1. Introduction
The question of the sources of ore matter and the mechanism of its concentration in the magmatic process is the most important in geology. One of the possible ways is the accumulation of ore components during the differentiation of melts at the stage of their liquation separation and the appearance of fluid, or salt melts. This idea was developed in Russia at the end of the XX century by A. A. Marakushev, N. I. Bezmen, E. N. Gramenitsky and others. Experimental studies have established that in granite systems, the main storage of some rare and rare earth elements (REE) in the process of silicate-salt immiscibility is a salt-based alkaline aluminofluoride melt. It was shown that all rare-earth elements are predominantly distributed in the salt melt with partition coefficients much greater than 1.
Previously, experiments were conducted in the sodium nepheline and quartz-normative areas of the compositions of the granite system [
1]. It was shown that the addition of fluorine leads to the equilibrium of the aluminosilicate melt with the fluoride phases, namely, cryolite, topaz and williomite. The aluminofluoride melt, which occupied a narrow region of alumina-rich nepheline-normative melts, was also in equilibrium with them. The saturation of the aluminosilicate melt by the salt aluminofluoride is achieved at a fluorine concentration of 3 wt.%. When potassium was added to the system, the equilibrium phases were silicate melt, K-Na modification of cryolite and feldspar.
The addition of lithium to the system dramatically affected the phase relations [
1,
2]. The field of stability of the salt aluminofluoride melt has expanded into the quartz-normative plumasite and agpaite regions of the composition of the silicate melt. It is shown that silicate-salt liquid immiscibility can be formed in fluorine-rich granite systems. The addition of potassium led to a greater variety of the resulting phases and brought the composition of the system closer to real rare-metal lithium-fluoride granites [
3].
This work is carried out in the field of stability of three non-crystalline phases: aluminosilicate, aluminofluoride melt and water fluid (Fl), at a water concentration of more than 10 wt.% and lithium content of 1.5 wt.%.
The aim of the work was to study the crystallization of a lithium-containing granite melt with high water and fluorine contents at temperatures of 800, 700, 600, 550, 500 and 400 °C and a pressure of 1 kbar.
The main task of the work was to conduct a series of experiments at different temperatures and pressures of 1 kbar and to investigate the change in the phase relations in a lithium-containing model granite system.
2. Materials and Methods
Six series of experiments were conducted at temperatures from 800 to 400 °C and 1 kbar. To study the model system, as the initial composition of the aluminosilicate melt, we used a composition close to the Qtz-Ab-Ort granite eutectic at a fluorine concentration of 1 wt% and a water pressure of 1 kbar [
4]. Salt components were introduced into the system in an amount of 50 wt.% of the charge weight, the composition of which corresponded to the stoichiometry (Li, K, Na) of cryolite. The concentration of Li in the system was 1.5 wt.%. The following reagents were used as the initial compositions of the experiments are: dried gel SiO
2, LiF, K
2SiF
6, NaF, AlF
3, Al
2SiO
5, Al
2O
3 and distilled water. In the composition of the charge for the experiments, set at 800 and 700 °C and a pressure of 1 kbar, REE was introduced, in the form of oxides, 0.5 wt.% of the element. For experiments performed at T = 600, 550, 500, and 400 °C and P = 1 kbar, REE oxides were introduced into the system in certain pairs so that the REE peaks did not overlap on the microanalyzer are: (1) Y
2O
3, La
2O
3; (2) Sm
2O
3, Gd
2O
3, Tb
2O
3; (3) CeO
2, Eu
2O
3 and Ho
2O
3; (4) Dy
2O
3; (5) Pr
2O
3, Lu
2O
3, Sc
2O
3; (6) Er
2O
3, Yb
2O
3; (7) Nd
2O
3, Tm
2O
3; (8) Sc
2O
3, Gd
2O
3 in quantities of 2 wt.% of the element by weight of the hitch. Distilled water was added to each ampoule by 5-15, in a number of experiments up to 20–50 wt.% of the weight of the charge.
The experiments were carried out on a high gas pressure unit with internal heating (“gas bomb”) at the Institute of Experimental Mineralogy of the Russian Academy of Sciences in Chernogolovka (IEM RAS). The accuracy of the temperature control and adjustment was ±5 °C; the pressure was ±50 bar. For each ampoule, weight control was performed before and after the experiment. The experiment was considered successful if the mass discrepancy was no more than 0.001 g. Lower temperature experiments were first performed at a temperature of 800 °C and maintained for 3 days, and then the temperature of the experiment was lowered to 600–400 °C at 1 kbar and maintained for another 3–4 days. The quenching rate at the plant was 150–200 °C per minute.
