Management of Pleural Infection: A Historical Review and Updates
Abstract
1. Introduction
1.1. Pathophysiology
1.2. Microbiology
1.3. Epidemiology
2. Evaluation
2.1. Imaging
2.2. Pleural Fluid Sampling
2.3. Diagnosis
3. Management
3.1. Antimicrobial Therapy
3.2. Tube Thoracostomy
3.3. Fibrinolytics
3.4. Surgical Management
4. Prognosis
Predictors of Outcomes
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, N.C.; Griffith, P.P.; Sorensen, J.S.; McCauley, L.; Jones, B.E.; Lee, Y.C.G. Pleural Effusions at First ED Encounter Predict Worse Clinical Outcomes in Patients with Pneumonia. Chest 2016, 149, 1509–1515. [Google Scholar] [CrossRef]
- Bedawi, E.O.; Ricciardi, S.; Hassan, M.; Gooseman, M.R.; Asciak, R.; Castro-Añón, O.; Armbruster, K.; Bonifazi, M.; Poole, S.; Harris, E.K.; et al. ERS/ESTS statement on the management of pleural infection in adults. Eur. Respir. J. 2023, 61, 2201062. [Google Scholar] [CrossRef] [PubMed]
- Weese, W.C.; Shindler, E.R.; Smith, I.M.; Rabinovich, S. Empyema of the thorax then and now. A study of 122 cases over four decades. Arch. Intern. Med. 1973, 131, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Light, R.W. Parapneumonic effusions and empyema. Proc. Am. Thorac. Soc. 2006, 3, 75–80. [Google Scholar] [CrossRef]
- Shen, K.R.; Bribriesco, A.; Crabtree, T.; Denlinger, C.; Eby, J.; Eiken, P.; Jones, D.R.; Keshavjee, S.; Maldonado, F.; Paul, S.; et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J. Thorac. Cardiovasc. Surg. 2017, 153, e129–e146. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Daum, R.S. Chapter 35—Bacterial Pneumonia, Lung Abscess, and Empyema. In Pediatric Respiratory Medicine, 2nd ed.; Taussig, L.M., Landau, L.I., Eds.; Mosby: Maryland Heights, MO, USA, 2008; pp. 501–553. [Google Scholar] [CrossRef]
- Alemán, C.; Alegre, J.; Monasterio, J.; Segura, R.M.; Armadans, L.; Anglés, A.; Varela, E.; Ruiz, E.; Fernández de Sevilla, T. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin. Sci. 2003, 105, 601–607. [Google Scholar] [CrossRef]
- Kroegel, C.; Antony, V.B. Immunobiology of pleural inflammation: Potential implications for pathogenesis, diagnosis and therapy. Eur. Respir. J. 1997, 10, 2411–2418. [Google Scholar] [CrossRef]
- Light, R.W.; Girard, W.M.; Jenkinson, S.G.; George, R.B. Parapneumonic effusions. Am. J. Med. 1980, 69, 507–512. [Google Scholar] [CrossRef]
- Fitzgerald, D.B.; Leong, S.L.; Budgeon, C.A.; Murray, K.; Rosenstengal, A.; Smith, N.A.; Bielsa, S.; Clive, A.O.; Maskell, N.A.; Porcel, J.M.; et al. Relationship of pleural fluid pH and glucose: A multi-centre study of 2971 cases. J. Thorac. Dis. 2019, 11, 123–130. [Google Scholar] [CrossRef]
- Hau, T.; Förster, E. Pleural Empyema. In Source Control: A Guide to the Management of Surgical Infections; Schein, M., Marshall, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 201–206. [Google Scholar] [CrossRef]
- Qureshi, N.R.; Gleeson, F.V. Imaging of pleural disease. Clin. Chest Med. 2006, 27, 193–213. [Google Scholar] [CrossRef]
- Davies, H.E.; Davies, R.J.O.; Davies, C.W.H. Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65 (Suppl. 2), ii41–ii53. [Google Scholar] [CrossRef] [PubMed]
- Maskell, N.A.; Batt, S.; Hedley, E.L.; Davies, C.W.H.; Gillespie, S.H.; Davies, R.J.O. The Bacteriology of Pleural Infection by Genetic and Standard Methods and Its Mortality Significance. Am. J. Respir. Crit. Care Med. 2006, 174, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Kanellakis, N.I.; Wrightson, J.M.; Gerry, S.; Ilott, N.; Corcoran, J.P.; Bedawi, E.O.; Asciak, R.; Nezhentsev, A.; Sundaralingam, A.; Hallifax, R.J.; et al. The bacteriology of pleural infection (TORPIDS): An exploratory metagenomics analysis through next generation sequencing. Lancet Microbe 2022, 3, e294–e302. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Gorbach, S.L.; Thadepalli, H.; Finegold, S.M. Bacteriology of empyema. Lancet 1974, 1, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Vladimir Djambazov Gergova, G.; Dragomir Iotov Petrov, D.; Osmanliev, D.; Minchev, Z.; Mitov, I. Anaerobic microbiology in 198 cases of pleural empyema: A Bulgarian study. Anaerobe 2004, 10, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Cargill, T.; Harriss, E.; Asciak, R.; Mercer, R.M.; Bedawi, E.O.; McCracken, D.J.; Psallidas, I.; Corcoran, J.P.; Rahman, N.M. The microbiology of pleural infection in adults: A systematic review. Eur. Respir. J. 2019, 54, 1900542. [Google Scholar] [CrossRef]
