Lung Cancer Staging—A Clinical Practice Review
Abstract
1. Introduction
2. TNM Staging System
3. Neuroendocrine Tumors
4. Imaging Techniques for Staging
5. Invasive Techniques to Stage the Mediastinum
5.1. Endoscopic Staging
5.2. Surgical Staging
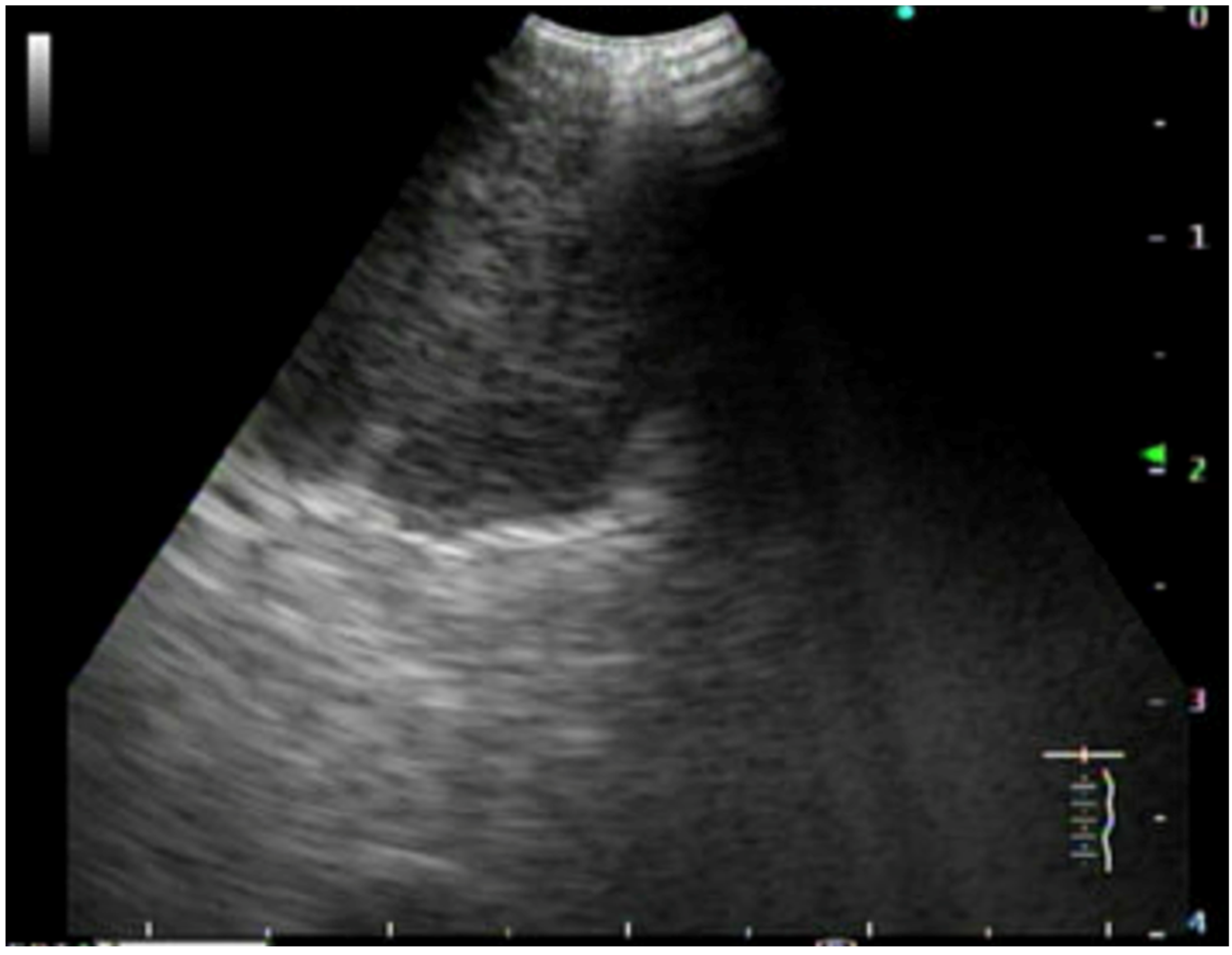
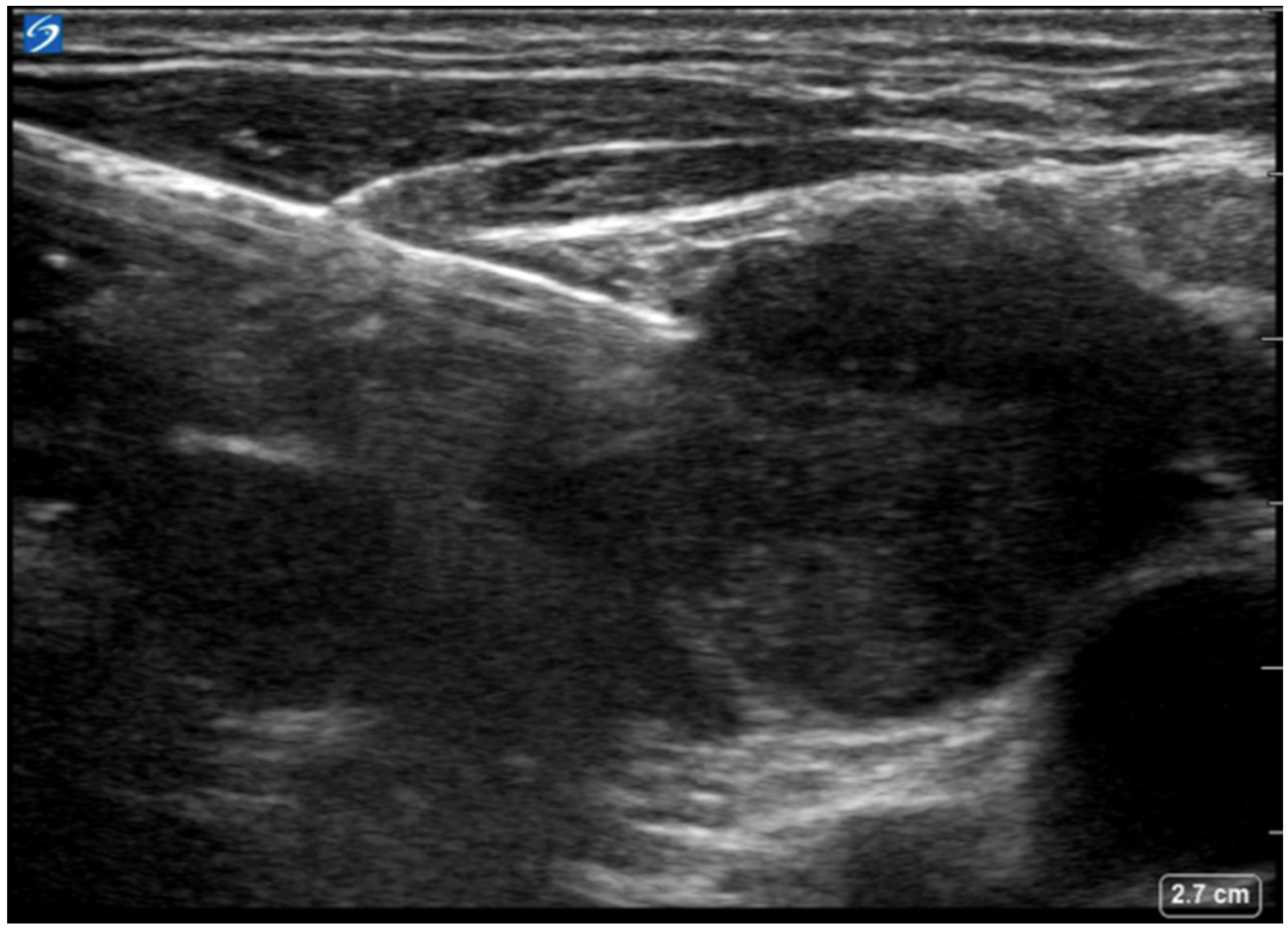
6. Supraclavicular Lymph Node Biopsy
7. Needle Aspiration Versus Core Biopsy
8. Thoracentesis and Pleuroscopy
9. Conclusions
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Asamura, H.; Travis, W.D.; Rusch, V.W. Lung cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 138–155. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Bolejack, V.; Giroux, D.J.; Chansky, K.; Crowley, J.; Asamura, H.; Goldstraw, P. The IASLC lung cancer staging project: The new database to inform the eighth edition of the TNM classification of lung cancer. J. Thorac. Oncol. 2014, 9, 1618–1624. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Bolejack, V.; Crowley, J.; Ball, D.; Kim, J.; Lyons, G.; Rice, T.; Suzuki, K.; Thomas, C.F.; Travis, W.D.; et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 990–1003. [Google Scholar] [CrossRef]
- Travis, W.D.; Asamura, H.; Bankier, A.A.; Beasley, M.B.; Detterbeck, F.; Flieder, D.B.; Goo, J.M.; MacMahon, H.; Naidich, D.; Nicholson, A.G.; et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1204–1223. [Google Scholar] [CrossRef]
- Huang, J.; Osarogiagbon, R.U.; Giroux, D.J.; Nishimura, K.K.; Bille, A.; Cardillo, G.; Detterbeck, F.; Kernstine, K.; Kim, H.K.; Lievens, Y.; et al. The International Association for the Study of Lung Cancer Staging Project for Lung Cancer: Proposals for the Revision of the N Descriptors in the Forthcoming Ninth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2023; in press. [Google Scholar] [CrossRef]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Nicholson, A.G.; Franklin, W.A.; Marom, E.M.; Travis, W.D.; Girard, N.; Arenberg, D.A.; Bolejack, V.; Donington, J.S.; Mazzone, P.J.; et al. The IASLC Lung Cancer Staging Project: Summary of Proposals for Revisions of the Classification of Lung Cancers with Multiple Pulmonary Sites of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J. Thorac. Oncol. 