi, M.; Kheirandish, F.; Ganjalikhani Hakemi, M.; Ghasemi, R.; Varzi, A.-M.; Sohrabi, S.-M.; Baharvand, P.A.; et al. Anti-allergic effects of vitamin E in allergic diseases: An updated review. Int. Immunopharmacol. 2021, 90, 107196. [Google Scholar] [CrossRef]
- Jespersen, T.; Kampmann, F.B.; Dantoft, T.M.; Jørgensen, N.R.; Kårhus, L.L.; Madsen, F.; Linneberg, A.; Thysen, S.M. The association of vitamin K status with lung function and disease in a general population. ERJ Open Res. 2023, 9, 00208-2023. [Google Scholar] [CrossRef] [PubMed]
- Vlašić, Ž.; Dodig, S.; Čepelak, I.; Topić, R.Z.; Živčić, J.; Nogalo, B.; Turkalj, M. Iron and ferritin concentrations in exhaled breath condensate of children with asthma. J. Asthma 2009, 46, 81–85. [Google Scholar] [CrossRef]
- Kadrabová, J.; Mad’arič, A.; Kovačiková, Z.; Podivínsky, F.; Ginter, E.; Gazdík, F. Selenium status is decreased in patients with intrinsic asthma. Biol. Trace Elem. Res. 1996, 52, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Uysalol, M.; Uysalol, E.P.; Yilmaz, Y.; Parlakgul, G.; Ozden, T.A.; Ertem, H.V.; Omer, B.; Uzel, N. Serum level of vitamin D and trace elements in children with recurrent wheezing: A cross-sectional study. BMC Pediatr. 2014, 14, 270. [Google Scholar] [CrossRef]
- Koumpagioti, D.; Boutopoulou, B.; Douros, K. Chapter 29—The Mediterranean diet and asthma. In The Mediterranean Diet, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 327–336. [Google Scholar]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Gribben, K.C.; Alam, M.; Lyden, E.R.; Hanson, C.K.; LeVan, T.D. Association of dietary fiber on asthma, respiratory symptoms, and inflammation in the adult national health and nutrition examination survey population. Ann. Am. Thorac. Soc. 2020, 17, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, K.; Son, S.; Kim, Y.-C.; Kwak, J.W.; Kim, H.G.; Lee, S.H.; Kim, T.H. Association of allergic diseases and related conditions with dietary fiber intake in Korean adults. Int. J. Environ. Res. Public Health 2021, 18, 2889. [Google Scholar] [CrossRef]
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between dietary fibre intake and asthma (symptoms and control): Results from the French national e-cohort NutriNet-Santé. Br. J. Nutr. 2019, 122, 1040–1051. [Google Scholar] [CrossRef]
- McLoughlin, R.; Berthon, B.S.; Rogers, G.B.; Baines, K.J.; Leong, L.E.; Gibson, P.G.; Williams, E.J.; Wood, L.G. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine 2019, 46, 473–485. [Google Scholar] [CrossRef]
- Klimanov, I.A.; Soodaeva, S.; Tush, E.; Obykhov, A.; Ovsyannikov, D.; Vanyakina, S.V.; Khaletskaya, O.; Glukhova, M.; Nikitina, L. Oxalate excretion in patients with allergic airway diseases. Eur. Respir. Soc. 2021, 58 (Suppl. 65), PA725. [Google Scholar]
- Shaĭlieva, L.O.; Fedoseev, G.B.; Zorina, M.L.; Petrova, M.A.; Trofimov, V.I.; Kakliugin, A.P. Clinical, laboratory, and functional characteristic of patients with bronchial asthma and chronic obstructive pulmonary disease with disturbances of oxalic acid metabolism. Klin. Meditsina 2013, 91, 36–40. [Google Scholar]
- Fedoseev, G.B.; Petrova, M.A.; Shaĭlieva, L.O.; Kakliugin, A.P.; Zorina, M.L.; Sakharov, A.N.; Pavliukova, N.O. Clinical characteristics and condition of the bronchial tree in patients with bronchial asthma and chronic obstructive pulmonary disease in combination with hyperoxaluria. Ter. Arkhiv 2007, 79, 37–41. [Google Scholar]