- Nigo, M.; Vial, M.R.; Munita, J.M.; Jiang, Y.; Tarrand, J.; Jimenez, C.A.; Kontoyiannis, D.P. Fungal empyema thoracis in cancer patients. J. Infect. 2016, 72, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.J.B.; Fisk, M.D.; Koo, C.Y.; Pavlou, M.; Peck, L.; Lee, S.F.; Lawrence, D.; Macrae, M.B.; Wilson, A.P.; Brown, J.S.; et al. Thoracic Empyema: A 12-Year Study from a UK Tertiary Cardiothoracic Referral Centre. PLoS ONE 2012, 7, e30074. [Google Scholar] [CrossRef]
- Iliopoulou, M.; Skouras, V.; Psaroudaki, Z.; Makarona, M.; Vogiatzakis, E.; Tsorlini, E.; Katsifa, E.; Spyratos, D.; Siopi, D.; Kotsiou, O.; et al. Bacteriology, antibiotic resistance and risk stratification of patients with culture-positive, community-acquired pleural infection. J. Thorac. Dis. 2021, 13, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Chen, K.Y.; Hsueh, P.R.; Luh, K.T.; Yang, P.C. Fungal Empyema Thoracis. Chest 2000, 117, 1672–1678. [Google Scholar] [CrossRef]
- Lin, K.H.; Liu, Y.M.; Lin, P.C.; Ho, C.M.; Chou, C.H.; Wang, J.H.; Chi, C.Y.; Ho, M.W.; Wang, J.H. Report of a 63-case series of Candida empyema thoracis: 9-year experience of two medical centers in central Taiwan. J. Microbiol. Immunol. Infect. 2014, 47, 36–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mummadi, S.R.; Stoller, J.K.; Lopez, R.; Kailasam, K.; Gillespie, C.T.; Hahn, P.Y. Epidemiology of Adult Pleural Disease in the United States. Chest 2021, 160, 1534–1551. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Morris, T.T.; Suri, T.; Morley, A.; Frost, V.; Vipond, I.B.; Medford, A.R.; Payne, R.A.; Muir, P.; et al. Epidemiology of pleural empyema in English hospitals and the impact of influenza. Eur. Respir. J. 2021, 57, 2003546. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Singanayagam, A.; Murray, M.P.; Scally, C.; Fawzi, A.; Hill, A.T. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 2009, 64, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Metersky, M.L. Is the lateral decubitus radiograph necessary for the management of a parapneumonic pleural effusion? Chest 2003, 124, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Moffett, B.K.; Panchabhai, T.S.; Anaya, E.; Nakamatsu, R.; Arnold, F.W.; Peyrani, P.; Wiemken, T.; Guardiola, J.; Ramirez, J.A. Computed tomography measurements of parapneumonic effusion indicative of thoracentesis. Eur. Respir. J. 2011, 38, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.L. Imaging of the pleura. Radiology 1993, 186, 297–309. [Google Scholar] [CrossRef]
- Stark, D.D.; Federle, M.P.; Goodman, P.C.; Podrasky, A.E.; Webb, W.R. Differentiating lung abscess and empyema: Radiography and computed tomography. AJR Am. J. Roentgenol. 1983, 141, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Jiménez, J.; Alonso-Charterina, S.; Sánchez-Payá, J.; Fernández-Latorre, F.; Gil-Sánchez, S.; Lloret-Llorens, M. Evaluation of CT findings for diagnosis of pleural effusions. Eur. Radiol. 2000, 10, 681–690. [Google Scholar] [CrossRef]
- Waite, R.J.; Carbonneau, R.J.; Balikian, J.P.; Umali, C.B.; Pezzella, A.T.; Nash, G. Parietal pleural changes in empyema: Appearances at CT. Radiology 1990, 175, 145–150. [Google Scholar] [CrossRef]
