Abstract
Cigarette combustion has the potential to generate over 7000 chemicals, the majority of which are reactive free radicals that are known to trigger pro-inflammatory and carcinogenic responses. Numerous contemporary investigations have proposed that the pathophysiological and cellular mechanisms underlying the release of extracellular vesicles (EVs) in response to cigarette smoke (CS) may serve as potential pathways for CS-induced pathogenesis, while also reflecting the physiological state of the originating cells. This review provides a concise overview of the pathophysiological mechanisms linked to CS-induced EVs in various lung diseases, including chronic obstructive pulmonary disease, lung cancer, pulmonary fibrosis, and pulmonary hypertension. Additionally, it explores the potential and prospects of EVs as diagnostic biomarkers for CS-related lung diseases.
1. Introduction
When cigarettes are burned, they can generate a multitude of chemicals, exceeding 7000 in number. These chemicals are found in various forms of cigarette smoke (CS), including mainstream, sidestream, secondhand, thirdhand, and discarded cigarette butts. The majority of these substances are highly reactive free radicals such as peroxyl radicals, nitrogen radicals, and other oxygen-derived species. These free radicals have the potential to induce pro-inflammatory and carcinogenic responses [1]. Consequently, the inhalation of CS poses a significant threat to the health of both smokers and nonsmokers. Smokers directly inhale mainstream smoke, while nonsmokers primarily inhale sidestream smoke passively [2]. The sales of cigarettes have experienced a notable growth trajectory, rising from $4.96 trillion in 1980 to $5.5 trillion in 2016, with projections indicating a further increase to $9 trillion by the year 2025 [2,3].
Based on the data disseminated by the World Health Organization (WHO), a staggering 8 million individuals succumbed to the adverse effects of CS on a global scale in the year 2018 [4]. Projections indicate that by 2030, 50% of smokers who have smoked for more than 30 years will die from diseases caused by cigarettes. The WHO highlights the dangers of cigarettes to lung health: more than 40% of cigarette-related deaths are caused by lung disease. Cigarettes have a significant impact on the lung health of people worldwide, including chronic obstructive pulmonary disease (COPD) and lung cancer. CS is the major cause of COPD, where pus accumulates in patients’ lungs causing coughing and difficulty breathing, and CS accounts for over two-thirds of lung cancer deaths worldwide.
There is a growing body of evidence suggesting that the link between CS and several chronic diseases may be mediated in part by extracellular vesicles (EVs). EVs include exosomes and microvesicles released by cells to the extracellular environment and are carriers of cytosolic proteins, lipids, and RNA. EVs represent an important mode of intercellular communication. Firstly, EVs transport proteins that bind to receptors on the cell surface receptors and trigger intracellular signal transduction. In addition, EVs fuse with target cell membranes to deliver functional RNA, nuclear DNA, proteins, and mitochondria that regulate target cell activity [5]. Finally, EVs carry certain proteins that function in the extracellular space, such as by remodeling the extracellular matrix (ECM) [6].
Exposure to CS stimulates the release of EVs into tissue cells and body circulation. Furthermore, CS-induced EVs secretion leads to several biochemical and cellular processes, including angiogenesis [7], endothelial dysfunction [8], tissue remodeling [9], pro-inflammation, oxidative stress, fibrosis, and thrombosis [10], contributing to the pathogenesis of CS-related lung diseases. The proteins, nucleic acids, and other molecules contained within EVs are known to change in response to changes in the state of the parental cells, suggesting that EVs may serve as diagnostic markers reflecting the physiological status of the originating cells [11].
CS is extremely destructive to the lungs and even poses a threat to the lung health of people worldwide. Tobacco dependence as a chronic disease is not given enough attention by many people, and, as a result, they tend to ignore CS-induced chronic illness and miss the best time to intervene for treatment. The lack of a thorough understanding of the effects of CS on the lung often prevents smokers from making a decision to stop smoking. In addition to the widely known pathogenic mechanisms of CS, numerous studies have recently suggested that the pathophysiological and cellular processes involved in the release of EVs triggered by CS are potential mechanisms of CS-induced pathogenesis [12]. Research in this area has advanced significantly over the past decade, and according to a review by Ryu et al. [13] it is possible to understand the pathophysiological and cellular processes that are involved in the release of EVs triggered by CS up to the year. The past 5 years have been marked by a lack of reviews, especially on the mechanisms of lung diseases involved in the release of CS-triggered EVs; therefore, here we review the role of EVs in the pathogenesis of CS-related lung diseases such as COPD, lung cancer, pulmonary fibrosis (PF) and pulmonary hypertension (PH), and look at the prospects and potential of EVs as biomarkers for the diagnosis of CS-related lung diseases (Figure 1).
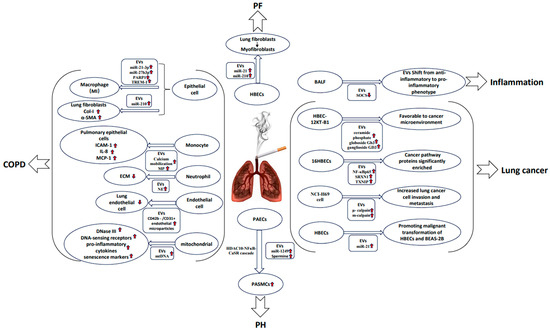
Figure 1.
Induction and modulation of EVs by CS and their relevance in lung disease. CS induces the secretion of EVs, which carry proteins, nucleic acids, and lipids that may contribute to the development of diseases of the lung (COPD, lung cancer, pulmonary fibrosis, and pulmonary hypertension). Red arrows indicate an increase/decrease in substance or an enhancement/weakening of a reaction.
2. Lung Diseases Associated with CS-Induced Release of EVs
2.1. COPD
COPD is a heterogeneous lung disease characterized by chronic respiratory symptoms (dyspnea, cough, sputum, exacerbations) caused by airway abnormalities (bronchitis) and/or alveolar (emphysema), resulting in a persistent, usually progressive airflow obstruction [14]. The major pathological features of the disease are emphysema and chronic bronchitis. In emphysema, the walls between the air sacs in the lungs are disrupted and gradually lose their shape and function, which reduces the amount of gas exchange in the lungs [15]. Chronic bronchitis is characterized by inflammation, narrowing of the small airways, and the associated production of mucus [16]. Both pathological features result in a progressive airway obstruction. COPD is expected to become one of the leading causes of death worldwide by 2030 [17]. Inflammation and oxidative stress are key factors in the pathogenesis of COPD. It is estimated that more than 90% of COPD is caused by CS [18]. CS causes an excess of reactive oxygen species and reactive nitrogen species (ROS and RNS, respectively), and increases the expression levels of pro-inflammatory genes, resulting in an increase in inflammation and a burden of oxidative stress [19].
