Robotic Bronchoscopy: A Comprehensive Review
Abstract
1. Introduction
2. History
3. Robotic Bronchoscopy
- Monarch™ Platform (FDA 2018);
- Ion™ Endoluminal RB Platform (FDA 2019);
- Galaxy System™ (FDA 2023).
- The planning phase—The systems require data from thin-slice CT scans to plan the pathway and navigate to the desired target using specialized software. This phase is typically performed on the same day or the day before the actual procedure.
- The guidance and biopsy phase—The bronchoscope is systematically guided and advanced through the bronchial branches until it reaches the target lesion, following a predetermined pathway generated by specialized software utilizing the initial CT scan data. Subsequently, based on procedural requirements, conventional biopsy methods like Transbronchial Needle Aspiration (TBNA), Cryobiopsy and/or forceps may be employed for biopsy of the identified lesion.
4. MONARCH™ by Auris Health
5. ION ™ ENDOLUMINAL SYSTEM by Intuitive Surgical
6. GALAXY SYSTEM™ by Noah Medical
7. Advantages over Other Biopsy Modalities
8. Limitations
9. Future Directions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Annual Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; Gierada, D.S.; Jones, G.C.; et al. Results of initial low-dose computed tomographic screening for lung cancer. New Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e93S–e120S. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. New Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. New Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Xu, Y.; Yang, M.; Huang, Z.; Lin, J.; Xie, S.; Sun, H. Comparison between percutaneous transthoracic co-axial needle CT-guided biopsy and transbronchial lung biopsy for the diagnosis of persistent pulmonary consolidation. Insights Into Imaging 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Gustav, F.Z. Killian, father of bronchoscopy. Arch. Otolaryngol. 1965, 82, 656–659. [Google Scholar]
- Baaklini, W.A.; Reinoso, M.A.; Gorin, A.B.; Sharafkaneh, A.; Manian, P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000, 117, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Trick, W.; Mba, B.I.; Mohananey, D.; Sethi, J.; Musani, A.I. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017, 22, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Vining, D.J.; Liu, K.; Choplin, R.H.; Haponik, E.F. Virtual bronchoscopy. Relationships of virtual reality endobronchial simulations to actual bronchoscopic findings. Chest 1996, 109, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Gildea, T.R.; Mazzone, P.J.; Karnak, D.; Meziane, M.; Mehta, A.C. Electromagnetic navigation diagnostic bronchoscopy: A prospective study. Am. J. Respir. Crit. Care Med. 2006, 174, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Folch, E.E.; Pritchett, M.A.; Nead, M.A.; Bowling, M.R.; Murgu, S.D.; Krimsky, W.S.; Murillo, B.A.; LeMense, G.P.; Minnich, D.J.; Bansal, S.; et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J. Thorac. Oncol. 2019, 14, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Ost, D.E.; Ernst, A.; Lei, X.; Kovitz, K.L.; Benzaquen, S.; Diaz-Mendoza, J.; Greenhill, S.; Toth, J.; Feller-Kopman, D.; Puchalski, J.; et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am. J. Respir. Crit. Care Med. 2016, 193, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Pastis, N.J.; Machuzak, M.S.; Gildea, T.R.; Simoff, M.J.; Gillespie, C.T.; Mahajan, A.K.; Oh, S.S.; Silvestri, G.A. Accuracy of a Robotic Endoscopic System in Cadaver Models with Simulated Tumor Targets: ACCESS Study. Respir. Int. Rev. Thorac. Dis. 2020, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Yarmus, L.; Akulian, J.; Wahidi, M.; Chen, A.; Steltz, J.P.; Solomon, S.L.; Yu, D.; Maldonado, F.; Cardenas-Garcia, J.; Molena, D.; et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020, 157, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Gillespie, C.T. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann. Thorac. Surg. 2018, 106, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Hogarth, D.K.; Murgu, S. Robotic bronchoscopy for pulmonary lesions: A review of existing technologies and clinical data. J. Thorac. Dis. 2020, 12, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Chaddha, U.; Kovacs, S.P.; Manley, C.; Hogarth, D.K.; Cumbo-Nacheli, G.; Bhavani, S.V.; Kumar, R.; Shende, M.; Egan, J.P.; Murgu, S. