Investigating the Effect of Reaction Temperature on the Extraction of Calcium from Ironmaking Slag: A Kinetics Study †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Characterisation Techniques
2.3.1. Atomic Absorption Spectroscopy (AAS)
2.3.2. X-ray Diffractometer (XRD)
2.3.3. Energy-Dispersive X-ray Spectrometry (EDX)
3. Results
4. Discussion
4.1. The Effect of Reaction Temperature on the Extraction Calcium from Ironmaking Slag
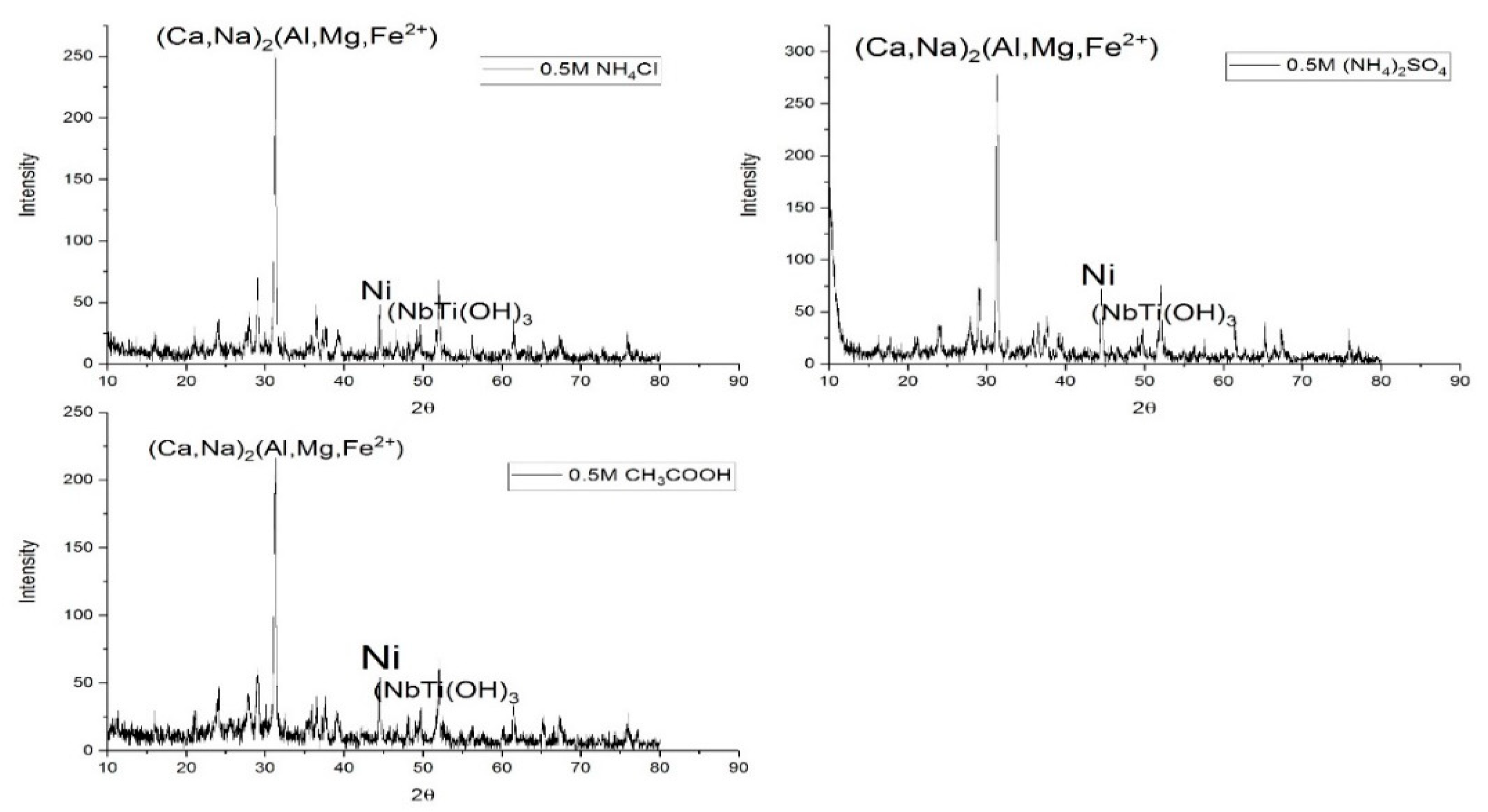