- Porcel, J.M.; Pardina, M.; Alemán, C.; Pallisa, E.; Light, R.W.; Bielsa, S. Computed tomography scoring system for discriminating between parapneumonic effusions eventually drained and those cured only with antibiotics. Respirology 2017, 22, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Svigals, P.Z.; Chopra, A.; Ravenel, J.G.; Nietert, P.J.; Huggins, J.T. The accuracy of pleural ultrasonography in diagnosing complicated parapneumonic pleural effusions. Thorax 2017, 72, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Shkolnik, B.; Judson, M.A.; Austin, A.; Hu, K.; D’Souza, M.; Zumbrunn, A.; Huggins, J.T.; Yucel, R.; Chopra, A. Diagnostic Accuracy of Thoracic Ultrasonography to Differentiate Transudative From Exudative Pleural Effusion. Chest 2020, 158, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Brogi, E.; Gargani, L.; Bignami, E.; Barbariol, F.; Marra, A.; Forfori, F.; Vetrugno, L. Thoracic ultrasound for pleural effusion in the intensive care unit: A narrative review from diagnosis to treatment. Crit. Care 2017, 21, 325. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.C.; Luh, K.T.; Chang, D.B.; Wu, H.D.; Yu, C.J.; Kuo, S.H. Value of sonography in determining the nature of pleural effusion: Analysis of 320 cases. AJR Am. J. Roentgenol. 1992, 159, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Arondi, S.; Baglivo, F.; Lonni, S.; Quadri, F.; Valsecchi, A.; Venturoli, N.; Ceruti, P. New insights in the use of pleural ultrasonography for diagnosis and treatment of pleural disease. Clin. Respir. J. 2018, 12, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Rocca, E.; Zanza, C.; Longhitano, Y.; Piccolella, F.; Romenskaya, T.; Racca, F.; Savioli, G.; Saviano, A.; Piccioni, A.; Mongodi, S. Lung Ultrasound in Critical Care and Emergency Medicine: Clinical Review. Adv. Respir. Med. 2023, 91, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Brutsche, M.H.; Solèr, M. Accuracy of pleural puncture sites: A prospective comparison of clinical examination with ultrasound. Chest 2003, 123, 436–441. [Google Scholar] [CrossRef] [PubMed]
- UK NPSA Rapid Response Report: Risks of Chest Drain Insertion. Available online: http://www.npsa.nhs.uk/EasySiteWeb/GatewayLink.aspx?alId=11636 (accessed on 10 February 2024).
- Roberts, M.E.; Rahman, N.M.; Maskell, N.A.; Bibby, A.C.; Blyth, K.G.; Corcoran, J.P.; Edey, A.; Evison, M.; de Fonseka, D.; Hallifax, R.; et al. British Thoracic Society Guideline for pleural disease. Thorax 2023, 78 (Suppl. 3), s1–s42. [Google Scholar] [CrossRef]
- Ferguson, A.D.; Prescott, R.J.; Selkon, J.B.; Watson, D.; Swinburn, C.R. The clinical course and management of thoracic empyema. QJM 1996, 89, 285–289. [Google Scholar] [CrossRef]
- Moriyama, B.; Torabi-Parizi, P.; Pratt, A.K.; Henning, S.A.; Pennick, G.; Shea, Y.R.; Roy Chowdhuri, S.; Rinaldi, M.G.; Barrett, A.J.; Walsh, T.J. Pharmacokinetics of Liposomal Amphotericin B in Pleural Fluid. Antimicrob. Agents Chemother. 2010, 54, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp, K.; O’Horo, J.C.; Kashyap, R.; Kloesel, B.; Lahr, B.D.; Daniels, C.E.; Nichols, F.C., 3rd; Baddour, L.M. Empyema management: A cohort study evaluating antimicrobial therapy. J. Infect. 2016, 72, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Towe, C.W.; Srinivasan, S.; Ho, V.P.; Bachmann, K.; Worrell, S.G.; Perry, Y.; Argote-Green, L.M.; Linden, P.A. Antibiotic Resistance Is Associated with Morbidity and Mortality after Decortication for Empyema. Ann. Thorac. Surg. 2021, 111, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Torbic, H.; Glasser, N.; Rostas, S.E.; Alquwaizani, M.; Hacobian, G. Intrapleural Antimicrobial Irrigation for Postpneumonectomy Empyema in Patients with Lung Cancer. J. Pharm. Pract. 2015, 28, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.; Skouras, V.S.; Rahman, N.M.; Psallidas, I.; Aliberti, S. Antibiotics for Pleural Infections; Aliberti, S., Chalmers, J.D., Pletz, M.W., Eds.; European Respiratory Society: Lausanne, Switzerland, 2017; pp. 253–263. [Google Scholar] [CrossRef]