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: Results from the initial multicenter experience. BMC Pulm. Med. 2019, 19, 243. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Pastis, N.J., Jr.; Mahajan, A.K.; Khandhar, S.J.; Simoff, M.J.; Machuzak, M.S.; Cicenia, J.; Gildea, T.R.; Silvestri, G.A. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021, 159, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Akulian, J.; Molena, D.; Wahidi, M.; Chen, A.; Yu, D.; Maldonado, F.; Lee, H.; Vachani, A.; Yarmus, L. A Direct Comparative Study of Bronchoscopic Navigation Planning Platforms for Peripheral Lung Navigation: The ATLAS Study. J. Bronchol. Interv. Pulmonol. 2022, 29, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Fielding, D.I.; Bashirzadeh, F.; Son, J.H.; Todman, M.; Chin, A.; Tan, L.; Steinke, K.; Windsor, M.N.; Sung, A.W. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respir. Int. Rev. Thorac. Dis. 2019, 98, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Benn, B.S.; Romero, A.O.; Lum, M.; Krishna, G. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021, 199, 177–186. [Google Scholar] [CrossRef]
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.-H.; Husta, B.C.; Adusumilli, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022, 161, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Pyarali, F.F.; Hakami-Majd, N.; Sabbahi, W.; Chaux, G. Robotic-assisted Navigation Bronchoscopy: A Meta-Analysis of Diagnostic Yield and Complications. J. Bronchol. Interv. Pulmonol. 2024, 31, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Rickman, O.B.; Mahajan, A.K.; Hogarth, D.K.; Bhadra, K. “Tool-in-lesion” Accuracy of Galaxy System—A Robotic Electromagnetic Navigation BroncHoscopy with Integrated Tool-in-lesion-Tomosynthesis Technology: The MATCH Study. J. Bronchol. Interv. Pulmonol. 2024, 31, 23–29. [Google Scholar] [CrossRef]
- Low, S.W.; Lentz, R.J.; Chen, H.; Katsis, J.; Aboudara, M.C.; Whatley, S.; Paez, R.; Rickman, O.B.; Maldonado, F. Shape-Sensing Robotic-Assisted Bronchoscopy vs Digital Tomosynthesis-Corrected Electromagnetic Navigation Bronchoscopy: A Comparative Cohort Study of Diagnostic Performance. Chest 2023, 163, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Lee-Mateus, A.Y.; Reisenauer, J.; Garcia-Saucedo, J.C.; Abia-Trujillo, D.; Buckarma, E.H.; Edell, E.S.; Grage, R.A.; Bowman, A.W.; Labarca, G.; Johnson, M.M.; et al. Robotic-assisted bronchoscopy versus CT-guided transthoracic biopsy for diagnosis of pulmonary nodules. Respirology 2023, 28, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Solano, J.R.; Ugalde-Gamboa, L.; Machuzak, M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J. Bronchol. Interv. Pulmonol. 2018, 25, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ho, E.; Chaddha, U.; Demirkol, B.; Bhavani, S.V.; Hogarth, D.K.; Murgu, S. Factors Associated with Diagnostic Accuracy of Robotic Bronchoscopy with 12-Month Follow-up. Ann. Thorac. Surg. 2023, 115, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Reisenauer, J.; Simoff, M.J.; Pritchett, M.A.; Ost, D.E.; Majid, A.; Keyes, C.; Casal, R.F.; Parikh, M.S.; Diaz-Mendoza, J.; Fernandez-Bussy, S.; et al. Ion: Technology and Techniques for Shape-sensing Robotic-assisted Bronchoscopy. Ann. Thorac. Surg. 2022, 113, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Simoff, M.J.; Pritchett, M.A.; Reisenauer, J.S.; Ost, D.E.; Majid, A.; Keyes, C.; Casal, R.F.; Parikh, M.S.; Diaz-Mendoza, J.; Fernandez-Bussy, S.; et al. Shape-sensing robotic-assisted bronchoscopy for pulmonary nodules: Initial multicenter experience using the Ion™ Endoluminal System. BMC Pulm. Med. 2021, 21, 322. [Google Scholar] [CrossRef] [PubMed]
- Kalchiem-Dekel, O.; Fuentes, P.; Bott, M.J.; Beattie, J.A.; Lee, R.P.; Chawla, M.; Husta, B.C. Multiplanar 3D fluoroscopy redefines tool-lesion relationship during robotic-assisted bronchoscopy. Respirology 2021, 26, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Leonard, K.M.; Low, S.W.; Echanique, C.S.; Swanner, B.A.; Johnson, J.; Dahlberg, G.; Paez, R.; Ratwani, A.P.; Shojaee, S.; Rickman, O.B.; et al. Diagnostic Yield vs Diagnostic Accuracy for Peripheral Lung Biopsy Evaluation: Evidence Supporting a Future Pragmatic End Point. Chest 2023, in press.