4.2. XRD Analyses of Blast Furnace Ironmaking Slag Leaching Residues
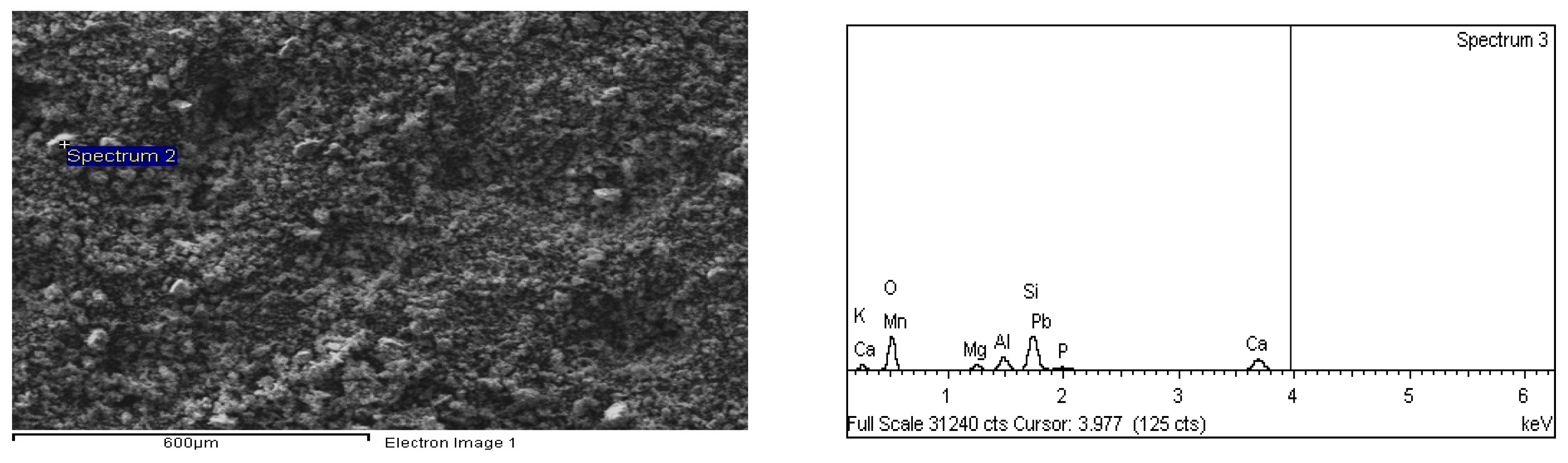
4.3. EDX Analyses of Blast Furnace Ironmaking Slag Leaching Residues