The experimental products were studied using a Jeol JSM-6480LV scanning electron microscope (Japan) with an energy-dispersive INCA Energy-350 and a crystal-diffraction INCA Wave-500 (Oxford Instrument Ltd., Oxford, UK) spectrometer in the Laboratory of Local Methods of Substance Research of the Department of Petrology and Volcanology of Lomonosov Moscow State University. The accelerating voltage was 20 keV at a current of 0.7 nA. The main and rare-earth elements in silicate glasses were studied using the Superprobe JXA-8230 electron probe microanalyzer (Japan). To prevent glass destruction, the analyses were carried out in the defocused beam mode (up to 10 microns) at an accelerating voltage of 10 kV and a current strength of 10 nA. The REE analysis was performed at an accelerating voltage of 20 kV and a current of 30 nA. The concentrations of REE, Y, Sc, and Li for experiments at 800 and 700 °C were determined by inductively coupled plasma mass spectrometry on the ICP MS device with double focus Element-2 in the Laboratory of Experimental Geochemistry of the Department of Geochemistry of the Faculty of Geology of Lomonosov Moscow State University. Due to the small amount of the analyzed substance, the concentrations were recalculated for the content of aluminum in the phases, determined in advance by microprobe analysis. The samples were also examined by inductively coupled plasma mass spectrometry and laser ablation at the Analytical Certification Testing Center of the Institute of Microelectronics Technology and High-Purity Materials of the Russian Academy of Sciences (ASIC IPTM RAS). The measurements were carried out on a quadrupole mass spectrometer with inductively coupled plasma X Series II (Thermo Scientific, Waltham, MA, USA) with an UP266 MACRO laser ablation attachment (New Wave Research, Fremont, CA, USA) with certain parameters: mass spectrometer-RF generator output power-1200 W, plasma flow rate Ar—13 L/min, auxiliary flow-0.90 L/min, carrier gas flow He—0.6 L/min, followed by mixing with Ar—0.6 L/min, resolution 0.4 and 0.8 M. The prefix for laser ablation is a laser wavelength of 266 nm, pulse repetition rate of 10 Hz, pulse energy of 3 MJ, pulse duration of 4 ns, crater diameter of 60 microns. The time of one measurement was 10 s.