The role of EVs derived from epithelial cells and their interaction with macrophages is an important pathway for CS induction of COPD via EVs. Cigarette smoke extracts (CSEs) exposure enhances exosome release from human bronchial epithelial cells (BEAS-2B) by depleting extracellular free thiols, while thiol protection inhibits the deleterious changes induced by EVs signaling under conditions of inflammation and oxidative stress [20]. CSEs induced significant changes in the expression of eight miRNAs (let-7a, let-7b, let-7c, let-7f, let-7g, let-7i, miR-100, and miR-210) in human bronchial epithelial cell (HBEC)-derived EVs. Among them, miR-210 increased the expression levels of type I collagen (Col-I) and α-smooth muscle actin (α-SMA) in lung fibroblasts (LFs), thereby promoting myofibroblast differentiation [21]. Alveolar macrophages (AMs) are the major population of immune cells that can regulate the local microenvironment by removing cellular debris, particulate matter, and pathogens, and participate in the progression of inflammation in the lung [22]. Depending on environmental stimuli, macrophages can be classified into pro-inflammatory classically activated (M1) and anti-inflammatory alternatively activated (M2) phenotypes, which are mutually antagonistically reversible [23]. As the M1 macrophages can produce pro-inflammatory mediators and proteases that damage tissues, excessive accumulation of M1 macrophages at sites of inflammation can lead to increased tissue damage and a more severe inflammatory response [24]. In addition, M1 macrophages may have an indirect role in COPD through impaired phagocytosis of bacterial pathogens and defective clearance of apoptotic cells [25,26]. Chen et al. [27,28] found that CS-treated BEAS-2B-derived EVs could promote M1-type polarization of macrophages through a mechanism associated with elevated miR-21-3p, miR-27b-3p, and poly ADP-ribose polymerase 1 (PARP1) protein levels. The effects of EVs on macrophages were extensively studied by Wang et al. [29]. They isolated exosomes ExoPBS and ExoCSE from PBS- or CSE-treated mouse airway epithelial cells. To explore the regulatory role of ExoCSE in macrophage polarization, they established a mouse macrophage cell line (RAW264.7) co-culture system with ExoCSE or ExoPBS. After 48 h of in vitro culture, the percentage of M1 macrophages, expression of iNOS (M1 macrophage marker) mRNA, and levels of TNF-α and IL-1β in cell supernatants were significantly upregulated in exosome-treated RAW264.7 macrophages, suggesting that ExoCSE promotes M1 macrophage polarization. The results also demonstrated that Trigger receptor-1 (TREM-1) mRNA and TREM-1 protein were highly expressed in ExoCSE-treated RAW264.7 macrophages. TREM-1 expressed on myeloid cells is a key innate immune receptor that triggers inflammation or amplifies inflammatory responses and plays an important role in the pathogenesis of several inflammatory and autoimmune diseases [30,31]. Furthermore, TREM-1 induces macrophage polarization towards the M1 phenotype [32]. To elucidate whether TREM-1 mediates the promoting effect of ExoCSE on the polarization of M1 macrophages, the researchers knocked down TREM-1 expression in RAW264.7 macrophages and then incubated the cells with ExoCSE [29]. Silencing TREM-1 was found to significantly reduce the proportion of M1 macrophages, inhibit iNOS mRNA expression, and decrease TNF-α and IL-1β production. Therefore, it was concluded that ExoCSE promotes M1 macrophage polarization by upregulating TREM-1 expression in RAW264.7 macrophages. In order to further investigate the in vivo role and mechanism of action of ExoCSE in COPD, the authors injected ExoCSE tail vein into COPD mice and observed a thickening of the bronchial wall, disruption of the alveolar structure, enlargement of the alveolar lumen, and infiltration of inflammatory cells in the lung tissue of COPD mice, and these ExoCSE-induced exacerbations of lung injury in COPD mice were partially reversed using TREM-1 silencing. An ExoCSE treatment further increased the expression of iNOS mRNA, TNF-α, and IL-1β in COPD mice, but a lentivirus-mediated downregulation of TREM-1 reversed the upregulation of these factors to some extent. In addition, ExoCSE further improved TREM-1 mRNA and TREM-1 protein levels in the lung tissue of COPD mice. These data therefore suggested that the effect of ExoCSE on lung injury in COPD mice is related to its upregulation of TREM-1 expression and promotion of M1 macrophage polarization. CS induces bronchial epithelial cells to produce excessive amounts of mature miR-93, which move to macrophages via EVs [33]. MiR-93 activates the JNK pathway in macrophages through the targeting of bispecific phosphatase 2, which increases the levels of matrix metalloproteinase 9 (MMP9) and matrix metalloproteinase 12 (MMP12), which induces the breakdown of elastin and leads to emphysema. This indicated that abnormal epithelial–macrophage crosstalk is associated with CS-associated emphysema. The miR-93 of EVs may serve as a novel risk biomarker for CS-induced emphysema. The expression of circ_0040929 in bronchial epithelial cells has also been reported in the literature to increase in a dose- and time-dependent manner with CSE treatment. Furthermore, circ_0040929 has been shown to play a facilitative role in CSE-induced COPD by regulating the miR-515-5p/IGFBP3 axis, suggesting that it may be a new potential target for COPD therapy [34]. In addition, α-1 antitrypsin deficient (AATD) macrophages had an enhanced inflammatory response upon exposure to CS-induced EVs released from airway epithelial cells. CS-induced EVs induced the expression of granulocyte-macrophage colony-stimulating factor and interleukin-8 (IL-8) in AATD macrophages, but did not affect normal macrophages. The release of AAT polymer (potent neutrophil granulocyte chemoattractant) from AATD macrophages was also enhanced following exposure to CS-induced EVs. This may therefore lead to progressive lung inflammation and injury in individuals with AATD [35].