- Pritchett, M.A. Prospective Analysis of a Novel Endobronchial Augmented Fluoroscopic Navigation System for Diagnosis of Peripheral Pulmonary Lesions. J. Bronchol. Interv. Pulmonol. 2021, 28, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Bhadra, K.; Mattingley, J.S. Electromagnetic Navigation Bronchoscopy with Tomosynthesis-based Visualization and Positional Correction: Three-dimensional Accuracy as Confirmed by Cone-Beam Computed Tomography. J. Bronchol. Interv. Pulmonol. 2021, 28, 20. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A.; Bhadra, K.; Calcutt, M.; Folch, E. Virtual or reality: Divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J. Thorac. Dis. 2020, 12, 1595–1611. [Google Scholar] [CrossRef]
- Sagar, A.E.S.; Sabath, B.F.; Eapen, G.A.; Song, J.; Marcoux, M.; Sarkiss, M.; Arain, M.H.; Grosu, H.B.; Ost, D.E.; Jimenez, C.A.; et al. Incidence and Location of Atelectasis Developed during Bronchoscopy under General Anesthesia: The I-LOCATE Trial. Chest 2020, 158, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Y.; Berkowitz, D.; Krimsky, W.S.; Hogarth, D.K.; Parks, C.; Bechara, R. Safety of pacemakers and defibrillators in electromagnetic navigation bronchoscopy. Chest 2013, 143, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Elicker, B.M. Lung Nodule Risk Calculator and Cost-Effectiveness of Different Lung Cancer Screening Algorithms. Radiol. Cardiothorac. Imaging 2021, 3, e210050. [Google Scholar] [CrossRef] [PubMed]
- Marino, K.A.; Sullivan, J.L.; Weksler, B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann. Thorac. Surg. 2016, 102, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Casutt, A.; Kinj, R.; Ozsahin, E.-M.; von Garnier, C.; Lovis, A. Fiducial markers for stereotactic lung radiation therapy: Review of the transthoracic, endovascular and endobronchial approaches. Eur. Respir. Rev. 2022, 31, 210149. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Argento, A.C.; Batra, H.; Benzaquen, S.; Bramley, K.; Chambers, D.; Desai, N.; Dincer, H.E.; Ferguson, J.S.; Kalanjeri, S.; et al. A Multicenter Study Assessing Interventional Pulmonary Fellow Competency in Electromagnetic Navigation Bronchoscopy. ATS Sch. 2022, 3, 220–228. [Google Scholar] [CrossRef] [PubMed]
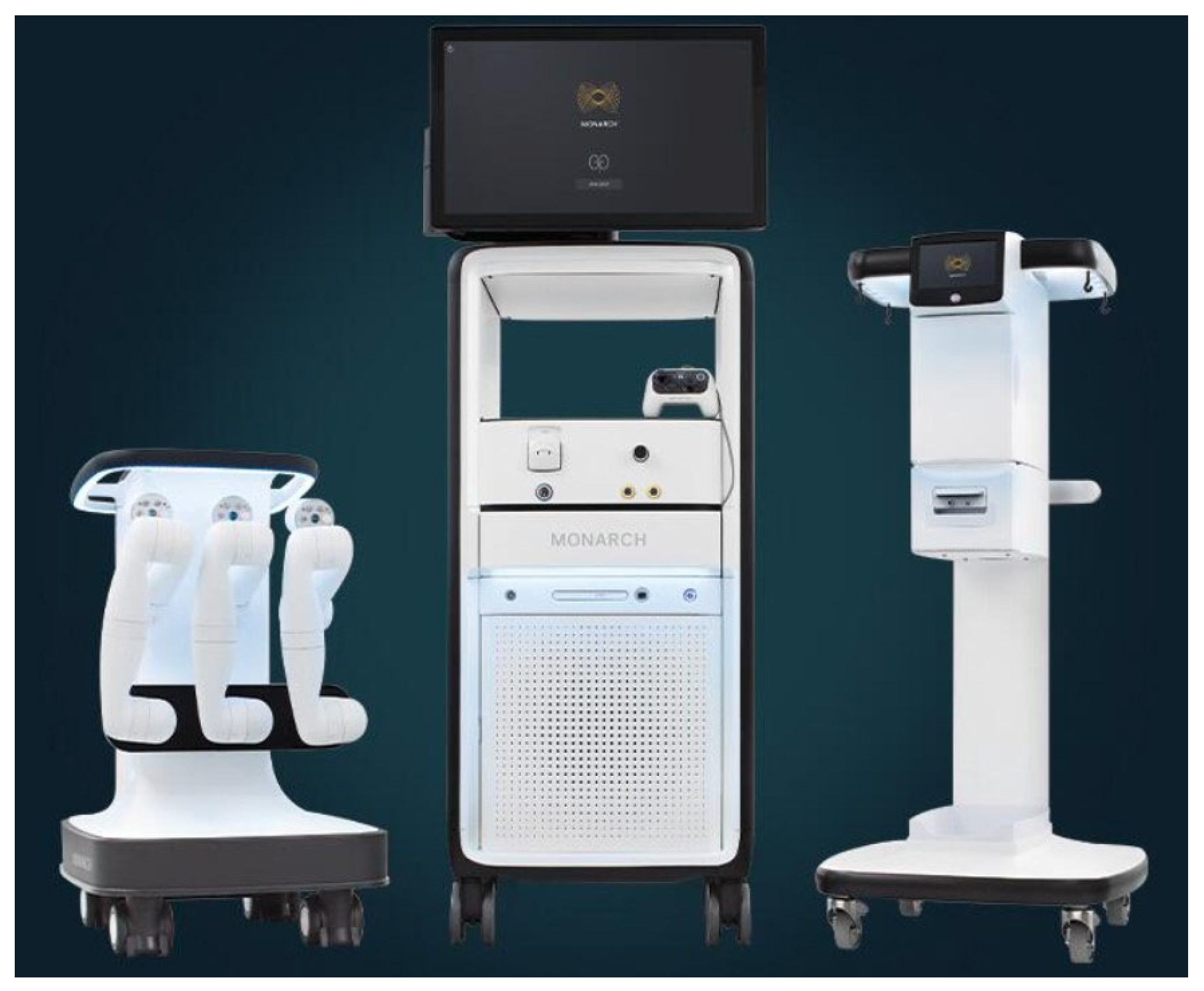


| Monarch Robotic Bronchoscopy System (Auris Health) | Ion Robotic Bronchoscopy System (Intuitive Surgical) | The Galaxy System (Noah Medical) | |
|---|---|---|---|
| FDA Approval | March 2018 | February 2019 | March 2023 |