- Berger, H.A.; Morganroth, M.L. Immediate Drainage Is Not Required for All Patients with Complicated Parapneumonic Effusions. Chest 1990, 97, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Eid, S.M.; Gillaspie, E.A.; Broderick, S.; Shafiq, M. Epidemiologic Trends in Pleural Infection. A Nationwide Analysis. Ann. ATS 2021, 18, 452–459. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, H.Y.; Chen, C.W.; Lee, C.H.; Hsiue, T.R. Predicting factors for outcome of tube thoracostomy in complicated parapneumonic effusion for empyema. Chest 1999, 115, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.M.; Maskell, N.A.; Davies, C.W.H.; Hedley, E.L.; Nunn, A.J.; Gleeson, F.V.; Davies, R.J. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010, 137, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Maskell, N.A.; Davies, C.W.H.; Nunn, A.J.; Hedley, E.L.; Gleeson, F.V.; Miller, R.; Gabe, R.; Rees, G.L.; Peto, T.E.; Woodhead, M.A.; et al. U.K. Controlled Trial of Intrapleural Streptokinase for Pleural Infection. N. Engl. J. Med. 2005, 352, 865–874. [Google Scholar] [CrossRef]
- Porcel, J.M.; Bielsa, S.; Esquerda, A.; Ruiz-González, A.; Falguera, M. Pleural fluid C-reactive protein contributes to the diagnosis and assessment of severity of parapneumonic effusions. Eur. J. Intern. Med. 2012, 23, 447–450. [Google Scholar] [CrossRef]
- Bielsa, S.; Valencia, H.; Ruiz-González, A.; Esquerda, A.; Porcel, J.M. Serum C-Reactive Protein as an Adjunct for Identifying Complicated Parapneumonic Effusions. Lung 2014, 192, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Colice, G.L.; Curtis, A.; Deslauriers, J.; Heffner, J.; Light, R.; Littenberg, B.; Sahn, S.; Weinstein, R.A.; Yusen, R.D. Medical and Surgical Treatment of Parapneumonic Effusions: An Evidence-Based Guideline. Chest 2000, 118, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Altmann, E.S.; Crossingham, I.; Wilson, S.; Davies, H.R. Intra-pleural fibrinolytic therapy versus placebo, or a different fibrinolytic agent, in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst. Rev. 2019, 2019, CD002312. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.M.; Maskell, N.A.; West, A.; Teoh, R.; Arnold, A.; Mackinlay, C.; Peckham, D.; Davies, C.W.; Ali, N.; Kinnear, W.; et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N. Engl. J. Med. 2011, 365, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, N.; Bintcliffe, O.; De Fonseka, D.; Blyth, K.G.; Smith, N.A.; Piccolo, F.; Martin, G.; Wong, D.; Edey, A.; Maskell, N.; et al. Dose De-escalation of Intrapleural Tissue Plasminogen Activator Therapy for Pleural Infection. The Alteplase Dose Assessment for Pleural Infection Therapy Project. Ann. ATS 2017, 14, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, N.; Ip, H.; Lau, E.P.M.; Piccolo, F.; Dootson, K.; Yeoh, C.; Phu, W.Y.; Brown, R.; West, A.; Ahmed, L.; et al. Alteplase Dose Assessment for Pleural infection Therapy (ADAPT) Study-2: Use of 2.5 mg alteplase as a starting intrapleural dose. Respirology 2022, 27, 510–516. [Google Scholar] [CrossRef] [PubMed]
- McClune, J.R.; Wilshire, C.L.; Gorden, J.A.; Louie, B.E.; Farviar, A.S.; Stefanski, M.J.; Vallieres, E.; Aye, R.W.; Gilbert, C.R. Safety and Efficacy of Intrapleural Tissue Plasminogen Activator and DNase during Extended Use in Complicated Pleural Space Infections. Can. Respir. J. 2016, 2016, e9796768. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.J.; Biswas, A.; Penley, A.M.; Cope, J.; Barnes, M.; Jantz, M.A. Management of Intrapleural Sepsis with Once Daily Use of Tissue Plasminogen Activator and Deoxyribonuclease. Respiration 2016, 91, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Awerbuch, E. Newer Paradigm in the Management of Intrapleural Sepsis: Once Daily Coadministration of Tissue Plasminogen Activator/Deoxyribonuclease. Chest 2017, 152, A528. [Google Scholar] [CrossRef]
- Chaddha, U.; Agrawal, A.; Feller-Kopman, D.; Kaul, V.; Shojaee, S.; Maldonado, F.; Ferguson, M.K.; Blyth, K.G.; Grosu, H.B.; Corcoran, J.P.; et al. Use of fibrinolytics and deoxyribonuclease in adult patients with pleural empyema: A consensus statement. Lancet Respir. Med. 2021, 9, 1050–1064. [Google Scholar] [CrossRef]