In addition to the interaction of epithelial cells with macrophages, monocytes, neutrophils, endothelial cells, and mitochondria release increased levels of EVs in the CS environment. EVs carry inclusions into the circulation to target adjacent and distal cells, further enhancing inflammation, oxidative stress, fibrosis, and ECM degradation, resulting in lung tissue damage and thus contributing to the development of COPD. For example, exposure of human mononuclear cells to CSE was shown to increase calcium mobilization and microparticle (MP) concentrations, and co-incubation of MP with lung epithelial cells resulted in a detectable upregulation of the expression of pro-inflammatory mediators intercellular including adhesion molecules-1 (ICAM-1), IL-8, and monocyte chemoattractant protein-1 (MCP-1) [36]. Neutrophils (PMN) are activated by the presence of acetylated proline–glycine–proline in bronchoalveolar lavage fluid (BALF) from mice exposed to CS, and the surface of PMN-derived activated exosomes contains neutrophil elastase (NE), which binds to integrin Mac-1 and degrades ECM, leading to COPD features [37]. The plasma levels of CD42b−/CD31+ endothelial microparticles (EMPs) were found to be significantly elevated in smoking rats, indicating that CS induces apoptosis and stress damage to the endothelial layer of the lung, which is involved in the early development of emphysema [38]. According to Saxena et al., CSE increases the secretion of immortalized human airway basal cell EVs, which contain vascular endothelial growth factor A that binds to vascular endothelial growth factor receptor 2, activates the MAPK signaling program in endothelial cells, and alters the interactions between bronchial epithelial cells and endothelial cells to promote airway remodeling associated with COPD pathogenesis [39,40]. Xu et al. [41] co-cultured untreated and CSE-treated HBECs with bronchial fibroblast cells (MRC-5) and found higher levels of exosomal miR-21 in CSE-treated HBECs than in untreated HBECs. Levels of miR-21, α-SMA, and Col-I were upregulated in MRC-5 co-cultured with CSE-treated HBEC-derived exosomes, indicating an increased differentiation of airway fibroblasts to myofibroblasts. The main pathogenic mechanism for CS toxicity in the lung is inhibition of mitochondrial respiration [42]. Exposure to CSE decreases mitochondrial membrane potential, increases oxidative stress, dysregulates mitochondrial dynamics, and triggers the release of mitochondrial DNA (mtDNA) from EVs. The release of mtDNA into EVs is accompanied by increased expression of markers associated with the DNA damage response including DNase III, DNA-sensing receptors (cGAS and NLRP3), pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-18, and CXCL2), and senescence markers (p16 and p21). Given that a high level of acellular mtDNA was detected in the plasma of COPD patients and the serum of emphysematous mice, it can be hypothesized that cell-free mtDNA acts as a mitochondrial stress marker in response to CS exposure-induced COPD [43].
According to the Global Initiative for Chronic Obstructive Lung Disease, the current diagnosis of COPD is based on three characteristics, including spirometry, observation of symptoms, and significant exposure to toxic stimuli [44]. As work progressed, it was found that EVs could provide a potential method for the diagnosis and monitoring of COPD progression. CS-induced upregulation of atypical pro-inflammatory WNT-5A and pro-inflammatory cytokine expression in serum EVs of COPD patients can affect M1/M2 macrophage polarization [45]. It has also been shown that treatment of BEAS-2B cells with CSE increases the content of oxidized proteins as well as the ratio of saturated fatty acids/monounsaturated fatty acids that can be used as a biomarker for the diagnosis of CS-induced lung injury [46]. Furthermore, miRNA levels in EVs were significantly different in COPD patients who smoked compared to nonsmoking COPD patients, suggesting that CS alters miRNA levels in circulating EVs and that specific miRNA have the potential to be identified as possible biomarkers for the diagnosis of COPD. For example, CS exposure can cause the upregulation of exosomal miR-21 and miR-210 in HBECs as well as upregulation of exosomal miR-21-3p and miR-27b-3p in BEAS-2B. It was also demonstrated that miRNAs let-7d, -126, -125-5p, and -22 levels were significantly upregulated in human cellular EMPs and mouse circulating particles exposed to CS, and that these miRNAs induced blastomycosis in receptor macrophage damage [8]. Singh et al. [47] identified seven differentially expressed microRNAs in plasma EVs when comparing the smoking and nonsmoking groups, namely, hsa-let-7a-5p, hsa-miR-21-5p, hsa-miR-29b-3p, hsa-let-7f-5p, hsa-miR-143-3p, hsa-miR-30a -5p, and hsa-let-7i-5p. Hsa-let-7a-5p had high sensitivity and specificity to distinguish the nonsmoking group from the smoking group based on ROC curve analysis. Ouyang et al. [48] found that the expression of miR-5-182p and miR-5-185p was significantly downregulated in lung tissue of COPD rats induced using CSE compared with control rats, suggesting that these two miRNAs may be involved in the development of COPD and may serve as potential biomarkers for COPD diagnosis. The expression of eight miRNAs (let-7a-5p, let-7i-5p, miR-29a-3p, miR-99a-5p, miR-100-5p, miR-151b, miR-375, and miR-486-5p) was significantly increased in CSE-derived EVs from BEAS-2B cells, whereas CSE-treated human monocytes (U937) showed a tendency to decrease the expression of most exosomal miRNAs (miR-let -7a-5p, miR-let7i-5p, miR-103a-3p, and miR-151b) [49]. EVs as a potential biomarker will aid in the identification of lung injury, diagnosis, and monitoring of COPD.
2.2. Lung Cancer
Epidemiological studies have shown that CS increases the risk of many cancers in humans. CS accounts for approximately 81% of human cancer incidence [50]. Lung cancer is the most common cancer induced by CS, and 85% of lung cancers are associated with CS [51].