2016, 11, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Zelen, M. Keynote address on biostatistics and data retrieval. Cancer Chemother. Rep. 3 1973, 4, 31–42. [Google Scholar] [PubMed]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e211S–e250S. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Ragusa, P.; Montemaggi, P.; Bona, C.M. Combining independent studies of diagnostic fluorodeoxyglucose positron-emission tomography and computed tomography in mediastinal lymph node staging for non-small cell lung cancer. Tumori 2006, 92, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hansen, M.; Baldwin, D.R.; Hasler, E.; Zamora, J.; Abraira, V.; Roqué i Figuls, M. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst. Rev. 2014, 2014, CD009519. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Schil, P.V.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef]
- Hochstenbag, M.M.; Twijnstra, A.; Wilmink, J.T.; Wouters, E.F.; ten Velde, G.P. Asymptomatic brain metastases (BM) in small cell lung cancer (SCLC): MR-imaging is useful at initial diagnosis. J. Neurooncol. 2000, 48, 243–248. [Google Scholar] [CrossRef]
- Novello, S.; Barlesi, F.; Califano, R.; Cufer, T.; Ekman, S.; Levra, M.G.; Kerr, K.; Popat, S.; Reck, M.; Senan, S.; et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v1–v27. [Google Scholar] [CrossRef]
- Beyaz, F.; Verhoeven, R.L.J.; Schuurbiers, O.C.J.; Verhagen, A.F.T.M.; van der Heijden, E.H.F.M. Occult lymph node metastases in clinical N0/N1 NSCLC; A single center in-depth analysis. Lung Cancer 2020, 150, 186–194. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Reck, M.; Rabe, K.F. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 849–861. [Google Scholar] [CrossRef]
- DuComb, E.A.; Tonelli, B.A.; Tuo, Y.; Cole, B.F.; Mori, V.; Bates, J.H.; Washko, G.R.; Estépar, R.S.J.; Kinsey, C.M. Evidence for Expanding Invasive Mediastinal Staging for Peripheral T1 Lung Tumors. Chest 2020, 158, 2192–2199. [Google Scholar] [CrossRef]
- Navani, N.; Spiro, S.G.; Janes, S.M. Mediastinal staging of NSCLC with endoscopic and endobronchial ultrasound. Nat. Rev. Clin. Oncol. 2009, 6, 278–286. [Google Scholar] [CrossRef][Green Version]