- Kheir, F.; Cheng, G.; Rivera, E.; Folch, A.; Folch, E.; Fernandez-Bussy, S.; Keyes, C.; Parikh, M.; Channick, C.; Chee, A.; et al. Concurrent Versus Sequential Intrapleural Instillation of Tissue Plasminogen Activator and Deoxyribonuclease for Pleural Infection. J. Bronchol. Interv. Pulmonol. 2018, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Bédat, B.; Plojoux, J.; Noel, J.; Morel, A.; Worley, J.; Triponez, F.; Karenovics, W. Comparison of intrapleural use of urokinase and tissue plasminogen activator/DNAse in pleural infection. ERJ Open Res. 2019, 5, 00084–02019. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, F.; Popowicz, N.; Wong, D.; Lee, Y.C.G. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. J. Thorac. Dis. 2015, 7, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.E.; Edey, A.J.; Wallis, A.; Clive, A.O.; Morley, A.; White, P.; Medford, A.R.; Harvey, J.E.; Darby, M.; Zahan-Evans, N.; et al. Pleural irrigation trial (PIT): A randomised controlled trial of pleural irrigation with normal saline versus standard care in patients with pleural infection. Eur. Respir. J. 2015, 46, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Tillett, W.S.; Sherry, S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J. Clin. Investig. 1949, 28, 173–190. [Google Scholar] [CrossRef]
- Mitchell, M.E.; Alberts, W.M.; Chandler, K.W.; Goldman, A.L. Intrapleural streptokinase in management of parapneumonic effusions. Report of series and review of literature. J. Fla. Med. Assoc. 1989, 76, 1019–1022. [Google Scholar]
- Moulton, J.S.; Moore, P.T.; Mencini, R.A. Treatment of loculated pleural effusions with transcatheter intracavitary urokinase. AJR Am. J. Roentgenol. 1989, 153, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Im, J.G.; Kim, Y.H.; Hwang, S.H.; Bae, W.K.; Lee, B.H. Treatment of thoracic multiloculated empyemas with intracavitary urokinase: A prospective study. Radiology 1991, 179, 771–775. [Google Scholar] [CrossRef]
- Rosen, H.; Nadkarni, V.; Theroux, M.; Padman, R.; Klein, J. Intrapleural streptokinase as adjunctive treatment for persistent empyema in pediatric patients. Chest 1993, 103, 1190–1193. [Google Scholar] [CrossRef]
- Taylor, R.F.; Rubens, M.B.; Pearson, M.C.; Barnes, N.C. Intrapleural streptokinase in the management of empyema. Thorax 1994, 49, 856–859. [Google Scholar] [CrossRef][Green Version]
- Jerjes-Sánchez, C.; Ramirez-Rivera, A.; Elizalde, J.J.; Delgado, R.; Cicero, R.; Ibarra-Perez, C.; Arroliga, A.C.; Padua, A.; Portales, A.; Villarreal, A.; et al. Intrapleural fibrinolysis with streptokinase as an adjunctive treatment in hemothorax and empyema: A multicenter trial. Chest 1996, 109, 1514–1519. [Google Scholar] [CrossRef]
- Bouros, D.; Schiza, S.; Patsourakis, G.; Chalkiadakis, G.; Panagou, P.; Siafakas, N.M. Intrapleural streptokinase versus urokinase in the treatment of complicated parapneumonic effusions: A prospective, double-blind study. Am. J. Respir. Crit. Care Med. 1997, 155, 291–295. [Google Scholar] [CrossRef]
- Davies, R.J.; Traill, Z.C.; Gleeson, F.V. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997, 52, 416–421. [Google Scholar] [CrossRef]
- Bouros, D.; Schiza, S.; Tzanakis, N.; Chalkiadakis, G.; Drositis, J.; Siafakas, N. Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double-blind study. Am. J. Respir. Crit. Care Med. 1999, 159, 37–42. [Google Scholar] [CrossRef]
- Thomson, A.H.; Hull, J.; Kumar, M.R.; Wallis, C.; Balfour Lynn, I.M. Randomised trial of intrapleural urokinase in the treatment of childhood empyema. Thorax 2002, 57, 343–347. [Google Scholar] [CrossRef]
- Simpson, G.; Roomes, D.; Reeves, B. Successful treatment of empyema thoracis with human recombinant deoxyribonuclease. Thorax 2003, 58, 365–366. [Google Scholar] [CrossRef]
- Diacon, A.H.; Theron, J.; Schuurmans, M.M.; Van de Wal, B.W.; Bolliger, C.T. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am. J. Respir. Crit. Care Med. 2004, 170, 49–53. [Google Scholar] [CrossRef]
- Skeete, D.A.; Rutherford, E.J.; Schlidt, S.A.; Abrams, J.E.; Parker, L.A.; Rich, P.B. Intrapleural tissue plasminogen activator for complicated pleural effusions. J. Trauma 2004, 57, 1178–1183. [Google Scholar] [CrossRef]
- Thommi, G.; Nair, C.K.; Aronow, W.S.; Shehan, C.; Meyers, P.; McLeay, M. Efficacy and safety of intrapleural instillation of alteplase in the management of complicated pleural effusion or empyema. Am. J. Ther. 2007, 14, 341–345. [Google Scholar] [CrossRef]