In healthy lungs, alveolar macrophages and the lung epithelium act synergistically to rapidly remove inhaled substances and maintain an anti-inflammatory state. The EVs of alveolar macrophages promote this anti-inflammatory state by delivering cytokine signaling inhibitor protein 1 (SOCS 1) and cytokine signaling inhibitor protein 3 (SOCS 3) to epithelial cells [52]. SOCS 1 inhibits interferon γ signaling stimulation through signal transducer and activator of transcription 1, whereas SOCS 3 inhibits the interleukin-6 (IL-6) signaling response through signal transducer and activator of transcription 3 (STAT 3). The concentration of SOCS in the BALF of humans exposed to CS was reduced, suggesting that smokers lose their EVs-dependent anti-inflammatory status [7]. CS not only interferes with EVs-dependent anti-inflammatory signaling but also actively promotes EVs-dependent pro-inflammatory signaling. CS shifts the function of EVs secreted by monocytes, epithelial cells, neutrophils, and endothelial cells from an anti-inflammatory to a pro-inflammatory phenotype, which is associated with lung cancer [7]. Increased WNT-5A in primary lung fibroblasts from COPD patients induced by CS exacerbated the elastase-induced airspace enlargement in emphysema in vivo [53]. CS treatment can induce tumorigenesis of healthy cells through the upregulation of WNT/β-catenin signaling in vitro and in human lung cancer patients [54]. Sphingolipids (SLs), glycosphingolipids (GSLs), and epoxylane-like lipids are bioactive lipids that have been shown to play an important role in the etiology of several diseases including cancer. Machala et al. [55] used HPLC-MS/MS to comparatively analyze the acquired mesenchymal phenotype of CS carcinogen benzo [a]pyrene (BaP)-transformed HBEC-12KT-B1 cells and parental human bronchial epithelial cells HBEC-12KT. It was found that large quantities of enediolones were produced in the transformed cell lines, and that the profile of SLs/GSLs was significantly altered. Moreover, the levels of ceramide phosphate, globoside Gb3, and ganglioside GD3 were increased in the exosomes derived from the transformed cells. Thus, these epithelial–mesenchymal transition processes may contribute to changes in the cancer microenvironment and receptor cells, which in turn may be involved in cancer development. Fujita et al. [21] investigated the mechanism of EVs-mediated intercellular communication between HBECs and LFs and found that CS-induced a relative upregulation of EVs miR-210 expression in HBECs, which in turn promoted myofibroblast differentiation in LFs. The abnormal increase in the number of myofibroblasts further induced cancer development.
HBECs are sensitive to CSE, producing a large number of differentially expressed exosomal proteins (DEEPs), and DEEPs are significantly enriched in cancer pathways, such as NF-κB p65, sulforaphane polysaccharide-1, and thioredoxin-interacting protein, suggesting a strong effect of DEEPs on the development of tumors and metastasis [56]. Nitrosamine 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK), an important component of CS, has been reported to mimic epidermal growth factor in enhancing cysteine protease (i.e., calpains) activity through mitogen-activated protein kinase/extracellular regulated protein kinases (MAPK/ERK) signaling pathway [57,58]. Xu et al. [59] found that NNK significantly enhanced μ- and m-calpain in NCI-H69 cell-derived EVs in a culture medium, which may have the potential to cleave the ECM, leading to increased lung cancer cell invasion and metastasis.
CS can regulate the expression of miRNAs in EVs that can be internalized or transferred to neighboring cells and elicit phenotypic effects similar to those of the parental cells. Exosomal miRNAs have been shown to affect cancer in two ways, by regulating the expression of protein-encoded oncogenes and tumor suppressors, such as anaplastic lymphoma kinase, tumor protein p53, vascular endothelial growth factor (VEGF) family, calcineurin-E; or as oncogenes and tumor suppressors such as let-7, miR-21, and miR-34 families [60]. Exosomal miR-21 has been shown to promote the malignant transformation of BEAS-2B and has a strong potential to become a good universal biomarker for cancer diagnosis [61,62]. A study manifested that the exosomal miR-21 levels were higher in CSE-treated HBECs than in untreated HBECs [41]. The miR-21 in exosomes leads to STAT3 activation, which increases VEGF levels in recipient cells, a process implicated in the angiogenesis and malignant transformation of HBECs [63]. Low let-7 expression in lung cancer patients is a risk factor for the prognosis of lung cancer patients. Fujita et al. [21] found that six members of the let-7 family (let-7a, let-7b, let-7c, let-7f, let-7g, and let-7i) were significantly downregulated in CSE-induced HBEC-derived EVs. Héliot et al. [64] quantified miRNAs in BALF-derived EVs, where let-7e, let-7g, and miR-26b were indeed significantly less present in EVs from smokers compared to nonsmokers. They exposed BEAS-2B to EVs from BALF isolated from 10 smokers and 10 nonsmokers over 24 h. The results were consistent with the above experimental results of Xu [41] and Fujita [21], where miR-21 and miR-27a expression was significantly increased in BEAS-2B exposed to smokers’ EVs and the expression of let-7e and let-7g was significantly reduced in the presence of smokers’ EVs. BEAS-2B exposure to smoking EVs was also shown to induce the release of the pro-inflammatory cytokines IL-6 and IL-8, as well as the specific lung cancer biomarker cytokeratin 19 fragment antigen 21-1. These exosomes have the potential to be biomarkers for lung cancer, and the detection of these biomarkers can be used for early diagnosis or prognostic assessment of lung cancer patients.
2.3. Pulmonary Fibrosis
PF is a prototype of a chronic, progressive, and fibrotic lung disease characterized by the deposition of ECM and destruction of alveolar structures. For most patients, the development of PF is associated with a history of CS. The secretion of EVs from CS-exposed airway epithelial cells modulates the level of autophagy in bronchial fibroblasts and induces the differentiation of bronchial fibroblasts into myofibroblasts [21]. Bai et al. [65] reported that the CS-induced downregulation of circRNA_0026344 in HBECs resulted in reduced sponginess of miR-21 in HBEC-derived EVs. Elevated miR-21 translocation to bronchial fibroblasts inhibits Smad7 and thereby activates the TGF-β1/Smads pathway. The activated signaling pathway induces fibroblast differentiation into myofibroblasts, leading to aberrant epithelial–fibroblast crosstalk involved in excessive ECM formation, resulting in PF. Fujita et al. [21] investigated the mechanism of EVs-mediated intercellular communication between HBECs and LFs and found that CS induced a relative upregulation of EV miR-210 expression in HBECs, which in turn promoted myofibroblast differentiation in LFs.
To date, there are no effective clinical treatments for CS-induced PF. However, miR-21 is a potential biomarker for CS-induced PF and may be useful in providing therapeutic targets [66].
2.4. Pulmonary Hypertension
PH is a severe pulmonary vascular disorder characterized by sustained vasoconstriction and pulmonary vascular remodeling leading to increased pulmonary circulatory resistance with right ventricular dysfunction. PH in smokers with COPD is the most prevalent complication driving emphysematous derangement of the pulmonary vascular bed resulting in a ventilation/perfusion mismatch and hypoxaemia [67,68].