| Technology used | Electromagnetic Navigation | Shape-sensing technology | Tilt technology/real-time navigation: electromagnetic navigation, digital tomosynthesis |
| Bronchoscope specifications | Outer sheath: 6.0 mm Inner scope: 4.4 mm OD Working Channel: 2.1 mm Integrated working camera: Yes Peripheral vision available during biopsy | Scope: 3.5 mm OD Working Channel: 2.0 mm Vision probe which goes through working channel. Shape-sensing fibers providing feedback Peripheral vision is not available during biopsy | Scope: 4 mm OD Working Channel: 2.1 mm Integrated vision |
| Controller | Gaming controller | Track ball and scroll wheel | Gaming controller |
| Cone-beam CT/rEBUS/fluoroscopy compatibility | Yes (EMN is sensitive to metal) | Yes (shape sensing not sensitive to metal) | Yes (EMN is sensitive to metals, but system has inbuilt digital tomosynthesis) |
| Scope | Reusable | Reusable | Disposable |
| # | Study | Platform | No. of Patients | Follow Up | Navigation Successful | Bronchus Sign | Adjuvant Imaging | Reported Diagnostic Yield |
|---|---|---|---|---|---|---|---|---|
| 1 | Chadda 2019 BMC Pulm Med [19] | Monarch | 165 | 6 months | 88.6% | 63% | rEBUS, 2D Fluoro | 69–77% |
| 2 | Chen 2020 (BENEFIT STUDY) Chest [20] | Monarch | 55 | 1 year | 96.2% | 60% | rEBUS, 2D Fluoro | 74% |
| 3 | Benn 2021 Lung [23] | Ion | 52 | 5–16 months | 85.0% | 46% | Cone-beam CT | 86% |
| 100.0% | ||||||||
| 4 | Fielding 2019 Respiration [22] | Ion | 29 | 6 months | 96.5% | 59% | rEBUS, 2D Fluoro | 79% |
| 93.0% | ||||||||
| 5 | Dekel 2021 Chest [24] | Ion | 131 | 1 year | 98.7% | 63% | rEBUS, 2D, 3D Fluoro | 81.7% |
| 6 | Pyarali 2024 (meta analysis) JOBIP [25] | Ion/Monarch | 1409 (23 studies) | Variable | NA | 25–70% | rEBUS, cone-beam CT | 81.90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, B.S.; Jain, A.; Sharma, S.; Rana, G.; Sabath, B.F. Robotic Bronchoscopy: A Comprehensive Review. J. Respir. 2024, 4, 128-139. https://doi.org/10.3390/jor4020011
Bhandari BS, Jain A, Sharma S, Rana G, Sabath BF. Robotic Bronchoscopy: A Comprehensive Review. Journal of Respiration. 2024; 4(2):128-139. https://doi.org/10.3390/jor4020011
Chicago/Turabian StyleBhandari, Bharat Singh, Akshita Jain, Soumit Sharma, Gunjan Rana, and Bruce Fernando Sabath. 2024. "Robotic Bronchoscopy: A Comprehensive Review" Journal of Respiration 4, no. 2: 128-139. https://doi.org/10.3390/jor4020011
APA StyleBhandari, B. S., Jain, A., Sharma, S., Rana, G., & Sabath, B. F. (2024). Robotic Bronchoscopy: A Comprehensive Review. Journal of Respiration, 4(2), 128-139. https://doi.org/10.3390/jor4020011