- Thommi, G.; Shehan, J.C.; Robison, K.L.; Christensen, M.; Backemeyer, L.A.; McLeay, M.T. A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir. Med. 2012, 106, 716–723. [Google Scholar] [CrossRef][Green Version]
- Piccolo, F.; Pitman, N.; Bhatnagar, R.; Popowicz, N.; Smith, N.A.; Brockway, B.; Nickels, R.; Burke, A.J.; Wong, C.A.; McCartney, R.; et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann. Am. Thorac. Soc. 2014, 11, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Kheir, F.; Folch, A.; Fernandez-Bussy, S.; Chatterji, S.; Maskey, A.; Fashjian, M.; Cheng, G.; Ochoa, S.; Alape, D.; et al. Concurrent Intrapleural Instillation of Tissue Plasminogen Activator and DNase for Pleural Infection. A Single-Center Experience. Ann. Am. Thorac. Soc. 2016, 13, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Aramini, B.; della Casa, G.; Ligabue, G.; Kaleci, S.; Casali, C.; Morandi, U. Preoperative Predictors of Successful Surgical Treatment in the Management of Parapneumonic Empyema. Ann. Thorac. Surg. 2013, 96, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Towe, C.W.; Carr, S.R.; Donahue, J.M.; Burrows, W.M.; Perry, Y.; Kim, S.; Kosinski, A.; Linden, P.A. Morbidity and 30-day mortality after decortication for parapneumonic empyema and pleural effusion among patients in the Society of Thoracic Surgeons’ General Thoracic Surgery Database. J. Thorac. Cardiovasc. Surg. 2019, 157, 1288–1297.e4. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, C.G.; Zhu, Y.; Nuorti, J.P.; Griffin, M.R. Emergence of parapneumonic empyema in the USA. Thorax 2011, 66, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Cargill, T.N.; Hassan, M.; Corcoran, J.P.; Harriss, E.; Asciak, R.; Mercer, R.M.; McCracken, D.J.; Bedawi, E.O.; Rahman, N.M. A systematic review of comorbidities and outcomes of adult patients with pleural infection. Eur. Respir. J. 2019, 54, 1900541. [Google Scholar] [CrossRef] [PubMed]
- Brims, F.; Popowicz, N.; Rosenstengel, A.; Hart, J.; Yogendran, A.; Read, C.A.; Lee, F.; Shrestha, R.; Franke, A.; Lewis, J.R.; et al. Bacteriology and clinical outcomes of patients with culture-positive pleural infection in Western Australia: A 6-year analysis. Respirology 2019, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Farjah, F.; Symons, R.G.; Krishnadasan, B.; Wood, D.E.; Flum, D.R. Management of pleural space infections: A population-based analysis. J. Thorac. Cardiovasc. Surg. 2007, 133, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Bobbio, A.; Bouam, S.; Frenkiel, J.; Zarca, K.; Fournel, L.; Canny, E.; Icard, P.; Porcher, R.; Alifano, M. Epidemiology and prognostic factors of pleural empyema. Thorax 2021, 76, 1117–1123. [Google Scholar] [CrossRef]
- Lai, S.W.; Lin, C.L.; Liao, K.F. Population-based cohort study investigating the correlation of diabetes mellitus with pleural empyema in adults in Taiwan. Medicine 2017, 96, e7763. [Google Scholar] [CrossRef]
- Rahman, N.M.; Kahan, B.C.; Miller, R.F.; Gleeson, F.V.; Nunn, A.J.; Maskell, N.A. A clinical score (RAPID) to identify those at risk for poor outcome at presentation in patients with pleural infection. Chest 2014, 145, 848–855. [Google Scholar] [CrossRef]
- Corcoran, J.P.; Psallidas, I.; Gerry, S.; Piccolo, F.; Koegelenberg, C.F.; Saba, T.; Daneshvar, C.; Fairbairn, I.; Heinink, R.; West, A.; et al. Prospective validation of the RAPID clinical risk prediction score in adult patients with pleural infection: The PILOT study. Eur. Respir. J. 2020, 56, 2000130. [Google Scholar] [CrossRef]
- Okiror, L.; Coltart, C.; Bille, A.; Guile, L.; Pilling, J.; Harrison-Phipps, K.; Routledge, T.; Lang-Lazdunski, L.; Hemsley, C.; King, J. Thoracotomy and decortication: Impact of culture-positive empyema on the outcome of surgery. Eur. J. Cardiothorac. Surg. 2014, 46, 901–906. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, W.; Chen, H.J.; Yu, Y.H.; Lin, Y.C.; Tu, C.Y.; Hsu, W.H. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med. Biol. 2009, 35, 1468–1474. [Google Scholar] [CrossRef]