Su et al. [69] used microRNA microarrays and real-time polymerase chain reactions to screen and validate microRNA profiles in plasma endothelial cell extracellular vesicles (eEVs) from rats and humans who smoked or did not smoke. The authors found that microR-1249 was highly expressed in eEVs from CS-exposed rat plasma and that miR-1249 expression was agreeably elevated in eEVs from human smoker plasma as well as in eEVs from CSE-treated pulmonary artery endothelial cells (PAECs). CSE-treated PAECs induced miR-1249 transfer to pulmonary artery smooth muscle cells (PASMCs) via eEVs in a paracrine manner [70]. MiR-1249 downregulated histone deacetylase 10 (HDAC10) expression in PASMCs, which in turn enhanced the level of the acetylated form of nuclear factor κB (NFκB) and its nuclear translocation, leading to increased expression of calcium-sensing receptors (CaSR). It was indicated that miR-1249 in CS-enriched eEVs promoted the high proliferative and anti-apoptotic state of PASMCs through the regulation of the HDAC10-NFκB-CaSR cascade, which promoted the development of PH. Furthermore, they found that in rats, the overexpression of micror1249 inhibitor and HDAC10, or knockdown of CaSR, inhibited the proliferative capacity of eEVs, reduced the anti-apoptotic capacity of PASMCs, and inhibited the development of PH. Zhu et al. [70] performed a metabolomic screen of eEVs from smokers and found significantly elevated spermidine levels. Spermine is a polyamine metabolite with potent agonist activity on extracellular CaSR [71]. Using immunohistochemical staining confocal imaging, they also showed that spermine-positive eEVs migrated from endothelial cells to smooth muscle cells in rat pulmonary arteries under CS exposure, which in turn caused smooth muscle cell contraction and proliferation, directly leading to PH. However, the inhibition of CaSR through the negative variable structure modulator Calhex231 or CaSR knockdown attenuated CS-induced pulmonary hypertension in rats without emphysematous changes or chronic hypoxemia.
3. Conclusions and Outlook
This article provides a review of the role of EVs in CS-induced lung diseases (Table 1). There is an extensive literature suggesting that EVs are a major risk factor for many lung diseases induced by CS. Prolonged exposure to environmental toxicants, such as CS, can trigger intracellular signaling, affecting EVs, and the subsequent release of EVs regulates a range of cellular events leading to disease progression. In the last few years, a large number of research results published on this topic have led to a significant development to the knowledge in this field. However, there are limitations in the current research, and a more diversified and comprehensive exploration is needed to improve the body of knowledge in this field. Firstly, the majority of the current exosome research leading to disease progression focuses on miRNAs, followed by proteins. Few studies have been conducted on the cellular events generated by exosomes such as lipids, DNA, and other small molecular substances. The development of isolation techniques to obtain pure and abundant exosomes is necessary to overcome the limitations of integrating and validating the present results. The specific mechanisms underlying the effect of CS on altering the secretion of EVs and their cargo specificity were not determined and warrant further investigation. Furthermore, there is a growing body of evidence supporting EVs’ role as potential diagnostic and prognostic biomarkers of CS-induced lung diseases, contributing to the diagnosis and treatment of related diseases. However, studies investigating EVs as biomarkers for CS-induced early disease-specific diagnosis have been relatively scarce so far. Clinical trials of extensive EVs studies describing their diagnostic or treatment feasibility are ongoing. Moreover, there are links and overlaps between EVs biomarkers in different lung diseases induced by CS; for example, pro-inflammatory factors play an important role in promoting the development of both COPD and lung cancer, and miR-21 and miR-210 have similar changes in the pathological processes of COPD, lung cancer, and lung fibrosis. It is anticipated that the continued exploration of EVs as biomarkers and the search for common markers among different diseases will promote the development of multi-targeted therapies and the development of drugs that act on multiple targets in the disease network, thereby creating a synergistic effect on the action of each target so that the total effect is greater than the sum of the individual effects to achieve the best therapeutic effect. Further, CS decreases the membrane potential of mitochondria and increases oxidative stress, leading to the dysregulation of mitochondrial dynamics and triggering mtDNA release from EVs. A large body of research has suggested that ROS originating from mitochondria play an important role as mediators of hypoxic pulmonary vasoconstriction, and that persistent hypoxic pulmonary vasoconstriction under widespread chronic hypoxia increases pulmonary vascular resistance and thus promotes the development of PH [72]. Comprehensive studies of EVs should focus not only on the direct effects of EVs on cells, but also on the question of whether EVs can affect third-party cellular functions by targeting functional organelles (e.g., mitochondria) to produce mediators. In conclusion, previous studies have collectively shown that EVs have a limited but strong role in CS-induced lung diseases by causing strong cellular effects. Therefore, further studies are required to help further define and understand the functional features of EVs in lung disease pathogenesis. Functional studies of CS-induced EVs pave the way for advances in the diagnosis and treatment of CS-related lung diseases.

Table 1.
CS regulates the secretion of EVs and the levels of proteins, nucleic acids, and lipids contained in EVs.
Author Contributions
Conceptualization, P.L.; writing—original draft preparation, M.Z. (Mengli Zhong); writing—review and editing, M.Z. (Muhan Zou), Y.Y., H.W., W.S., Y.W. and P.L.; project administration, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Open Competition Program of Ten Major Directions of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2022SDZG07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mossina, A.; Lukas, C.; Merl-Pham, J.; Uhl, F.E.; Mutze, K.; Schamberger, A.; Staab-Weijnitz, C.; Jia, J.; Yildirim, A.Ö.; Königshoff, M.; et al. Cigarette Smoke Alters the Secretome of Lung Epithelial Cells. Proteomics 2017, 17, 1600243. [Google Scholar] [CrossRef]
- Soleimani, F.; Dobaradaran, S.; De-la-Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of Toxic Components of Cigarette, Cigarette Smoke vs. Cigarette Butts: A Comprehensive Systematic Review. Sci. Total Environ. 2022, 813, 152667. [Google Scholar] [CrossRef] [PubMed]
- Mackay, J.; Eriksen, M.; Shafey, O. The Tobacco Atlas, 2nd ed.; American Cancer Society: Atlanta, GA, USA, 2006; pp. 1106–1107. [Google Scholar]
- World Health Statistics 2019: Monitoring Health for the SDGs, Sustainable Development Goals. Available online: https://www.who.int/publications-detail-redirect/9789241565707 (accessed on 14 September 2022).