3. Results
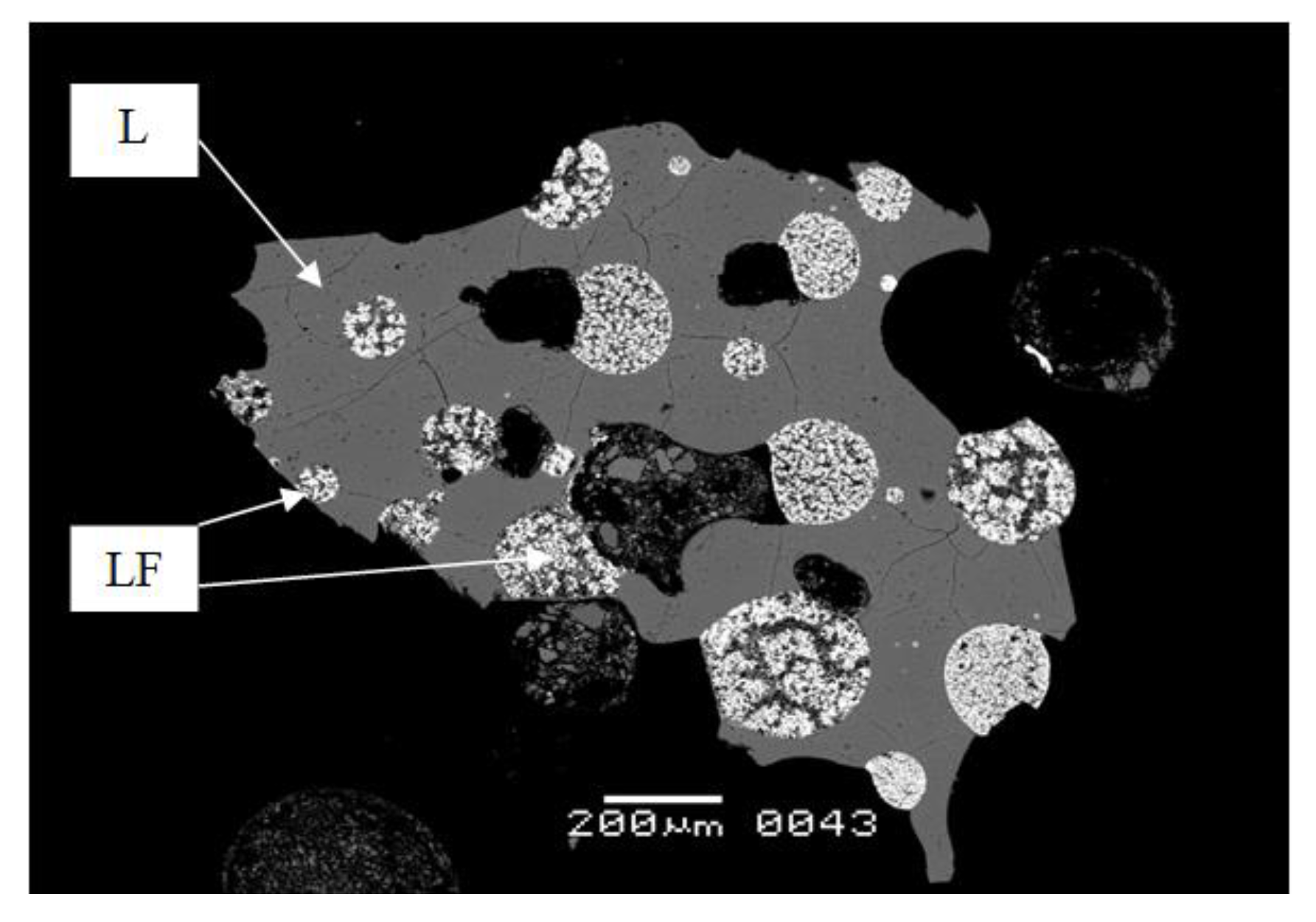
As a result of the experiments conducted in the temperature range from 800 to 400 °C and a pressure of 1 kbar, the equilibrium phases were aluminosilicate (L) and aluminofluoride (LF) melts, as well as an aqueous fluid at a water concentration in the system greater than 10 wt.% (
Figure 1).
Legend to
Figure 1: L—aluminosilicate melt, LF—aluminofluoride melt, pores—loss of water by the melt during quenching of the samples.
In experiments at a temperature of 800 °C and a pressure of 1 and 2 kbar, the aluminosilicate melt is represented by a gray tempered glass in BSE, in which small (<10 microns) quenching phases of light gray color are evenly distributed. Rounded globules (gray in BSE) of a size from 50 to >200 microns are formed from the aluminofluoride melt, inside which, during quenching, alkaline-aluminofluoride phases crystallize, stoichiometrically close to cryolithionite and cryolite (Crl). In addition to Crl, crystals of rare earth element fluorides (LnF
3) and lithium (LiF) are also formed in the salt melt. Sometimes the salt melt captured the silicate melt, which indicates their joint existence in the experimental conditions. Pores were found in both melts, which indicates that the melt lost water during the quenching of the samples. When the temperature decreases by 100 °C, large crystals of Na-K cryolite > 200 microns in diameter begin to form in the salt melt, but the melt itself is still in the liquid state, saturated with REE, Sc, Y and Li. Rare-earth element fluorides (white colors in BSE) are concentrated at the edges of the globules. At this temperature, different types of globules that were found are, firstly, in the above, the salt globule observed polymineral aggregate, in the center of which is cryolite, it traces the assembly phases tempered salt melt and the edges crystallize fluoride REE (
Figure 2); secondly, globules mosaic structure, inside of which are unevenly distributed fluoride REE, Sc, Y, Li, thirdly, uniformly crystallized globles in which Crl and (Ln,Sc,Y)F
3 (
Figure 3).
Legend to
Figure 2: L—aluminosilicate melt, LF—aluminofluoride melt, Crl—cryolite ((Na,K)
3AlF
6), REE—rare earth fluorides.
Legend to
Figure 3: L—aluminosilicate melt, LF—aluminofluoride melt.
