Low Oxygen Saturation of COVID-19 in Patient Case Fatalities, Limpopo Province, South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting, Period and Design
2.2. Statistical Analysis
2.3. Data Collection and Validation
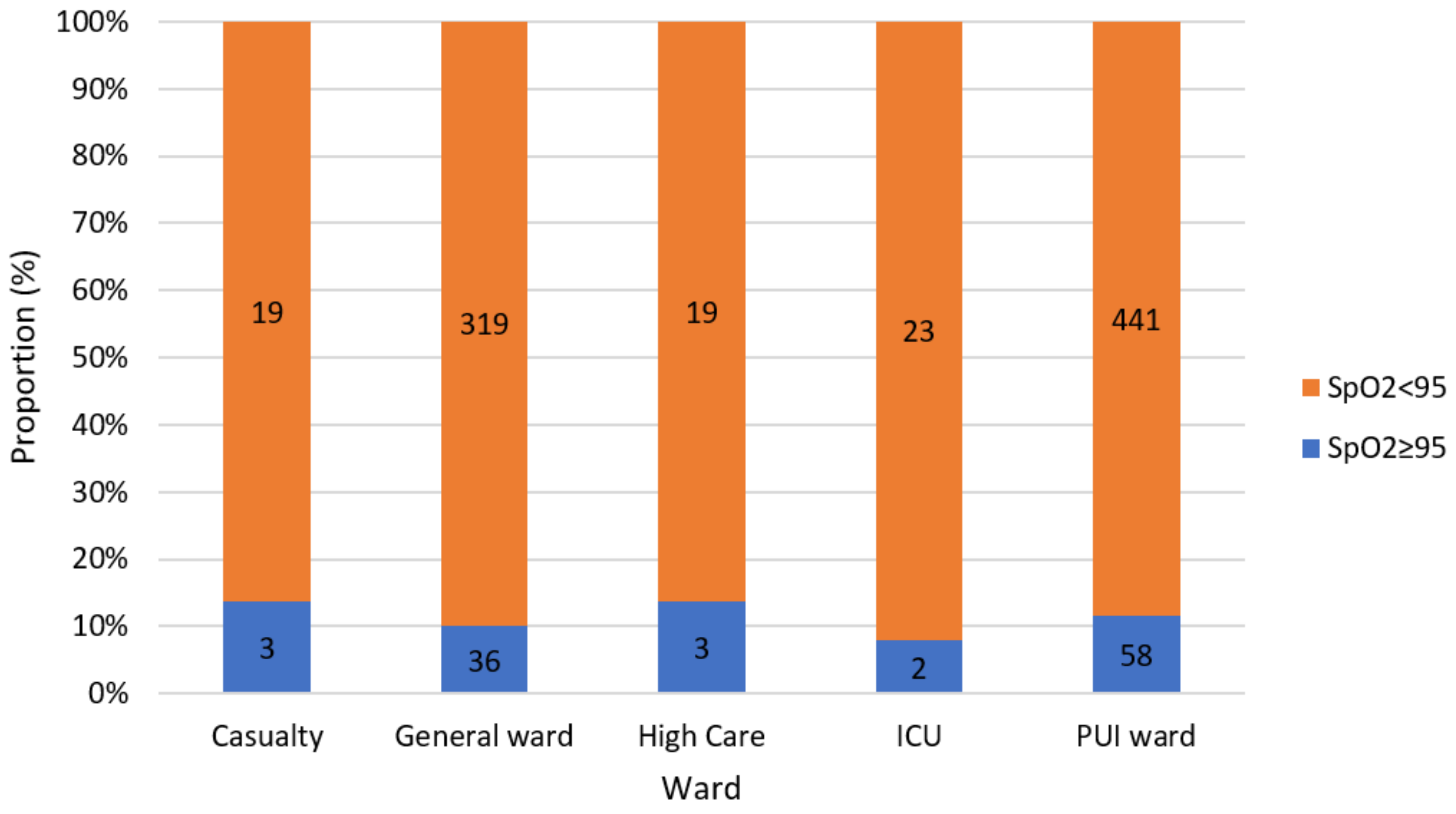
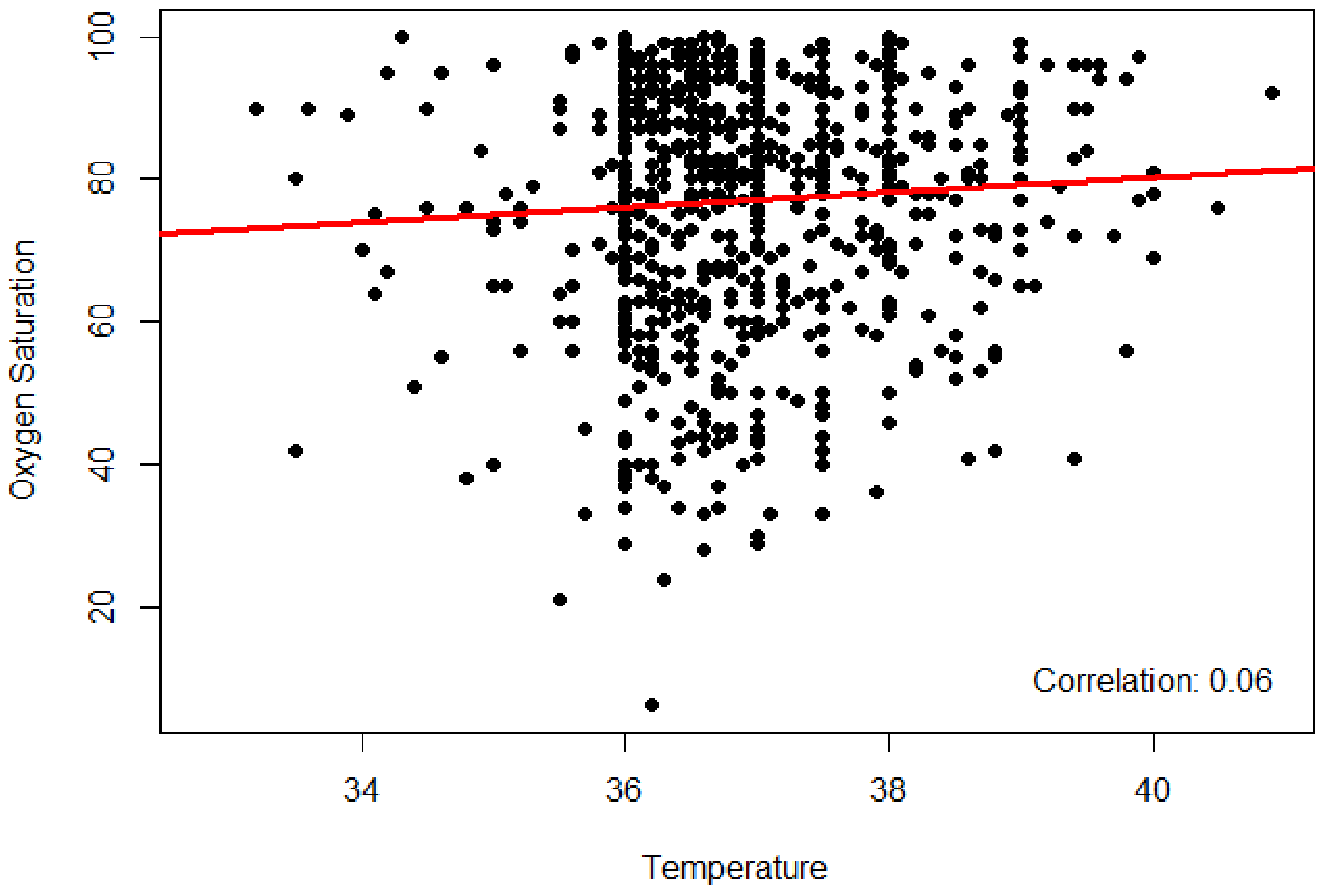
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef] [PubMed]
- Mkhwanazi, E. Does COVID-19 Rupture Theodicy? Theo-philosophical Musings. Phronimon 2021, 22, 1–14. [Google Scholar] [CrossRef]
- Greyling, T.; Rossouw, S.; Adhikari, T. The good, the bad and the ugly of lockdowns during COVID-19. PLoS ONE 2021, 16, e0245546. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int (accessed on 12 August 2021).
- Rogerson, C.M.; Rogerson, J.M. COVID-19 tourism impacts in South Africa: Government and industry responses. Geo J. Tour. Geosites 2020, 31, 1083–1091. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19): Weekly Epidemiological Update. 14 September 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/334304/nCoV-weekly-sitrep13Sep20-eng.pdf (accessed on 12 August 2021).
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, T.W.; Talbot, N.P.; Nickol, A.; Chadwick, A.J.; Lawton, O. Respiratory failure and non-invasive respiratory support during the COVID-19 pandemic: An update for re-deployed hospital doctors and primary care physicians. BMJ 2020, 369, m2446. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines. Oxygenation and Ventilation. 2020. Available online: https://www.covid19treatmentguidelines.nih.gov/management/critical-care/oxygenation-and-ventilation/ (accessed on 20 March 2022).
