What Can Be Learned about the Relationships between Water Discharge and Composition during Flood Events in a Forested Karstic Catchment from the Pyrenees Mountains (Southwestern France)? †
Abstract
:1. Introduction
2. Materials and Methods
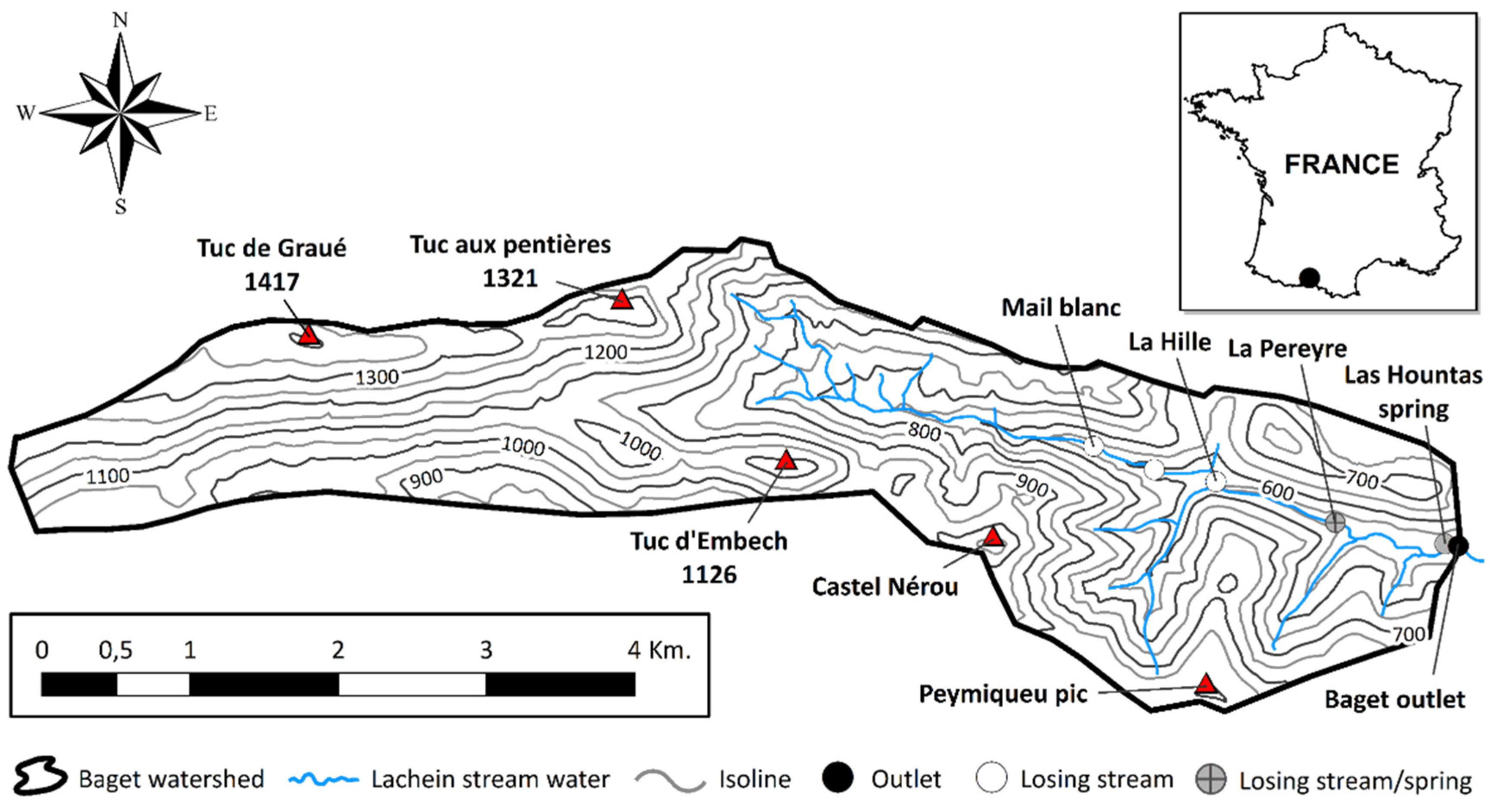
2.1. Study Area
2.2. Sampling and Analysis
3. Results and Discussion
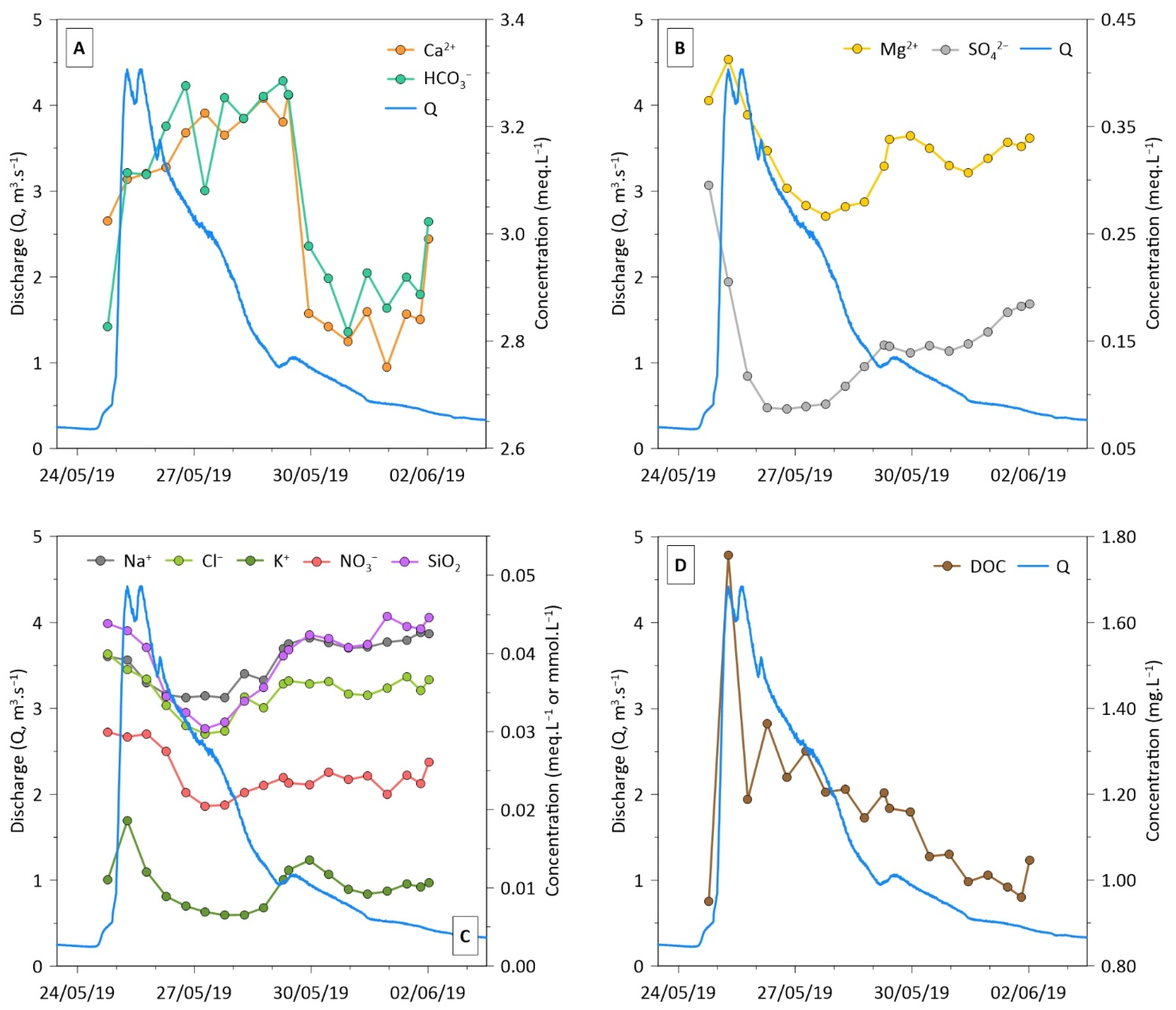
3.1. Hydrochemical Characteristics
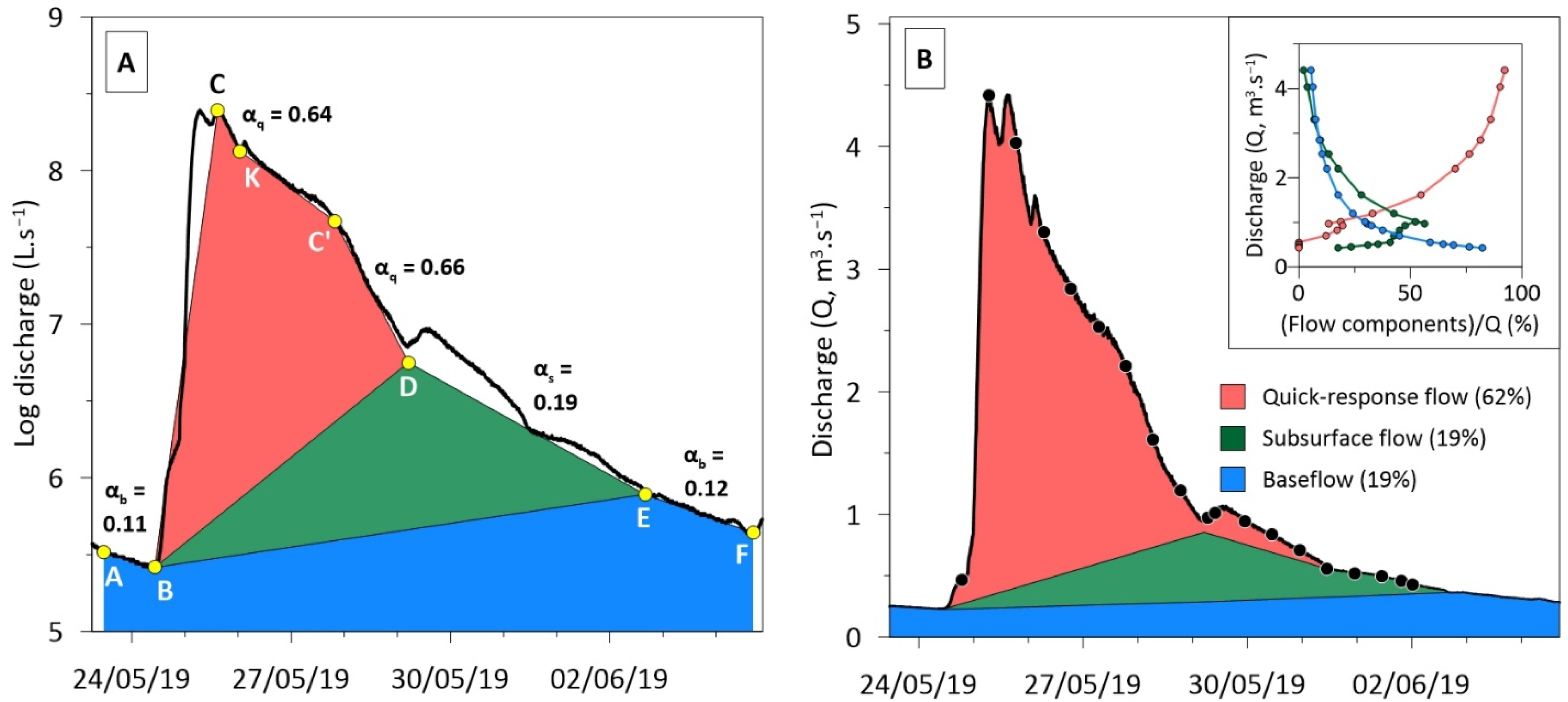
3.2. Separation of the Flood Flow Components
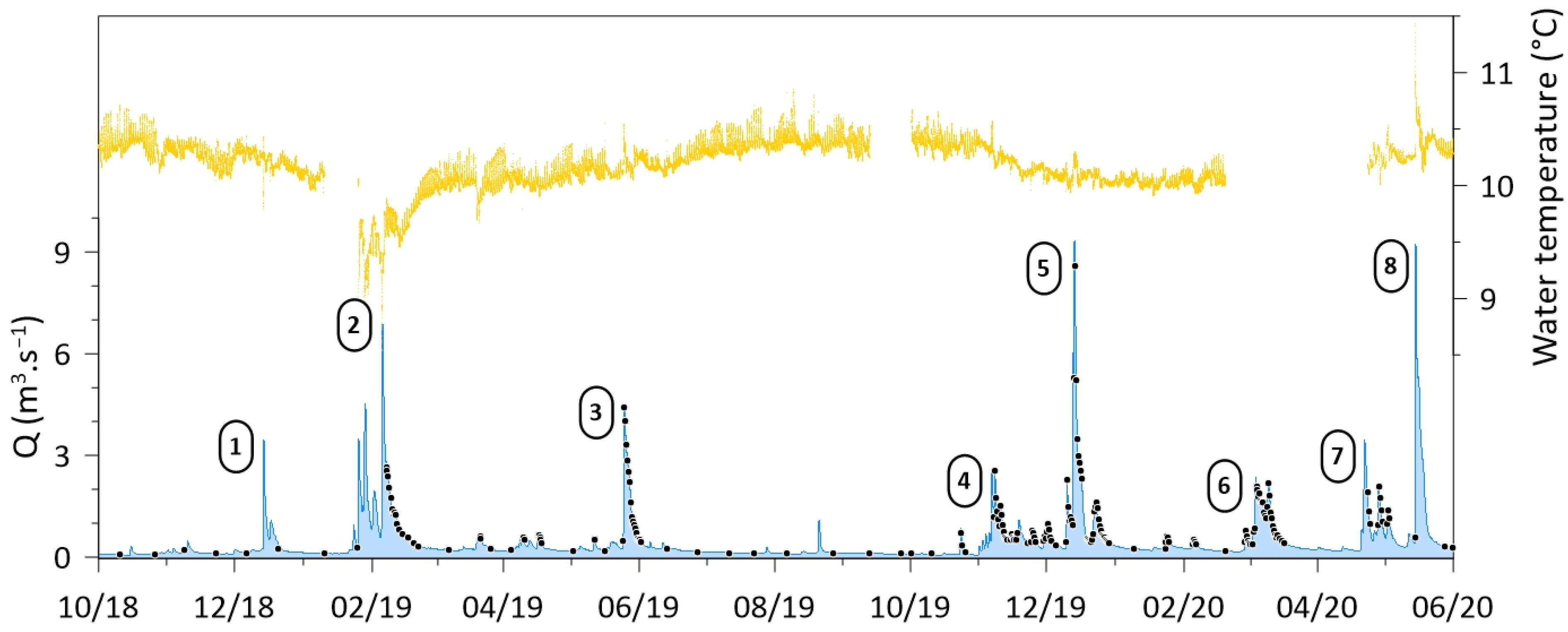
3.3. Temporal Variations and C-Q Relationships
3.4. Hysteresis Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CZ | Critical Zone |
| Q | Discharge |
| BC | Baget Catchment |
| T | Water Temperature |
| SC | Specific Conductivity |
| DOC | Dissolved Organic Carbon |
| CV | Coefficient of Variation |
| DCR | Discharge Change Rate |
| HI | Hysteresis Index |
| RL | Rising Limb |
| FL | Falling Limb |
References
- Bakalowicz, M. Epikarst. In Encyclopedia of Caves, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 284–288. [Google Scholar]
- Beaulieu, E.; Goddéris, Y.; Donnadieu, Y.; Labat, D.; Roelandt, C. High sensitivity of the continental-weathering carbon dioxide sink to future climate change. Nat. Clim. Change 2012, 2, 346–349. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupre, B.; Louvat, P.; Allegre, C.J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Amiotte-Suchet, P.; Probst, J.