- Antonio Buendia, J.; Acuña-Cordero, R.; Patiño, D.G. The role of high carbohydrate-rich food intake and severity of asthma exacerbation in children between 2 to 6 years aged. J. Asthma 2023, 60, 412–418. [Google Scholar] [CrossRef]
- Musiol, S.; Harris, C.P.; Karlina, R.; Gostner, J.M.; Rathkolb, B.; Schnautz, B.; Schneider, E.; Mair, L.; Vergara, E.E.; Flexeder, C. Dietary digestible carbohydrates are associated with higher prevalence of asthma in humans and with aggravated lung allergic inflammation in mice. Allergy 2023, 78, 1218–1233. [Google Scholar] [CrossRef]
- Kim, H.J.; Dinh, D.T.T.; Yang, J.; Herath, K.H.I.N.M.; Seo, S.H.; Son, Y.-O.; Kang, I.; Jee, Y. High sucrose intake exacerbates airway inflammation through pathogenic Th2 and Th17 response in ovalbumin (OVA)-induced acute allergic asthma in C57BL/6 mice. J. Nutr. Biochem. 2024, 124, 109504. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Jiménez, A.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Bsc, S.N.; Bsc, G.S. Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Reiser, P. Sucrose: Properties and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Jackman, R.; Smith, J. Anthocyanins and betalains. In Natural Food Colorants; Springer: Berlin/Heidelberg, Germany, 1996; pp. 244–309. [Google Scholar]
- Kumar, A.; Patel, A.A.; Singh, R.B.; Desai, K. Alkali pre-soaking effects on acridity, colour parameters and oxalate content of elephant foot yam. J. Root Crops 2013, 39, 88–95. [Google Scholar]
- Panpae, K.; Jaturonrusmee, W.; Mingvanish, W.; Nuntiwattanawong, C.; Chunwiset, S.; Santudrob, K.; Triphanpitak, S. Minimization of sucrose losses in sugar industry by pH and temperature optimization. Malays. J. Anal. Sci. 2008, 12, 513–519. [Google Scholar]
- Rodríguez-Sevilla, M.D.; Villanueva-Suárez, M.J.; Redondo-Cuenca, A. Effects of processing conditions on soluble sugars content of carrot, beetroot and turnip. Food Chem. 1999, 66, 81–85. [Google Scholar] [CrossRef]
- Özyurt, G.; Uslu, L.; Durmuş, M.; Sakarya, Y.; Uzlaşir, T.; Küley, E. Chemical and physical characterization of microencapsulated Spirulina fermented with Lactobacillus plantarum. Algal Res. 2023, 73, 103149. [Google Scholar] [CrossRef]
- Czyzowska, A.; Siemianowska, K.; Sniadowska, M.; Nowak, A. Bioactive compounds and microbial quality of stored fermented red beetroots and red beetroot juice. Pol. J. Food Nutr. Sci. 2020, 70, 35–44. [Google Scholar] [CrossRef]
- Wadamori, Y.; Vanhanen, L.; Savage, G.P. Effect of Kimchi Fermentation on Oxalate Levels in Silver Beet (Beta vulgaris var. cicla). Foods 2014, 3, 269–278. [Google Scholar] [CrossRef]
- Otegbayo, B.O.; Akwa, I.M.; Tanimola, A.R. Physico-chemical properties of beetroot (Beta vulgaris L.) wine produced at varying fermentation days. Sci. Afr. 2020, 8, e00420. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; He, J.; Liu, T.; Guo, X. Influences of Ultrasonic Treatments on the Structure and Antioxidant Properties of Sugar Beet Pectin. Foods 2023, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Bong, W.-C.; Vanhanen, L.P.; Savage, G.P. Addition of calcium compounds to reduce soluble oxalate in a high oxalate food system. Food Chem. 2017, 221, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Pannetier, N.; Khoukh, A.; François, J. Physico-chemical study of sucrose and calcium ions interactions in alkaline aqueous solutions. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2001. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).