- Shenoy, N.; Luchtel, R.; Gulani, P. Considerations for target oxygen saturation in COVID-19 patients: Are we under-shooting? BMC Med. 2020, 18, 260. [Google Scholar] [CrossRef]
- Mejía, F.; Medina, C.; Cornejo, E.; Morello, E.; Vásquez, S.; Alave, J.; Schwalb, A.; Málaga, G. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS ONE 2020, 15, e0244171. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, K.; Tang, M.; Kong, F.; Zhou, J.; Diao, L.; Wu, S.; Jiao, P.; Su, T.; Dong, Y. Prevalence and factors associated with depression and anxiety of hospitalized patients with COVID-19. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Lahav, D.Z.; Picard, E.; Mimouni, F.; Joseph, L.; Goldberg, S. The effect of fever on blood oxygen saturation in children. Harefuah 2015, 154, 162–165. [Google Scholar]
- Sohrabi, M.R.; Amin, R.; Maher, A.; Bahadorimonfared, A.; Janbazi, S.; Hannani, K.; Kolahi, A.A.; Zali, A.R. Sociodemographic determinants and clinical risk factors associated with COVID-19 severity: A cross-sectional analysis of over 200,000 patients in Tehran, Iran. BMC Infect. Dis. 2021, 21, 474. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Zali, A.; Gholamzadeh, S.; Mohammadi, G.; Looha, M.A.; Akrami, F.; Zarean, E.; Vafaee, R.; Maher, A.; Khodadoost, M. Baseline characteristics and associated factors of mortality in COVID-19 patients; an analysis of 16,000 cases in Tehran, Iran. Arch. Acad. Emerg. Med. 2020, 8, e70. [Google Scholar]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Alizadehsani, R.; Alizadeh Sani, Z.; Behjati, M.; Roshanzamir, Z.; Hussain, S.; Abedini, N.; Hasanzadeh, F.; Khosravi, A.; Shoeibi, A.; Roshanzamir, M.; et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J. Med. Virol. 2021, 93, 2307–2320. [Google Scholar] [CrossRef]