- Holty, J.E.; Kuschner, W.G.; Gould, M.K. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: A meta-analysis. Thorax 2005, 60, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; Santoriello, C.; Di Natale, D.; Cascone, R.; Musella, V.; Mastromarino, R.; Serra, N.; Vicidomini, G.; Polverino, M.; Santini, M. In the era of ultrasound technology, could conventional trans-bronchial needle aspiration still play a role in lung cancer mediastinal staging? J. Thorac. Dis. 2017, 9 (Suppl. S5), S386–S394. [Google Scholar] [CrossRef][Green Version]
- Lee, H.S.; Lee, G.K.; Lee, H.-S.; Kim, M.S.; Lee, J.M.; Kim, H.Y.; Nam, B.-H.; Zo, J.I.; Hwangbo, B. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: How many aspirations per target lymph node station? Chest 2008, 134, 368–374. [Google Scholar] [CrossRef]
- Yasufuku, K.; Pierre, A.; Darling, G.; de Perrot, M.; Waddell, T.; Johnston, M.; Santos, G.d.C.; Geddie, W.; Boerner, S.; Le, L.W.; et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J. Thorac. Cardiovasc. Surg. 2011, 142, 1393–1400.e1. [Google Scholar] [CrossRef] [PubMed]
- Wahidi, M.M.; Herth, F.; Yasufuku, K.; Shepherd, R.W.; Yarmus, L.; Chawla, M.; Lamb, C.; Casey, K.R.; Patel, S.; Silvestri, G.A.; et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 816–835. [Google Scholar] [CrossRef]
- Elmufdi, F.S.; Peterson, M.K.; Niccum, D.; Asche, S.; Ham, K. Evaluating Yield of 19 Versus 21 G EBUS-TBNA Needles: A Prospective Study. J. Bronchol. Interv. Pulmonol. 2021, 28, 29–33. [Google Scholar] [CrossRef]
- von Bartheld, M.B.; van Breda, A.; Annema, J.T. Complication rate of endosonography (endobronchial and endoscopic ultrasound): A systematic review. Respiration 2014, 87, 343–351. [Google Scholar] [CrossRef]
- Shingyoji, M.; Nakajima, T.; Yoshino, M.; Yoshida, Y.; Ashinuma, H.; Itakura, M.; Tatsumi, K.; Iizasa, T. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann. Thorac. Surg. 2014, 98, 1762–1767. [Google Scholar] [CrossRef]
- Ong, P.; Grosu, H.; Eapen, G.A.; Rodriguez, M.; Lazarus, D.; Ost, D.; Jimenez, C.A.; Morice, R.; Bandi, V.; Tamara, L.; et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann. Am. Thorac. Soc. 2015, 12, 415–419. [Google Scholar] [CrossRef]