-L.; Ludwig, W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Z.; Goldscheider, N.; Frank, S.; Goeppert, N.; Kaufmann, G.; Zeng, C.; Zeng, Q.; Sun, H. Comparisons on the effects of temperature, runoff, and land-cover on carbonate weathering in different karst catchments: Insights into the future global carbon cycle. Hydrogeol. J. 2021, 29, 331–345. [Google Scholar] [CrossRef]
- Bakalowicz, M. Contribution de la Géochimie des Eaux a la Connaissance de L’aquifère Karstique et de la Karstification. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 1979. [Google Scholar]
- Huang, X.; Fang, N.F.; Zhu, T.X.; Wang, L.; Shi, Z.H.; Hua, L. Hydrological response of a large-scale mountainous watershed to rainstorm spatial patterns and reforestation in subtropical China. Sci. Total Environ. 2018, 645, 1083–1093. [Google Scholar] [CrossRef]
- Musolff, A.; Schmidt, C.; Selle, B.; Fleckenstein, J.H. Catchment controls on solute export. Adv. Water Resour. 2015, 86, 133–146. [Google Scholar] [CrossRef]
- Williams, G.P. Sediment concentration versus water discharge during single hydro- logic events in rivers. J. Hydrol. 1989, 111, 89–106. [Google Scholar] [CrossRef]
- Evans, C.; Davies, T.D. Causes of concentration/discharge hysteresis and its potential as a tool for analysis of episode hydrochemistry. Water Resour. Res. 1998, 34, 129–137. [Google Scholar] [CrossRef]