- Singh, S.; Hu, X.; Dixelius, C. Dynamics of Nucleic Acid Mobility. Genetics 2023, 225, iyad132. [Google Scholar] [CrossRef]
- Lacroix, R.; Dignat-George, F. Microparticles: New Protagonists in Pericellular and Intravascular Proteolysis. Semin. Thromb. Hemost. 2013, 39, 033–039. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Wouters, E.F.M.; Savelkoul, P.H.M.; Rohde, G.G.U.; Stassen, F.R.M. Extracellular Vesicles Released in Response to Respiratory Exposures: Implications for Chronic Disease. J. Toxicol. Environ. Health B 2018, 21, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Serban, K.A.; Rezania, S.; Petrusca, D.N.; Poirier, C.; Cao, D.; Justice, M.J.; Patel, M.; Tsvetkova, I.; Kamocki, K.; Mikosz, A.; et al. Structural and Functional Characterization of Endothelial Microparticles Released by Cigarette Smoke. Sci. Rep. 2016, 6, 31596. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Liu, Y.; Chen, Y.; Yu, D.; Williams, K.J.; Liu, M.-L. Novel Proteolytic Microvesicles Released from Human Macrophages after Exposure to Tobacco Smoke. Am. J. Pathol. 2013, 182, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Mobarrez, F.; Antoniewicz, L.; Bosson, J.A.; Kuhl, J.; Pisetsky, D.S.; Lundbäck, M. The Effects of Smoking on Levels of Endothelial Progenitor Cells and Microparticles in the Blood of Healthy Volunteers. PLoS ONE 2014, 9, e90314. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, E.; Lee, M.Y. Exosomes as Diagnostic Biomarkers in Cancer. Mol. Cell. Toxicol. 2018, 14, 113–122. [Google Scholar] [CrossRef]
- Fujita, Y.; Kosaka, N.; Araya, J.; Kuwano, K.; Ochiya, T. Extracellular Vesicles in Lung Microenvironment and Pathogenesis. Trends Mol. Med. 2015, 21, 533–542. [Google Scholar] [CrossRef]
- Ryu, A.-R.; Kim, D.H.; Kim, E.; Lee, M.Y. The Potential Roles of Extracellular Vesicles in Cigarette Smoke-Associated Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4692081. [Google Scholar] [CrossRef]
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes De Oca, M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef]
- Orozco-Levi, M.; Colmenares-Mejia, C.; Ruiz, J.; Valencia-Baron, Y.D.; Ramirez-Sarmiento, A.; Quintero-Lesmes, D.C.; Serrano, N.C. Effect of Antioxidants in the Treatment of COPD Patients: Scoping Review. J. Nutr. Metab. 2021, 2021, 7463391. [Google Scholar] [CrossRef] [PubMed]
- Jehan Peerzada, K. Chronic Obstructive Pulmonary Disease: An Update on Therapeutics and Pathophysiological Understanding. In Chronic Lung Diseases; Springer: Singapore, 2020; pp. 157–180. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated Role of Cigarette Smoking, Oxidative Stress, and Immune Response in COPD and Corresponding Treatments. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 307, L205–L218. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, E.; Zinellu, A.; Fois, A.G.; Pau, M.C.; Scano, V.; Piras, B.; Carru, C.; Pirina, P. Oxidative Stress Biomarkers in Chronic Obstructive Pulmonary Disease Exacerbations: A Systematic Review. Antioxidants 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Benedikter, B.J.; Volgers, C.; Van Eijck, P.H.; Wouters, E.F.M.; Savelkoul, P.H.M.; Reynaert, N.L.; Haenen, G.R.M.M.; Rohde, G.G.U.; Weseler, A.R.; Stassen, F.R.M. Cigarette Smoke Extract Induced Exosome Release Is Mediated by Depletion of Exofacial Thiols and Can Be Inhibited by Thiol-Antioxidants. Free Radic. Biol. Med. 2017, 108, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Araya, J.; Ito, S.; Kobayashi, K.; Kosaka, N.; Yoshioka, Y.; Kadota, T.; Hara, H.; Kuwano, K.; Ochiya, T. Suppression of Autophagy by Extracellular Vesicles Promotes Myofibroblast Differentiation in COPD Pathogenesis. J. Extracell. Vesicles 2015, 4, 28388. [Google Scholar] [CrossRef]
- Puttur, F.; Gregory, L.G.; Lloyd, C.M. Airway Macrophages as the Guardians of Tissue Repair in the Lung. Immunol. Cell Biol. 2019, 97, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nurakhayev, S.; Nurkesh, A.; Zharkinbekov, Z.; Saparov, A. Macrophage Polarization in Cardiac Tissue Repair Following Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 2715. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Digilio, F.A.; Galderisi, U.; Peluso, G. The Emerging Role of Macrophages in Chronic Obstructive Pulmonary Disease: The Potential Impact of Oxidative Stress and Extracellular Vesicle on Macrophage Polarization and Function. Antioxidants 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Finney-Hayward, T.K.; Quint, J.K.; Thomas, C.M.R.; Tudhope, S.J.; Wedzicha, J.A.; Barnes, P.J.; Donnelly, L.E. Defective Macrophage Phagocytosis of Bacteria in COPD. Eur. Respir. J. 2010, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Smoking Alters Alveolar Macrophage Recognition and Phagocytic Ability. Am. J. Respir. Cell Mol. Biol. 2007, 37, 748–755. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, H.; Shi, R.; Fan, W.; Zhang, J.; Su, W.; Wang, Y.; Li, P. MiRNAomics Analysis Reveals the Promoting Effects of Cigarette Smoke Extract-Treated Beas-2B-Derived Exosomes on Macrophage Polarization. Biochem. Biophys. Res. Commun. 2021, 572, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, H.; Fan, W.; Zhang, J.; Yao, Y.; Su, W.; Wang, Y.; Li, P. Naringenin Suppresses BEAS-2B-Derived Extracellular Vesicular Cargoes Disorder Caused by Cigarette Smoke Extract Thereby Inhibiting M1 Macrophage Polarization. Front. Immunol. 2022, 13, 930476. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Q.; Yu, Q.; Xiao, J.; Zhao, H. Cigarette Smoke Extract-Treated Airway Epithelial Cells-Derived Exosomes Promote M1 Macrophage Polarization in Chronic Obstructive Pulmonary Disease. Int. Immunopharmacol. 2021, 96, 107700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, W.; Wang, Z.; Chen, J.; Ding, M.; Han, L. TREM-1 Deficiency Attenuates the Inflammatory Responses in LPS-Induced Murine Endometritis. Microb. Biotechnol. 2019, 12, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; He, X.; Bian, Y.; Guo, Q.; Zheng, K.; Zhao, Y.; Lu, C.; Liu, B.; Xu, X.; Zhang, G.; et al. Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 498. [Google Scholar] [CrossRef]
- Tan, C.; Gurien, S.D.; Royster, W.; Aziz, M.; Wang, P. Extracellular CIRP Induces Inflammation in Alveolar Type II Cells via TREM-1. Front. Cell Dev. Biol. 2020, 8, 579157. [Google Scholar] [CrossRef]
- Xia, H.; Wu, Y.; Zhao, J.; Li, W.; Lu, L.; Ma, H.; Cheng, C.; Sun, J.; Xiang, Q.; Bian, T.; et al. The Aberrant Cross-Talk of Epithelium-Macrophages via METTL3-Regulated Extracellular Vesicle MiR-93 in Smoking-Induced Emphysema. Cell Biol. Toxicol. 2022, 38, 167–183. [Google Scholar] [CrossRef]
- Miao, Y.; Wu, J.; Wu, R.; Wang, E.; Wang, J. Circ_0040929 Serves as Promising Biomarker and Potential Target for Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, N.; Oshins, R.; Mehrad, B.; Lascano, J.E.; Qiang, X.; West, J.R.; Holliday, L.S.; Lee, J.; Wiesemann, G.; Eydgahi, S.; et al. Cigarette Smoke Exposed Airway Epithelial Cell-Derived EVs Promote Pro-Inflammatory Macrophage Activation in Alpha-1 Antitrypsin Deficiency. Respir. Res. 2022, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Cordazzo, C.; Petrini, S.; Neri, T.; Lombardi, S.; Carmazzi, Y.; Pedrinelli, R.; Paggiaro, P.; Celi, A. Rapid Shedding of Proinflammatory Microparticles by Human Mononuclear Cells Exposed to Cigarette Smoke Is Dependent on Ca2+ Mobilization. Inflamm. Res. 2014, 63, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Abdul Roda, M.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef]
- Liu, H.; Ding, L.; Zhang, Y.; Ni, S. Circulating Endothelial Microparticles Involved in Lung Function Decline in a Rat Exposed in Cigarette Smoke Maybe from Apoptotic Pulmonary Capillary Endothelial Cells. J. Thorac. Dis. 2014, 6, 649–655. [Google Scholar] [CrossRef]
- Saxena, A.; Walters, M.S.; Shieh, J.-H.; Shen, L.-B.; Gomi, K.; Downey, R.J.; Crystal, R.G.; Moore, M.A.S. Extracellular Vesicles from Human Airway Basal Cells Respond to Cigarette Smoke Extract and Affect Vascular Endothelial Cells. Sci. Rep. 2021, 11, 6104. [Google Scholar] [CrossRef] [PubMed]
- Curradi, G.; Walters, M.S.; Ding, B.-S.; Rafii, S.; Hackett, N.R.; Crystal, R.G. Airway Basal Cell Vascular Endothelial Growth Factor-Mediated Cross-Talk Regulates Endothelial Cell-Dependent Growth Support of Human Airway Basal Cells. Cell. Mol. Life Sci. 2012, 69, 2217–2231. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Ling, M.; Xue, J.; Dai, X.; Sun, Q.; Chen, C.; Liu, Y.; Zhou, L.; Liu, J.; Luo, F.; et al. Exosomal MicroRNA-21 Derived from Bronchial Epithelial Cells Is Involved in Aberrant Epithelium-Fibroblast Cross-Talk in COPD Induced by Cigarette Smoking. Theranostics 2018, 8, 5419–5433. [Google Scholar] [CrossRef]
- Giordano, L.; Farnham, A.; Dhandapani, P.K.; Salminen, L.; Bhaskaran, J.; Voswinckel, R.; Rauschkolb, P.; Scheibe, S.; Sommer, N.; Beisswenger, C.; et al. Alternative Oxidase Attenuates Cigarette Smoke–induced Lung Dysfunction and Tissue Damage. Am. J. Respir. Cell Mol. Biol. 2019, 60, 515–522. [Google Scholar] [CrossRef]
- Giordano, L.; Gregory, A.D.; Pérez Verdaguer, M.; Ware, S.A.; Harvey, H.; DeVallance, E.; Brzoska, T.; Sundd, P.; Zhang, Y.; Sciurba, F.C.; et al. Extracellular Release of Mitochondrial DNA: Triggered by Cigarette Smoke and Detected in COPD. Cells 2022, 11, 369. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease Incorporated. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2018. Available online: https://goldcopd.org/ (accessed on 2 September 2023).