- Vial, M.R.; O’Connell, O.J.; Grosu, H.B.; Hernandez, M.; Noor, L.; Casal, R.F.; Stewart, J.; Sarkiss, M.; Jimenez, C.A.; Rice, D.; et al. Diagnostic performance of endobronchial ultrasound-guided mediastinal lymph node sampling in early stage non-small cell lung cancer: A prospective study. Respirology 2018, 23, 76–81. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Call, S.; Dooms, C.; Obiols, C.; Sánchez, M.; Travis, W.D.; Vollmer, I. Lung cancer staging: A concise update. Eur. Respir. J. 2018, 51, 1800190. [Google Scholar] [CrossRef]
- Eggeling, S.; Martin, T.; Böttger, J.; Beinert, T.; Gellert, K. Invasive staging of non-small cell lung cancer--a prospective study. Eur. J. Cardiothorac. Surg. 2002, 22, 679–684. [Google Scholar] [CrossRef]
- Sebastián-Quetglás, F.; Molins, L.; Baldó, X.; Buitrago, J.; Vidal, G.; Spanish Video-assisted Thoracic Surgery Study Group. Clinical value of video-assisted thoracoscopy for preoperative staging of non-small cell lung cancer. A prospective study of 105 patients. Lung Cancer 2003, 42, 297–301. [Google Scholar] [CrossRef]
- Massone, P.P.; Lequaglie, C.; Magnani, B.; Ferro, F.; Cataldo, I. The real impact and usefulness of video-assisted thoracoscopic surgery in the diagnosis and therapy of clinical lymphadenopathies of the mediastinum. Ann. Surg. Oncol. 2003, 10, 1197–1202. [Google Scholar] [CrossRef]
- De Giacomo, T.; Rendina, E.A.; Venuta, F.; Della Rocca, G.; Ricci, C. Thoracoscopic staging of IIIB non-small cell lung cancer before neoadjuvant therapy. Ann. Thorac. Surg. 1997, 64, 1409–1411. [Google Scholar] [CrossRef]
- Kumaran, M.; Benamore, R.E.; Vaidhyanath, R.; Muller, S.; Richards, C.J.; Peake, M.D.; Entwisle, J.J. Ultrasound guided cytological aspiration of supraclavicular lymph nodes in patients with suspected lung cancer. Thorax 2005, 60, 229–233. [Google Scholar] [CrossRef][Green Version]
- Takashima, S.; Sone, S.; Nomura, N.; Tomiyama, N.; Kobayashi, T.; Nakamura, H. Nonpalpable lymph nodes of the neck: Assessment with US and US-guided fine-needle aspiration biopsy. J. Clin. Ultrasound 1997, 25, 283–292. [Google Scholar] [CrossRef]