- Darwiche-Criado, N.; Comín, F.A.; Sorando, R.; Sánchez-Pérez, J.M. Seasonal variability of NO3− mobilization during flood events in a Mediterranean catchment: The influence of intensive agricultural irrigation. Agric. Ecosyst. Environ. 2015, 200, 208–218. [Google Scholar] [CrossRef]
- Baker, E.B.; Showers, W.J. Hysteresis analysis of nitrate dynamics in the Neuse River, NC. Sci. Total Environ. 2019, 652, 889–899. [Google Scholar] [CrossRef]
- Qin, C.; Li, S.-L.; Waldron, S.; Yue, F.-J.; Wang, Z.-J.; Zhong, J.; Ding, H.; Liu, C.-Q. High-frequency monitoring reveals how hydrochemistry and dissolved carbon respond to rainstorms at a karstic critical zone, Southwestern China. Sci. Total Environ. 2020, 714, 136833. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.A.; East, J.W. Cyclical relationships between river discharge and chemical concentrations during flood events. J. Hydrol. 1982, 57, 93–106. [Google Scholar] [CrossRef]
- Ulloa-Cedamanos, F.; Probst, J.-L.; Binet, S.; Camboulive, T.; Payre-Suc, V.; Pautot, C.; Bakalowicz, M.; Beranger, S.; Probst, A. A Forty-Year Karstic Critical Zone Survey (Baget Catchment, Pyrenees-France): Lithologic and Hydroclimatic Controls on Seasonal and Inter-Annual Variations of Stream Water Chemical Composition, pCO2, and Carbonate Equilibrium. Water 2020, 12, 1227. [Google Scholar] [CrossRef]
- Probst, J.-L. Hydrologie du Bassin de la Garonne. Modèle de Mélanges. Bilan de L’érosion. Exportation des Phosphates et des Nitrates. Ph.D. Thesis, Toulouse III, Toulouse, France, 1983. [Google Scholar]