- Feller, D.; Kun, J.; Ruzsics, I.; Rapp, J.; Sarosi, V.; Kvell, K.; Helyes, Z.; Pongracz, J.E. Cigarette Smoke-Induced Pulmonary Inflammation Becomes Systemic by Circulating Extracellular Vesicles Containing Wnt5a and Inflammatory Cytokines. Front. Immunol. 2018, 9, 1724. [Google Scholar] [CrossRef]
- Chiaradia, E.; Sansone, A.; Ferreri, C.; Tancini, B.; Latella, R.; Tognoloni, A.; Gambelunghe, A.; dell’Omo, M.; Urbanelli, L.; Giovagnoli, S.; et al. Phospholipid Fatty Acid Remodeling and Carbonylated Protein Increase in Extracellular Vesicles Released by Airway Epithelial Cells Exposed to Cigarette Smoke Extract. Eur. J. Cell Biol. 2023, 102, 151285. [Google Scholar] [CrossRef]
- Singh, K.P.; Maremanda, K.P.; Li, D.; Rahman, I. Exosomal MicroRNAs Are Novel Circulating Biomarkers in Cigarette, Waterpipe Smokers, E-Cigarette Users and Dual Smokers. BMC Med. Genom. 2020, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Wang, W.; Wu, D.; Wang, W.; Ye, X.; Yang, Q. Analysis of Serum Exosome MicroRNAs in the Rat Model of Chronic Obstructive Pulmonary Disease. Am. J. Transl. Res. 2023, 15, 138–150. [Google Scholar] [PubMed]
- Sundar, I.K.; Li, D.; Rahman, I. Small RNA-Sequence Analysis of Plasma-Derived Extracellular Vesicle MiRNAs in Smokers and Patients with Chronic Obstructive Pulmonary Disease as Circulating Biomarkers. J. Extracell. Vesicles 2019, 8, 1684816. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Peto, R. Mortality in Relation to Smoking: 20 Years’ Observations on Male British Doctors. Br. Med. J. 1976, 2, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Bourdonnay, E.; Zasłona, Z.; Penke, L.R.K.; Speth, J.M.; Schneider, D.J.; Przybranowski, S.; Swanson, J.A.; Mancuso, P.; Freeman, C.M.; Curtis, J.L.; et al. Transcellular Delivery of Vesicular SOCS Proteins from Macrophages to Epithelial Cells Blunts Inflammatory Signaling. J. Exp. Med. 2015, 212, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Baarsma, H.A.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Günther, A.; Bracke, K.R.; Dagouassat, M.; et al. Noncanonical WNT-5A Signaling Impairs Endogenous Lung Repair in COPD. J. Exp. Med. 2017, 214, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Malyla, V.; Paudel, K.R.; De Rubis, G.; Hansbro, N.G.; Hansbro, P.M.; Dua, K. Cigarette Smoking Induces Lung Cancer Tumorigenesis via Upregulation of the WNT/β-Catenin Signaling Pathway. Life Sci. 2023, 326, 121787. [Google Scholar] [CrossRef] [PubMed]
- Machala, M.; Slavik, J.; Kovac, O.; Prochazkova, J.; Pencikova, K.; Parenicova, M.; Strakova, N.; Kotoucek, J.; Kulich, P.; Mollerup, S.; et al. Changes in Sphingolipid Profile of Benzo[a]Pyrene-Transformed Human Bronchial Epithelial Cells Are Reflected in the Altered Composition of Sphingolipids in Their Exosomes. Int. J. Mol. Sci. 2021, 22, 9195. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, R.; Liu, M.; Chen, M.; Wei, S.; Li, B.; Yu, S. Exosome Proteomics Study of the Effects of Traditional Cigarettes and Electronic Cigarettes on Human Bronchial Epithelial Cells. Toxicol. In Vitro 2023, 86, 105516. [Google Scholar] [CrossRef]
- Schuller, H.M. Mechanisms of Smoking-Related Lung and Pancreatic Adenocarcinoma Development. Nat. Rev. Cancer 2002, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Glading, A.; Überall, F.; Keyse, S.M.; Lauffenburger, D.A.; Wells, A. Membrane Proximal ERK Signaling Is Required for M-Calpain Activation Downstream of Epidermal Growth Factor Receptor Signaling. J. Biol. Chem. 2001, 276, 23341–23348. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Deng, X.M. Tobacco-Specific Nitrosamine 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone Induces Phosphorylation of μ- and m-Calpain in Association with Increased Secretion, Cell Migration, and Invasion. J. Biol. Chem. 2004, 279, 53683–53690. [Google Scholar] [CrossRef] [PubMed]
- Momi, N.; Kaur, S.; Rachagani, S.; Ganti, A.K.; Batra, S.K. Smoking and MicroRNA Dysregulation: A Cancerous Combination. Trends Mol. Med. 2014, 20, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. Considering Exosomal MiR-21 as a Biomarker for Cancer. J. Clin. Med. 2016, 5, 42. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G. MicroRNA-21 (MiR-21) Represses Tumor Suppressor PTEN and Promotes Growth and Invasion in Non-Small Cell Lung Cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, F.; Wang, B.; Li, H.; Xu, Y.; Liu, X.; Shi, L.; Lu, X.; Xu, W.; Lu, L.; et al. STAT3-Regulated Exosomal MiR-21 Promotes Angiogenesis and Is Involved in Neoplastic Processes of Transformed Human Bronchial Epithelial Cells. Cancer Lett. 2016, 370, 125–135. [Google Scholar] [CrossRef]
- Héliot, A.; Landkocz, Y.; Roy Saint-Georges, F.; Gosset, P.; Billet, S.; Shirali, P.; Courcot, D.; Martin, P.J. Smoker Extracellular Vesicles Influence Status of Human Bronchial Epithelial Cells. Int. J. Hyg. Environ. Health 2017, 220, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Deng, J.; Han, Z.; Cui, Y.; He, R.; Gu, Y.; Zhang, Q. CircRNA_0026344 via Exosomal MiR-21 Regulation of Smad7 Is Involved in Aberrant Cross-Talk of Epithelium-Fibroblasts during Cigarette Smoke-Induced Pulmonary Fibrosis. Toxicol. Lett. 2021, 347, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Zhang, Y.; Wan, R.; Jiang, M.; Xu, Y.; Zhang, Q. MiR-21 Mediates Nickel Nanoparticle-Induced Pulmonary Injury and Fibrosis. Nanotoxicology 2020, 14, 1175–1197. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Peinado, V.I.; Ramirez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; Barbera, J.A. Characterization of Pulmonary Vascular Remodelling in Smokers and Patients with Mild COPD. Eur. Respir. J. 2002, 19, 632–638. [Google Scholar] [CrossRef]
- Albert Barbera, J. Mechanisms of Development of Chronic Obstructive Pulmonary Disease-Associated Pulmonary Hypertension. Pulm. Circ. 2013, 3, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tan, R.; Sun, M.; Yuan, L.; Ruiz, M.; Dupuis, J.; Hu, Q.; Zhu, L. MiR-1249 on Endothelial Extracellular Vesicles Mediates Cigarette Smoke–Induced Pulmonary Hypertension by Inhibiting HDAC10 (Histone Deacetylase 10)-NFκB (Nuclear Factor ΚB)-CaSR (Calcium-Sensing Receptor) Cascade. Hypertension 2022, 79, 2721–2732. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xiao, R.; Zhang, X.; Lang, Y.; Liu, F.; Yu, Z.; Zhang, J.; Su, Y.; Lu, Y.; Wang, T.; et al. Spermine on Endothelial Extracellular Vesicles Mediates Smoking-Induced Pulmonary Hypertension Partially through Calcium-Sensing Receptor. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.X.; Geibel, J.P.; Hebert, S.C. Extracellular Polyamines Regulate Fluid Secretion in Rat Colonic Crypts via the Extracellular Calcium-Sensing Receptor. Gastroenterology 2004, 126, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Pak, O.; Nolte, A.; Knoepp, F.; Giordano, L.; Pecina, P.; Hüttemann, M.; Grossman, L.I.; Weissmann, N.; Sommer, N. Mitochondrial Oxygen Sensing of Acute Hypoxia in Specialized Cells-Is There a Unifying Mechanism? Biochim. Biophys. Acta (BBA)-Bioenerg. 2022, 1863, 148911. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).