- Davies, C.W.; Kearney, S.E.; Gleeson, F.V.; Davies, R.J. Predictors of outcome and long-term survival in patients with pleural infection. Am. J. Respir. Crit. Care Med. 1999, 160 Pt 1, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Senger, S.S.; Thompson, G.R., 3rd; Samanta, P.; Ahrens, J.; Clancy, C.J.; Nguyen, M.H. Candida Empyema Thoracis at Two Academic Medical Centers: New Insights into Treatment and Outcomes. Open Forum Infect. Dis. 2021, 8, ofaa656. [Google Scholar] [CrossRef] [PubMed]
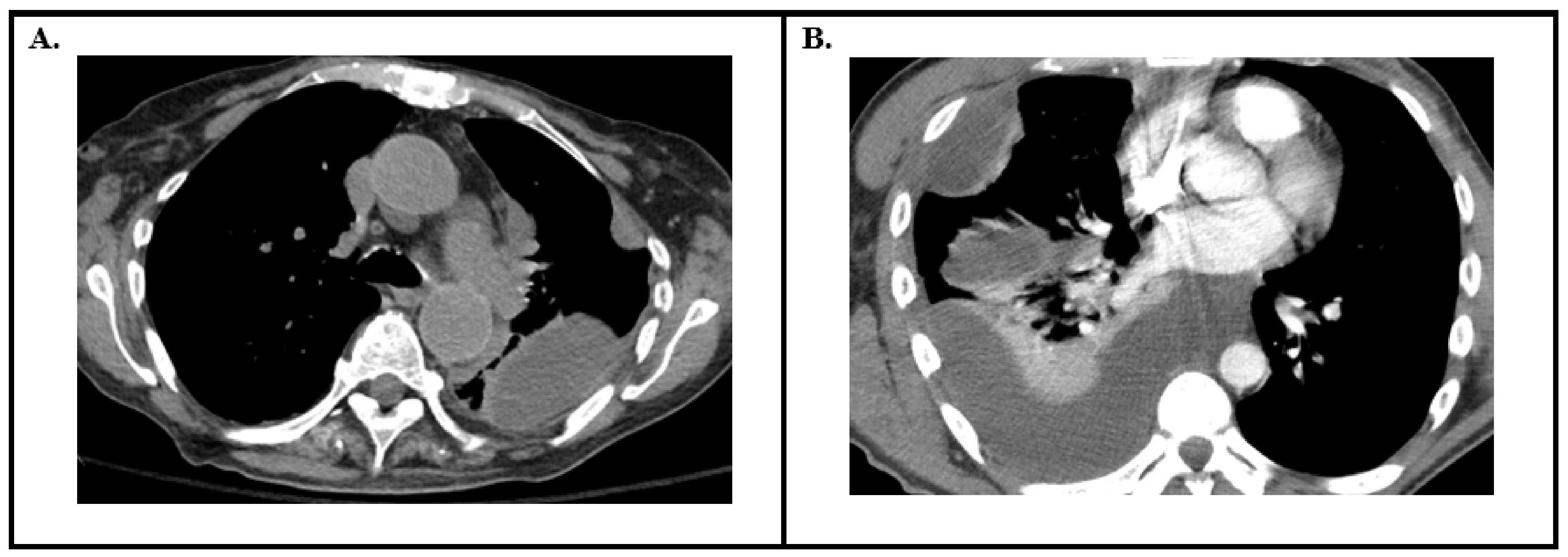

| Imaging Modality | Characteristic Findings Suggestive of a Parapneumonic Effusion or That Intervention Is Needed |
|---|---|
| Chest Radiography | Minimum size to drain: >5 cm height on upright or >1 cm depth on lateral decubitus |
| Computed Tomography of the Chest | Minimum size to drain: >2.5 cm depth High risk findings: “Split Pleura” sign Increased attenuation of extra-pleural fat Large volume effusion Pleural thickening |
| Thoracic Ultrasound | High risk findings: Homogenous echogenicity Hyperechoic septation Thickened parietal pleura |
| Pleural Effusion | Diagnosis | |
|---|---|---|
| Simple Pleural Effusion | Lung Parenchyma Imaging: No pneumonia Effusion Imaging: simple Pleural Fluid Analysis: Transudative | |
| Uncomplicated Parapneumonic Effusion | Lung Parenchyma Imaging: Pneumonia ipsilateral to the pleural effusion Effusion Imaging: May appear simple or complex Pleural Fluid Analysis: Exudative, pH > 7.2, and glucose > 2.2 mmol/L | |
| Pleural Infection | Complicated Parapneumonic Effusion | Lung Parenchyma Imaging: Pneumonia ipsilateral to the pleural effusion Effusion Imaging: More often complex Pleural Fluid Analysis: Exudative, pH < 7.2, or glucose < 2.2 mmol/L |
| Empyema | Lung Parenchyma Imaging: Most often there is a pneumonia ipsilateral to the pleural effusion. In rare cases of empyema outside of parapneumonic effusions, i.e., spontaneous bacterial empyema, this may not be the case Effusion Imaging: Complex Pleural Fluid Analysis: Frank pus or positive pleural fluid culture | |
| Study | Study Type | Interventions | Highlighted Outcomes |
|---|---|---|---|
| Tillett et al. 1949 [69] | Prospective Observational 23 Subjects | Intrapleural Streptokinase | Reduced fibrinogen levels, increased proteolysis, and reduced viscosity No bleeding complications reported |
| Mitchell et al. 1989 [70] | Case Series 9 Subjects | Intrapleural Streptokinase | “of nine patient” there was “obvious increase in chest tube output in 6” |
| Moulton et al. 1989 [71] | Case Series 11 Subjects | Intrapleural Urokinase | A total of 12/13 collections drained completely. No bleeding complications reported |
| Lee et al. 1991 [72] | Prospective Cohort 10 Subjects | Intrapleural Urokinase | A total of 9/10 subjects had complete drainage |
| Rosen et al. 1993 [73] | Case Series 5 Subjects (pediatric) | Intrapleural Streptokinase | Increased chest tube drainage and clinical improvement in all five subjects |
| Taylor et al. 1994 [74] | Case Series 11 Subjects | Intrapleural Streptokinase | Increased chest tube drainage in all patients. A total of 8/11 demonstrated complete resolution of empyema. In total two patients underwent decortication No bleeding complications reported |
| Jerjes-Sanchez et al. 1996 [75] | Prospective Cohort 48 Subjects | Intrapleural Streptokinase | A total of 44/48 subjects had increased drainage with clinical improvement. Only four required surgery. No bleeding complications reported |
| Bouros et al. 1997 [76] | Randomized Double Blind Clinical Trial 50 Subjects | Intrapleural Streptokinase vs. Urokinase | No difference in efficacy. Both result in increased drainage No bleeding complications reported |
| Davies et al. 1997 [77] | Randomized Double Blind Placebo Controlled Trial 24 Subjects | Intrapleural Streptokinase vs. Saline | Intrapleural streptokinase resulted in greater pleural fluid drainage, radiographic improvement, and no decortication (three in saline group) |
| Bouros et al. 1999 [78] | Randomized Double Blind Placebo Controlled Trial 31 Subjects | Intrapleural Urokinase vs. Saline | Urokinase resulted in increased radiographic improvement, and the volume of pleural fluid drained |