- Fultz, P.J.; Feins, R.H.; Strang, J.G.; Wandtke, J.C.; Johnstone, D.W.; Watson, T.J.; Gottlieb, R.H.; Voci, S.L.; Rubens, D.J. Detection and diagnosis of nonpalpable supraclavicular lymph nodes in lung cancer at CT and US. Radiology 2002, 222, 245–251. [Google Scholar] [CrossRef]
- van Overhagen, H.; Brakel, K.; Heijenbrok, M.W.; van Kasteren, J.H.L.M.; van de Moosdijk, C.N.F.; Roldaan, A.C.; van Gils, A.P.; Hansen, B.E. Metastases in supraclavicular lymph nodes in lung cancer: Assessment with palpation, US, and CT. Radiology 2004, 232, 75–80. [Google Scholar] [CrossRef]
- Ahmed, M.; Daneshvar, C.; Breen, D. Ultrasound-Guided Cervical Lymph Node Sampling Performed by Respiratory Physicians. Biomed. Hub. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Folch, E.; Costa, D.B.; Wright, J.; VanderLaan, P.A. Lung cancer diagnosis and staging in the minimally invasive age with increasing demands for tissue analysis. Transl. Lung Cancer Res. 2015, 4, 392–403. [Google Scholar] [CrossRef]
- Coley, S.M.; Crapanzano, J.P.; Saqi, A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: Obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol. 2015, 123, 318–326. [Google Scholar] [CrossRef]
- Schneider, F.; Smith, M.A.; Lane, M.C.; Pantanowitz, L.; Dacic, S.; Ohori, N.P. Adequacy of core needle biopsy specimens and fine-needle aspirates for molecular testing of lung adenocarcinomas. Am. J. Clin. Pathol. 2015, 143, 193–306. [Google Scholar] [CrossRef]
- Perrotta, F.; Nankivell, M.; Adizie, J.B.; Maqsood, U.; Elshafi, M.; Jafri, S.; Lerner, A.D.; Woolhouse, I.; Munavvar, M.; Evison, M.; et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for PD-L1 Testing in Non-small Cell Lung Cancer. Chest 2020, 158, 1230–1239. [Google Scholar] [CrossRef]
- The American Thoracic Society; The European Respiratory Society. Pretreatment evaluation of non-small-cell lung cancer. Am. J. Respir. Crit. Care Med. 1997, 156, 320–332. [Google Scholar] [CrossRef]
- Vial, M.R.; Eapen, G.A.; Casal, R.F.; Sarkiss, M.G.; Ost, D.E.; Vakil, E.; Grosu, H.B. Combined pleuroscopy and endobronchial ultrasound for diagnosis and staging of suspected lung cancer. Respir. Med. Case Rep. 2017, 23, 49–51. [Google Scholar] [CrossRef]