- Eskandarian, R.; Sani, Z.A.; Behjati, M.; Zahmatkesh, M.; Haddadi, A.; Kakhi, K.; Roshanzamir, M.; Shoeibi, A.; Alizadehsani, R.; Hussain, S.; et al. Identification of clinical features associated with mortality in COVID-19 patients. medRxiv 2021. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, J.Y. Walk-through screening center for COVID-19: An accessible and efficient screening system in a pandemic situation. J. Korean Med. Sci. 2020, 35, e154. [Google Scholar] [CrossRef] [Green Version]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Vanhems, P.; Gustin, M.; Elias, C.; Henaff, L.; Dananché, C.; Grisi, B.; Marion, E.; Khanafer, N.; Hilliquin, D.; Gardes, S.; et al. Factors associated with admission to intensive care units in COVID-19 patients in Lyon-France. PLoS ONE 2021, 16, e0243709. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, S.; Nomoto, K.; Miyashita, S.; Ishikawa, K. Body temperature correlates with mortality in COVID-19 patients. Crit. Care 2020, 24, 298. [Google Scholar] [CrossRef] [PubMed]
- Iftimie, S.; López-Azcona, A.F.; Vicente-Miralles, M.; Descarrega-Reina, R.; Hernández-Aguilera, A.; Riu, F.; Simó, J.M.; Garrido Joven, J.; Camps, J.; Castro, A. Risk factors associated with mortality in hospitalized patients with SARS-CoV-2 infection. A prospective, longitudinal, unicenter study in Reus, Spain. PLoS ONE 2020, 15, e0234452. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Zhou, Y.; Wang, F.; Wang, H.; Zhang, M.; Pan, X.; Zhao, Q.; Liu, J. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2020, 32, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Maistry, S. South Africa’s Comorbidity: A Chronic Affliction of Intersecting Education, Economic and Health Inequalities. Educ. Change 2021, 25, 1–21. [Google Scholar]
- Li, J.; Fink, J.B.; Ehrmann, S. High-flow nasal cannula for COVID-19 patients: Low risk of bio-aerosol dispersion. Eur. Respir. J. 2020, 55, 2000892. [Google Scholar] [CrossRef]
- Gürün, A. High flow nasal cannula in COVID-19: A literature review. Tuberk. Toraks 2020, 68, 168–174. [Google Scholar] [CrossRef]
- Montiel, V.; Robert, A.; Robert, A.; Nabaoui, A.; Marie, T.; Mestre, N.M.; Guillaume, M.; Laterre, F.; Wittebole, X. Surgical mask on top of high-flow nasal cannula improves oxygenation in critically ill COVID-19 patients with hypoxemic respiratory failure. Ann. Intensive Care 2020, 10, 125. [Google Scholar] [CrossRef]
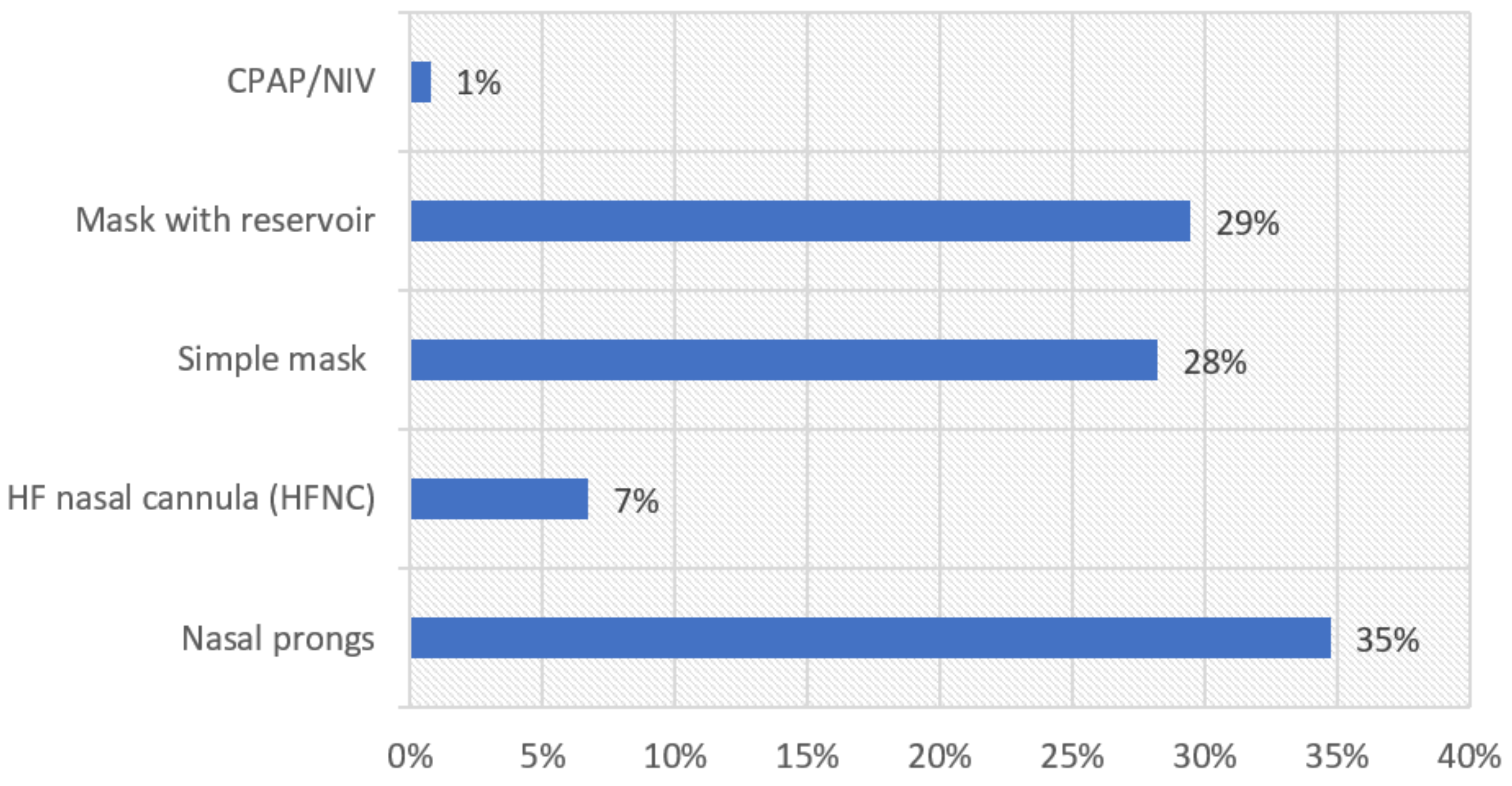
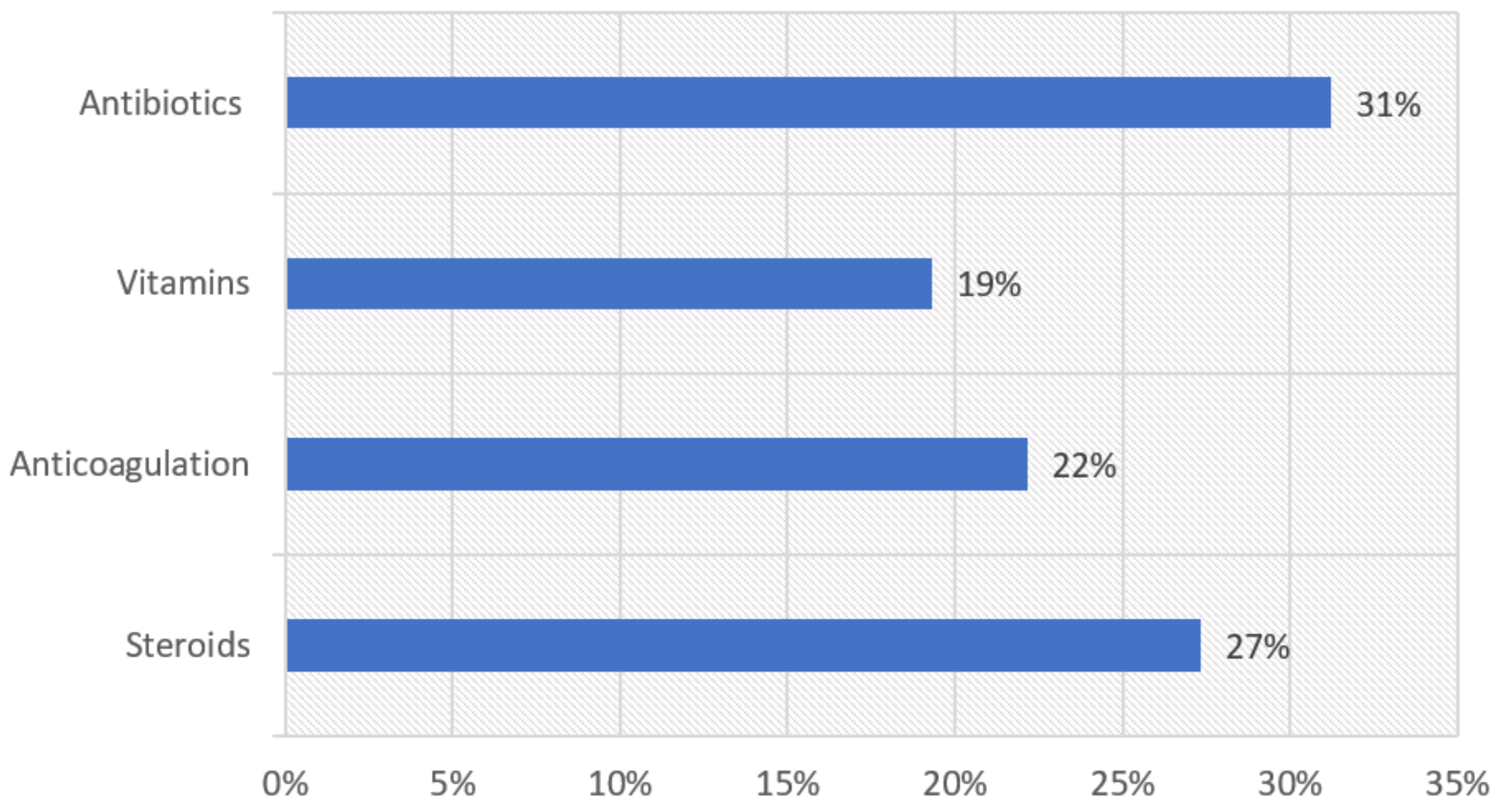
| Overall | Percentage | Capricorn | Mopani | Sekhukhune | Vhembe | Waterberg | |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| 20–29 | 10 | 1% | 9 (1.8%) | 2 (0.9%) | 0 (0%) | 2 (0.7%) | 0 (0%) |
| 30–39 | 62 | 6% | 17 (3.4%) | 14 (6.4%) | 6 (6.4%) | 22 (7.9%) | 3 (5.3%) |
| 40–49 | 101 | 9% | 46 (9.2%) | 23 (10.5%) | 8 (8.5%) | 24 (8.6%) | 5 (8.8%) |
| 50–59 | 172 | 15% | 65 (13%) | 31 (14.2%) | 12 (12.8%) | 55 (19.7%) | 11 (19.3%) |
| 60+ | 774 | 69% | 362 (72.5%) | 149 (68%) | 68 (72.3%) | 176 (63.1%) | 38 (66.7%) |