- Probst, J.-L. Nitrogen and phosphorous exportation in the Garonne basin (France). Hydrology 1985, 76, 281–305. [Google Scholar] [CrossRef]
- Probst, J.-L. Dissolved and suspended matter transported by the Girou River (France): Mechanical and chemical erosion rates in a calcareous molasse basin. Hydrol. Sci. J. 1986, 31, 61–79. [Google Scholar]
- Maillet, E. Essai D’hydraulique Souterraine et Fluviale; Libraire Sci. A. Herman: Paris, France, 1905. [Google Scholar]
- Dewandel, B.; Lachassagne, P.; Bakalowicz, M.; Weng, P.; Al-Malki, A. Evaluation of aquifer thickness by analysing recession hydrographs. Application to the Oman ophiolite hard-rock aquifer. J. Hydrol. 2003, 274, 248–269. [Google Scholar] [CrossRef]
- Godesy, S.E.; Kirchner, J.W.; Clow, D.W. Concentration–discharge relationships reflect chemostatic characteristics of US catchments. Hydrol. Process. 2009, 23, 1844–1864. [Google Scholar] [CrossRef]
- Clow, D.W.; Mast, M.A. Mechanisms for chemostatic behavior in catchments: Implications for CO2 consumption by mineral weathering. Chem. Geol. 2010, 269, 40–51. [Google Scholar] [CrossRef]
- Monnin, C.; Tamborski, J.; Bejannin, S.; Souhaut, M.; Rogues, M.; Olivier, P.; van Beek, P. Freshening of a Coastal Karst Aquifer Revealed by the Temporal Changes in a Spring Water Composition (La Palme, Southern France). Hydrology 2019, 6, 17. [Google Scholar] [CrossRef]