| Thomson et al. 2002 [79] | Randomized Placebo Controlled Trial (pediatrics) 60 Subjects | Intrapleural Urokinase vs. Saline | Urokinase resulted in reduced hospital length of stay |
| Simpson et al. 2003 [80] | Case Report 1 Subject | Intrapleural Deoxyribonuclease (DNase) | Increased chest tube drainage and improved lung expansion |
| Diacon et al. 2004 [81] | Randomized Placebo Controlled Trial 53 Subjects | Intrapleural Streptokinase vs. Saline | Streptokinase resulted in fewer surgical referrals and increased treatment success No bleeding complications reported |
| Skeete et al. 2004 [82] | Case Series 41 Subjects | Intrapleural t-PA | All patients managed nonoperatively and had radiographic improvement following the administration of t-PA |
| Maskell et al. 2005 [53] | Double Bline Placebo Controlled Trial 454 Subjects | Intrapleural Streptokinase vs. Saline | No difference in mortality, the rate of surgery, radiographic changes, or the length of stay No increase in bleeding between groups |
| Thommi et al. 2007 [83] | Retrospective Cohort 120 Subjects | Intrapleural Alteplase | In total, >90% had complete or partial response with favorable safety profile Two subjects had bleeding complications at doses of 25 mg and 50 mg each |
| Rahman et al. 2011 [58] | Double Blind 2 × 2 Factorial Trial 210 Subjects | Intrapleural Placebo vs. t-PA + DNase vs. t-PA vs. DNase | The combination of intrapleural t-PA and DNase resulted in greater radiographic improvement, fewer surgical referrals, and a shorter hospital length of stay Two subjects had intrapleural bleeding and one had hemoptysis in the t-PA + DNase group. No such events in the control arm |
| Thommi et al. 2012 [84] | Randomized Double Blind Placebo Controlled Trial 68 Subjects | Intrapleural Alteplase vs. Saline | Alteplase resulted in improved clinical resolution |
| Piccolo et al. 2014 [85] | Retrospective Cohort 107 Subjects | Intrapleural t-PA + DNase | Regimen is safe and effective in real world use Two subjects had intrapleural bleeding complications requiring transfusion |
| Majid et al. 2016 [86] | Retrospective Cohort 73 Subjects | Simultaneous Intrapleural t-PA + DNase | Simultaneous administration is safe and efficacious Four subjects had intrapleural bleeding complications requiring transfusion |
| Popowicz et al. 2017 [59] | Observational Open-label Study 61 Subjects | Reduced Dose t-PA (5 mg) + DNase | Reduced dose resulted in increased pleural fluid drainage and reduced CRP. Three patients still underwent surgery Three subjects had intrapleural bleeding complications requiring transfusion |
| Dosing | Schedule | Suggested Usage |
|---|---|---|
| Simultaneous administration of 5 mg DNase and 10 mg tPA followed by a flush and clamped for 60–120 min [58,85,86] | Twice daily for up to six doses | Standard dosing for patients with low risk of bleeding |
| Simultaneous administration of 5 mg DNase and 5 mg tPA followed by a flush and clamped for 40–60 min [59,83] | Twice daily, duration determined by clinical response | Alternative dosing for patients with increased risk of bleeding (i.e., on systemic anticoagulation, synthetic liver disfunction etc.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presti, T.; Asghar, A.; Ravikumar, N. Management of Pleural Infection: A Historical Review and Updates. J. Respir. 2024, 4, 112-127. https://doi.org/10.3390/jor4020010
Presti T, Asghar A, Ravikumar N. Management of Pleural Infection: A Historical Review and Updates. Journal of Respiration. 2024; 4(2):112-127. https://doi.org/10.3390/jor4020010
Chicago/Turabian StylePresti, Thomas, Aleezay Asghar, and Nakul Ravikumar. 2024. "Management of Pleural Infection: A Historical Review and Updates" Journal of Respiration 4, no. 2: 112-127. https://doi.org/10.3390/jor4020010
APA StylePresti, T., Asghar, A., & Ravikumar, N. (2024). Management of Pleural Infection: A Historical Review and Updates. Journal of Respiration, 4(2), 112-127. https://doi.org/10.3390/jor4020010