| Gender | |||||||
| Male | 544 | 47.3% | 226 (45.3%) | 102 (47%) | 52 (55.3%) | 133 (47.7%) | 30 (52.6%) |
| Female | 604 | 52.7% | 273 (54.7%) | 116 (53%) | 42 (44.7%) | 146 (52.3%) | 27 (47.4%) |
| Comorbid Conditions | |||||||
| HIV/AIDS | 141 | 19% | 60 (20.3%) | 31 (24.4%) | 15 (18.8%) | 27 (13.2%) | 8 (16.7%) |
| TB | 37 | 5% | 15 (6%) | 4 (3.9%) | 1 (1.3%) | 10 (5%) | 7 (14.6%) |
| COPD | 18 | 3% | 13 (5.3%) | 1 (1%) | 0 (0%) | 2 (1%) | 2 (4.2%) |
| Hypertension | 586 | 64% | 263 (69.9% | 127 (73%) | 57 (62%) | 111 (49.1%) | 28 (53.8%) |
| Diabetes Mellitus | 450 | 52% | 199 (56.9%) | 95 (62.5%) | 28 (33.3%) | 105 (46.9%) | 23 (42.6%) |
| Asthma | 35 | 5% | 17 (6.6%) | 5 (5.2%) | 2 (2.7%) | 8 (4.1%) | 3 (6.3%) |
| Obesity | 81 | 12% | 42 (15.3%) | 22 (20.4%) | 3 (4.1%) | 6 (3%) | 8 (16%) |
| Cancer | 23 | 4% | 9 (3.5%) | 5 (5.2%) | 4 (5.6%) | 4 (2%) | 1 (2.1%) |
| Respiratory distress | 919 | 91.5% | 432 (93.5%) | 164 (87.2%) | 76 (95%) | 199 (91.3%) | 48 (85.7%) |
| Clinical Presentations | |||||||
| Fever | 266 | 55% | 108 (41%) | 72 (27%) | 26 (10%) | 31 (12%) | 29 (11%) |
| Chills | 148 | 33% | 54 (36%) | 34 (23%) | 25 (17%) | 26 (18%) | 9 (6%) |
| Cough | 650 | 81% | 313 (48%) | 114 (18%) | 55 (8%) | 138 (21%) | 30 (5%) |
| Sore throat | 137 | 32% | 52 (38%) | 25 (18%) | 29 (21%) | 15 (11%) | 15 (11%) |
| Shortness of breath | 714 | 84% | 309 (43%) | 116 (16%) | 63 (9%) | 180 (25%) | 46 (6%) |
| Anosmia | 65 | 16% | 21 (32%) | 9 (14%) | 20 (31%) | 6 (9%) | 9 (14%) |
| Dysgeusia | 83 | 20% | 24 (29%) | 13 (16%) | 19 (23%) | 18 (22%) | 9 (11%) |