At a temperature of 600 °C and a pressure of 1 kbar, the crystallization processes continue. Quartz and Li-mica crystallize from the aluminosilicate melt and Li-K-Na cryolite forms polygonal crystals (in cross-section up to 50 microns) from the salt melt. At the same time, there is still a melt in the salt globules, which, when quenched, turns into an aggregate of small crystals of Li fluorides (grisite LiF, polylithionite) and REE, Y, and Sc (
Figure 4 and
Figure 5). Grisite forms elongated or round teardrop-shaped crystals of black color in BSE, more or less evenly distributed in the salt melt. Elongated quartz crystals are formed from the aluminosilicate melt, as well as whitish gray crystals in BSE, a fluorine-containing alkaline aluminosilicate, presumably polylithionite—Pol (
Figure 5).
Legend to
Figure 4: L—aluminosilicate melt, LF—aluminofluoride melt, Crl—cryolite ((Na,K)
3AlF
6), Qtz—quartz.
Legend to
Figure 5: L—aluminosilicate melt, LF—aluminofluoride melt, Crl—cryolite ((Na,K)
3AlF
6), LiF—graysite, Pol—polylithionite.
At 550 and 500 °C, the phase relations observed at 600 °C are preserved, but the amount of salt melt, which is a concentrate of fluorine, lithium and REE, is significantly reduced. In the aluminofluoride melt, Crl crystallizes in the form of two varieties—practically sodium and essentially potassium. The cryolite crystals in the central part of the globules are larger in size than at the edges. In the center, the Crl size reaches > 150 microns, and at the edges—no more than 50 microns (
Figure 6). At the boundary of the silicate and salt phases, prismatic crystals continue to crystallize, similar in stoichiometry to polylithionite (
Figure 6 and
Figure 7). The thickness of the Li-mica border between the globules and the silicate glass reaches 200 microns. Large quartz crystals, often of a rounded shape crystallize in the silicate melt, which can be considered analogs of pea-shaped quartz in rare-metal granites. Na- and Na-K cryolites crystallize inside the quartz grains (
Figure 7), which indicates their joint crystallization from the aluminosilicate melt. Such structures are similar to those observed in cryolite-containing granites, which indicates the igneous nature of cryolite in granites.
Legend to
Figure 6: LF—aluminofluoride melt, Crl—cryolite ((Na,K)
3AlF
6), Pol—polylithionite.
Despite the relatively low temperature (500 °C), the temperature of these experiments in comparison with the experiments conducted at T = 800 °C, complete crystallization in the system does not occur. It still contains a portion of the uncrystallized, supercooled, apparently metastable silicate melt. At the same time, the residual salt melt in the globules is probably a stable phase.
Legend to
Figure 7: L—aluminosilicate melt, Crl—cryolite ((Na,K)
3AlF
6), Qtz—quartz.
At 400 °C (
Figure 8), the aluminosilicate melt is preserved, which is in a metastable (supercooled) state in the form of glass. The salt melt completely crystallized. The salt phase is present in the form of crystal formations of a bizarre shape with a length of up to 100 microns, composed essentially of sodium cryolite in the center of these secretions (dark gray colors in BSE) and sodium-potassium cryolite on the periphery (light gray colors in BSE). K-Na Crl also crystallizes in the form of individual isometric, rounded grains, and Na-K Crl often forms crystals with a clear edges. Most REE, Sc, and Y fluorides form separate crystalline phases in the form of (Ln,Sc,Y)F
3, often confined to the marginal sites of alkali aluminofluoride releases.
Legend to
Figure 8: L—aluminosilicate melt, Crl—cryolite ((Na,K)
3AlF
6), pores—loss of water by the melt during quenching of the samples.
4. Discussion
In the experiments, new data were obtained on the phase relations in the quartz-normative, agpaite part of the Si-Al-Na-K-Li-F-O-H model granite system in the presence of potassium and lithium. In the process of lowering the temperature, three non-crystalline phases remained in equilibrium in the system are L, LF, and Fl. The main concentrator of REE, as well as lithium, scandium and yttrium, is the aluminofluoride melt in the entire range of studied temperatures, pressures and water contents. At temperatures below 600 °C, quartz and a new phase, corresponding to the stoichiometry of lithium mica(polylithionite), crystallize from aluminosilicate glass. At 550–500 °C, separately crystallizing Na- and Na-K-cryolite are added to them. Na- and Na-K-cryolites crystallize from the salt aluminofluoride melt, and with them in equilibrium, up to a temperature of 500 °C, there is a residual salt melt enriched in Li and REE. At 400 °C, the salt melt completely crystallizes, while the silicate melt still remains as a metastable, supercooled melt.