- Diddams, M.J.; Lee, H.J. Robotic Bronchoscopy: Review of Three Systems. Life 2023, 13, 354. [Google Scholar] [CrossRef]

| Category | Definition |
|---|---|
| Tis | Carcinoma in situ |
| T1a | Tumor ≤ 1 cm (cm) |
| T1b | Tumor > 1 cm to ≤2 cm |
| T1c | Tumor > 2 cm to ≤3 cm |
| T2a | Tumor > 3 cm to ≤4 cm; or any size involving the main bronchus or visceral pleura, or leading to obstructive atelectasis |
| T2b | Tumor > 4 cm to ≤5 cm |
| T3 | Tumor > 5 cm to ≤7 cm; or any size involving the parietal pleura, parietal pericardium, chest wall, T1–T2 nerve roots, phrenic nerve; or satellite tumor in same lobe as primary tumor |
| T4 | Tumor > 7 cm; or any size invading the mediastinum, diaphragm, trachea, main carina, recurrent laryngeal nerve, esophagus, visceral pericardium, vertebral body, great vessels, heart, cervical nerve roots; or satellite tumor in separate lobe of ipsilateral lung |
| N0 | No reginal lymph nodes involved |
| N1 | Ipsilateral peribronchial, hilar, and intrapulmonary nodes |
| N2 | Ipsilateral mediastinal and subcarinal nodes |
| N3 | Contralateral mediastinal, hilar, or any scale or supraclavicular node |
| M1a | Separate tumor nodule in contralateral lung, pleural, or pericardial involvement |
| M1b | Single extrathoracic metastasis |
| M1c | Multiple extrathoracic metastases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueschhoff, A.B.; Moore, A.W.; Jasahui, M.R.P. Lung Cancer Staging—A Clinical Practice Review. J. Respir. 2024, 4, 50-61. https://doi.org/10.3390/jor4010005
Rueschhoff AB, Moore AW, Jasahui MRP. Lung Cancer Staging—A Clinical Practice Review. Journal of Respiration. 2024; 4(1):50-61. https://doi.org/10.3390/jor4010005
Chicago/Turabian StyleRueschhoff, Ali B., Andrew W. Moore, and Maykol R. Postigo Jasahui. 2024. "Lung Cancer Staging—A Clinical Practice Review" Journal of Respiration 4, no. 1: 50-61. https://doi.org/10.3390/jor4010005
APA StyleRueschhoff, A. B., Moore, A. W., & Jasahui, M. R. P. (2024). Lung Cancer Staging—A Clinical Practice Review. Journal of Respiration, 4(1), 50-61. https://doi.org/10.3390/jor4010005