| Myalgia/body aches | 374 | 60% | 186 (50%) | 69 (18%) | 40 (11%) | 54 (14%) | 25 (7%) |
| Diarrhoea | 145 | 31% | 59 (41%) | 27 (19%) | 26 (18%) | 22 (15%) | 11 (8%) |
| Chest Pain | 55 | 8% | 28 (51%) | 6 (11%) | 10 (18%) | 10 (18%) | 1 (2%) |
| Loss of appetite | 65 | 9% | 32 (49%) | 10 (15%) | 9 (14%) | 10 (15%) | 4 (6%) |
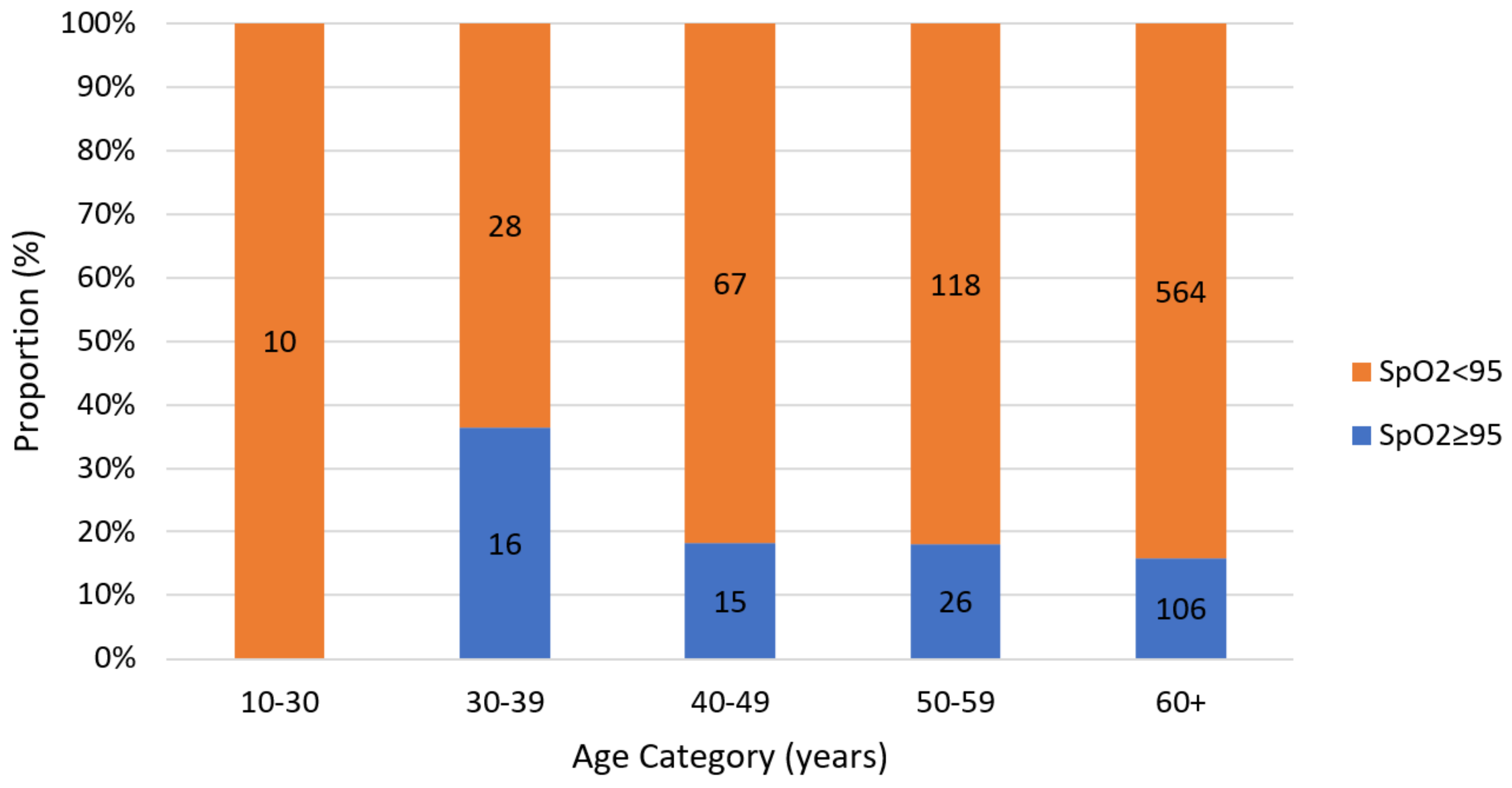
| SpO2 < 95 n(%) | SpO2 ≥ 95 n(%) | Chi-Square Test Value | p-Value | |
|---|---|---|---|---|
| Age | 14.488 | 0.006 * | ||
| 20–29 | 10 (1.3%) | 0 (0.0%) | ||
| 30–39 | 28 (3.6%) | 16 (9.8%) | ||
| 40–49 | 67 (8.5) | 15 (9.2%) | ||
| 50–59 | 118 (15.0%) | 26 (16.0%) | ||
| 60+ | 564 (71.7%) | 106 (65.0%) | ||
| Gender | 0.007 | 0.935 | ||
| Male | 369 (46.9%) | 86 (52.8%) | ||
| Female | 418 (53.1%) | 77 (47.2%) | ||
| Comorbid Conditions | ||||
| HIV/AIDS | 92 (17.5%) | 27 (26.5%) | 4.526 | 0.033 * |
| TB | 24 (5.1%) | 6 (6.7%) | 0.368 | 0.544 |
| COPD | 13 (2.8%) | 2 (2.3%) | 0.061 | 0.806 |
| Hypertension | 411 (64.2%) | 94 (68.6%) | 0.958 | 0.328 |
| Diabetes Mellitus | 317 (52.7%) | 71 (56.8%) | 0.714 | 0.398 |
| Asthma | 28 (5.9%) | 5 (5.7%) | 0.005 | 0.943 |
| Obesity | 62 (12.4%) | 11 (12.4%) | 0.001 | 0.986 |
| Cancer | 19 (4.0%) | 3 (3.6%) | 0.041 | 0.839 |
| Clinical Presentations | ||||
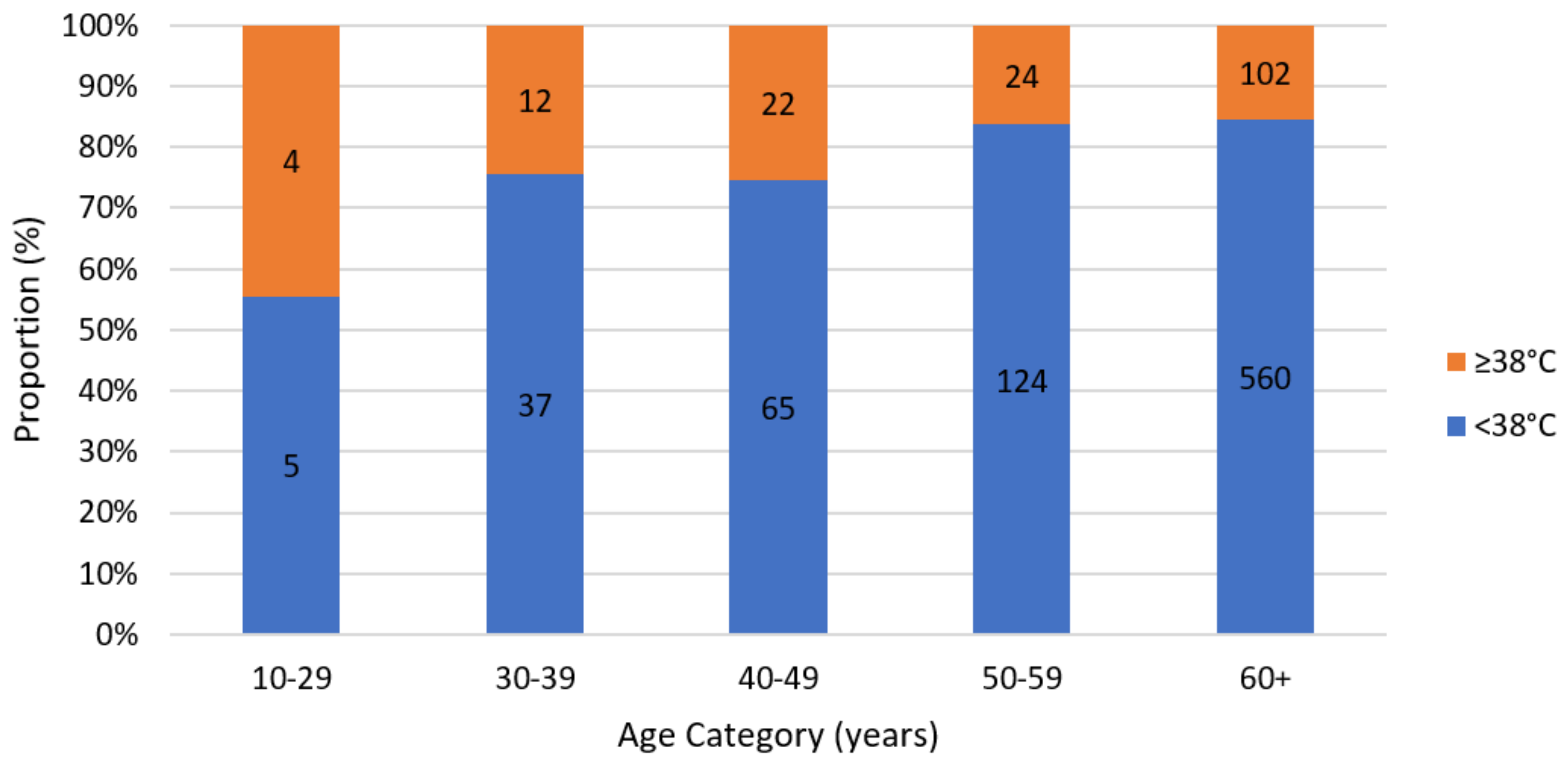
| Fever | 191 (55.7%) | 40 (64.5%) | 1.671 | 0.196 |
| Chills | 101 (32.7%) | 31 (50.0%) | 6.754 | 0.009 * |
| Cough | 491 (83.1%) | 86 (81.9%) | 0.087 | 0.768 |
| Sore throat | 88 (29.5%) | 31 (54.4%) | 13.265 | <0.001 * |
| Shortness of breath | 520 (84.1%) | 108 (87.1%) | 0.693 | 0.405 |