- El Azzi, D.; Probst, J.L.; Teisserenc, R.; Merlina, G.; Baque, D.; Julien, F.; Guiresse, M. Trace element and pesticide dynamics during a flood event in the save agriculture watershed: Soil-river transfer pathways and controlling factors. Water Air Soil Pollut. 2016, 227, 227–442. [Google Scholar] [CrossRef]
- Lloyd, C.E.; Freer, J.E.; Johnes, P.J.; Collins, A.L. Using hysteresis analysis of high- resolution water quality monitoring data, including uncertainty, to infer controls on nutrient and sediment transfer in catchments. Sci. Total Environ. 2016, 543, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.E.M.; Freer, J.E.; Johnes, P.J.; Collins, A.L. Technical note: Testing an improved index for analysing storm discharge-concentration hysteresis. Hydrol. Earth Syst. Sci. 2016, 20, 625–632. [Google Scholar] [CrossRef]
- Boy, J.; Valarezo, C.; Wilcke, W. Water flow paths in soil control element exports in an Andean tropical montane forest. Eur. J. Soil Sci. 2008, 59, 1209–1227. [Google Scholar] [CrossRef]
- Ulloa-Cedamanos, F.; Probst, A.; Dos-Santos, V.; Camboulive, T.; Granouillac, F.; Probst, J.-L. Stream Hydrochemical Response to Flood Events in a Multi-Lithological Karstic Catchment from the Pyrenees Mountains (SW France). Water 2021, 13, 1818. [Google Scholar] [CrossRef]
- Ulloa-Cedamanos, F.; Probst, A.; Moussa, I.; Probst, J.-L. Chemical weathering and CO2 consumption in a multi-lithological karstic critical zone: Long term hydrochemical trends and isotopic survey. Chem. Geol. 2021, 585, 120567. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa-Cedamanos, F.; Probst, A.; Dos-Santos, V.; Probst, J.-L. What Can Be Learned about the Relationships between Water Discharge and Composition during Flood Events in a Forested Karstic Catchment from the Pyrenees Mountains (Southwestern France)? Environ. Sci. Proc. 2021, 7, 9. https://doi.org/10.3390/ECWS-5-08058
Ulloa-Cedamanos F, Probst A, Dos-Santos V, Probst J-L. What Can Be Learned about the Relationships between Water Discharge and Composition during Flood Events in a Forested Karstic Catchment from the Pyrenees Mountains (Southwestern France)? Environmental Sciences Proceedings. 2021; 7(1):9. https://doi.org/10.3390/ECWS-5-08058
Chicago/Turabian StyleUlloa-Cedamanos, Francesco, Anne Probst, Vanessa Dos-Santos, and Jean-Luc Probst. 2021. "What Can Be Learned about the Relationships between Water Discharge and Composition during Flood Events in a Forested Karstic Catchment from the Pyrenees Mountains (Southwestern France)?" Environmental Sciences Proceedings 7, no. 1: 9. https://doi.org/10.3390/ECWS-5-08058
APA StyleUlloa-Cedamanos, F., Probst, A., Dos-Santos, V., & Probst, J.-L. (2021). What Can Be Learned about the Relationships between Water Discharge and Composition during Flood Events in a Forested Karstic Catchment from the Pyrenees Mountains (Southwestern France)? Environmental Sciences Proceedings, 7(1), 9. https://doi.org/10.3390/ECWS-5-08058