| Anosmia | 28 (10.2%) | 24 (43.6%) | 38.439 | <0.001 * |
| Dysgeusia | 46 (16.1%) | 24 (43.6%) | 21.465 | <0.001 * |
| Myalgia/body aches | 251 (59.1%) | 68 (72.3%) | 5.733 | 0.017 * |
| Diarrhoea | 88 (27.2%) | 34 (54.0%) | 17.559 | <0.001 * |
| Chest Pain | 47 (8.9%) | 6 (7.4%) | 0.206 | 0.650 |
| Loss of appetite | 46 (8.7%) | 9 (11.1%) | 0.492 | 0.483 |
| Frequency | Percentage | |
|---|---|---|
| Duration on oxygen | ||
| 24 h | 14 | 46% |
| 24–72 h | 145 | 19% |
| 3–7 days | 185 | 25% |
| 8–14 days | 61 | 8% |
| >14 days | 14 | 2% |
| Mechanical ventilation | 67 | 6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mphekgwana, P.M.; Sono-Setati, M.E.; Maluleke, A.F.; Matlala, S.F. Low Oxygen Saturation of COVID-19 in Patient Case Fatalities, Limpopo Province, South Africa. J. Respir. 2022, 2, 77-86. https://doi.org/10.3390/jor2020006
Mphekgwana PM, Sono-Setati ME, Maluleke AF, Matlala SF. Low Oxygen Saturation of COVID-19 in Patient Case Fatalities, Limpopo Province, South Africa. Journal of Respiration. 2022; 2(2):77-86. https://doi.org/10.3390/jor2020006
Chicago/Turabian StyleMphekgwana, Peter M., Musa E. Sono-Setati, Abdul F. Maluleke, and Sogo F. Matlala. 2022. "Low Oxygen Saturation of COVID-19 in Patient Case Fatalities, Limpopo Province, South Africa" Journal of Respiration 2, no. 2: 77-86. https://doi.org/10.3390/jor2020006
APA StyleMphekgwana, P. M., Sono-Setati, M. E., Maluleke, A. F., & Matlala, S. F. (2022). Low Oxygen Saturation of COVID-19 in Patient Case Fatalities, Limpopo Province, South Africa. Journal of Respiration, 2(2), 77-86. https://doi.org/10.3390/jor2020006