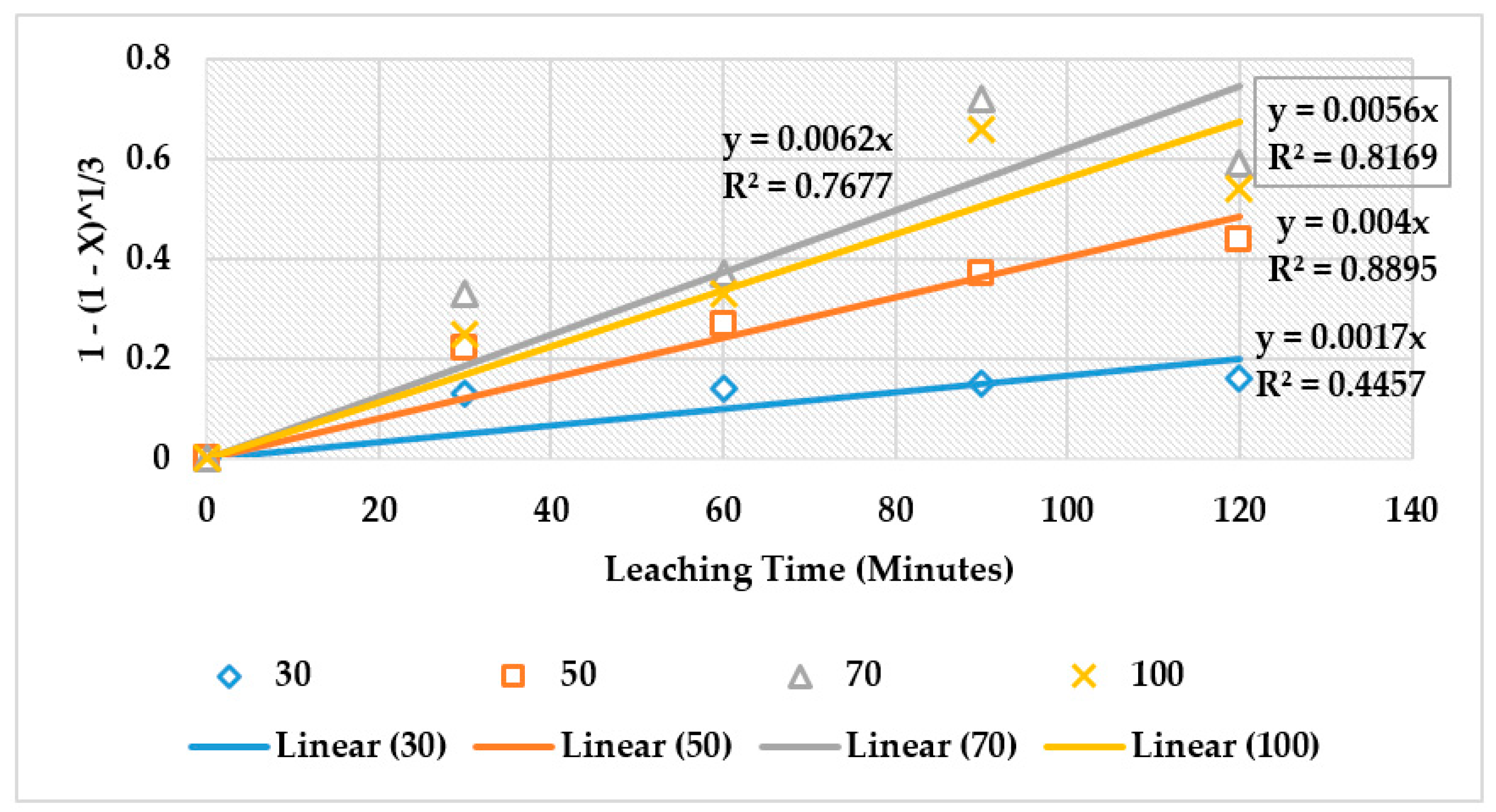
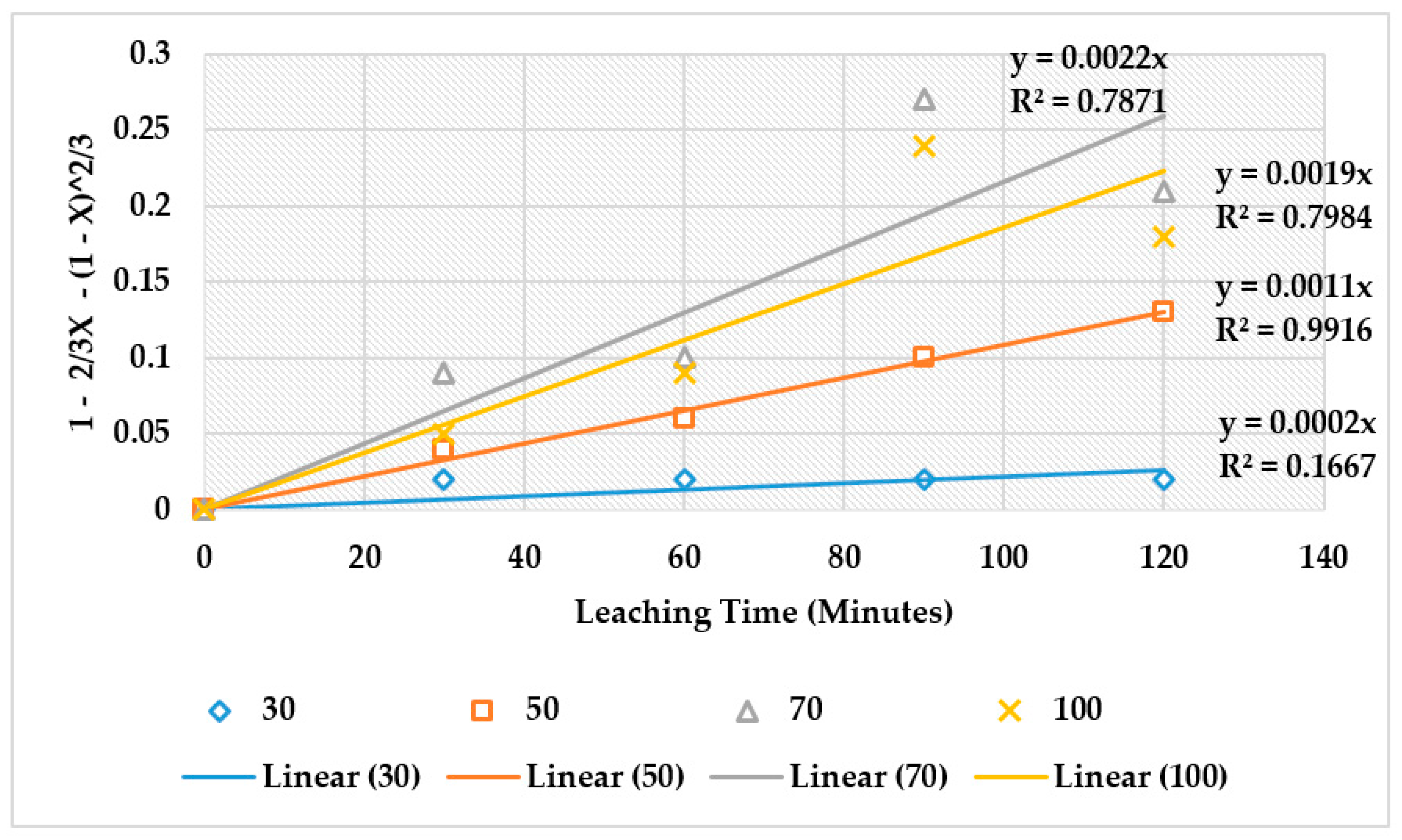
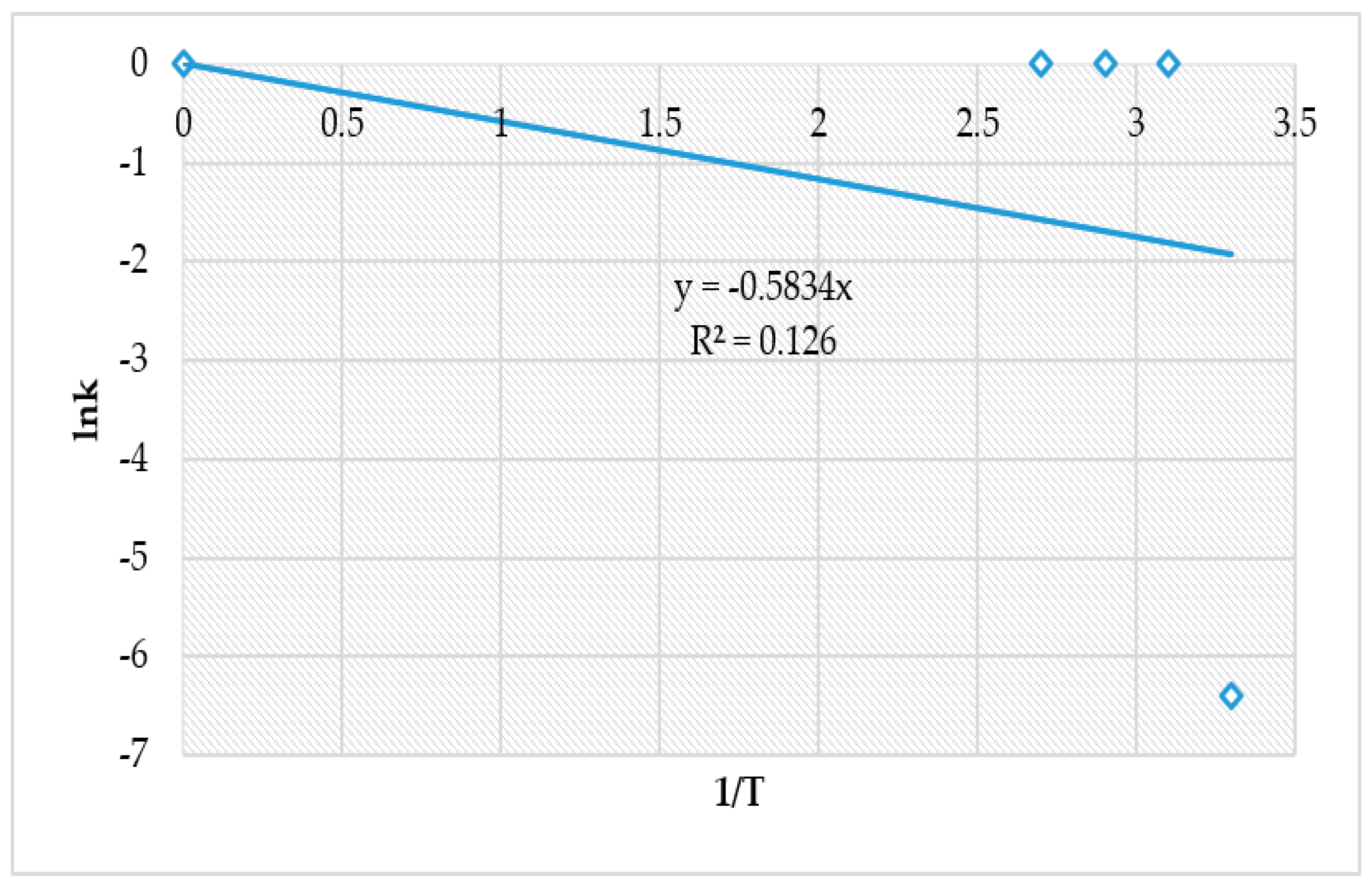
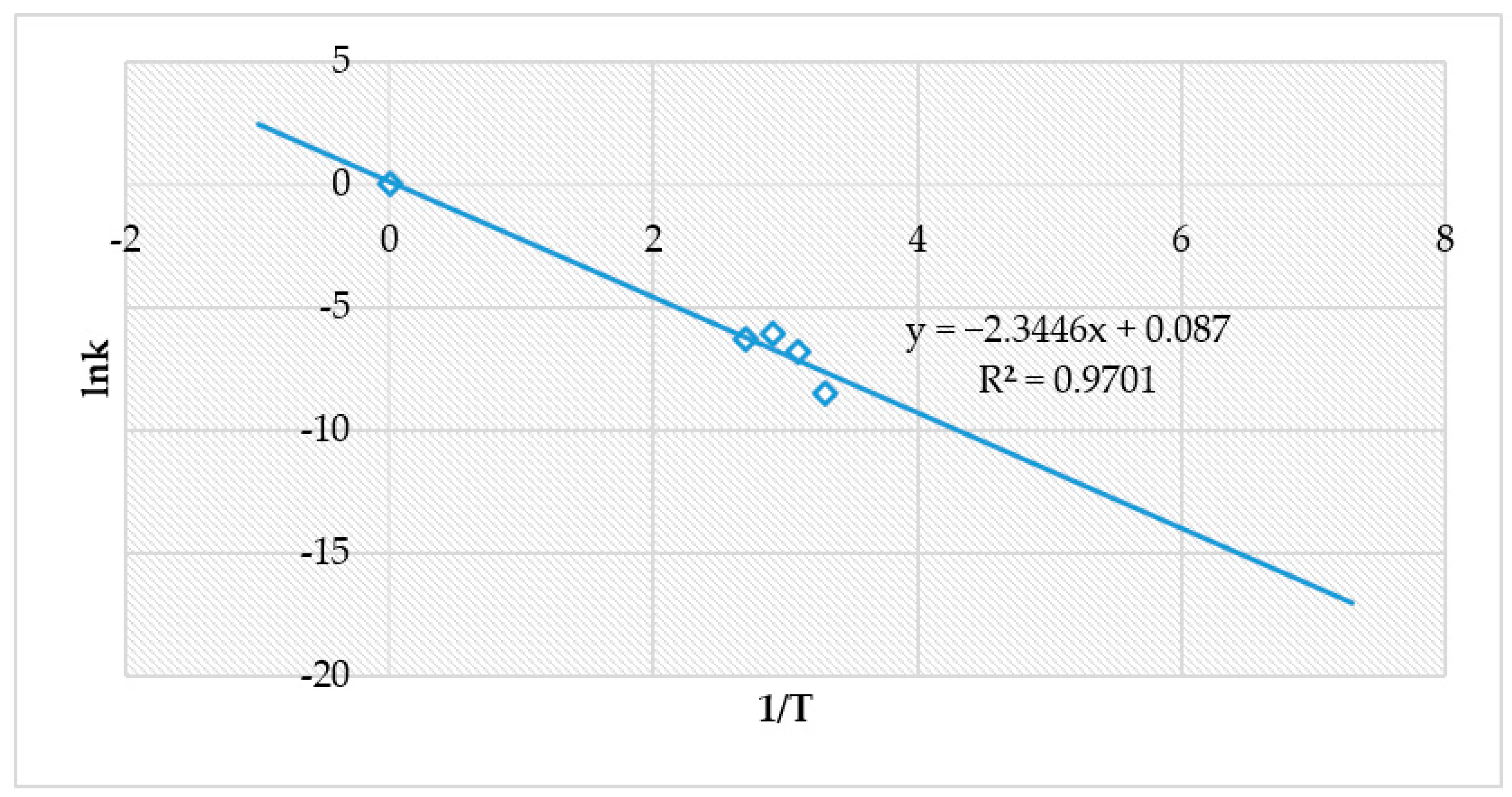
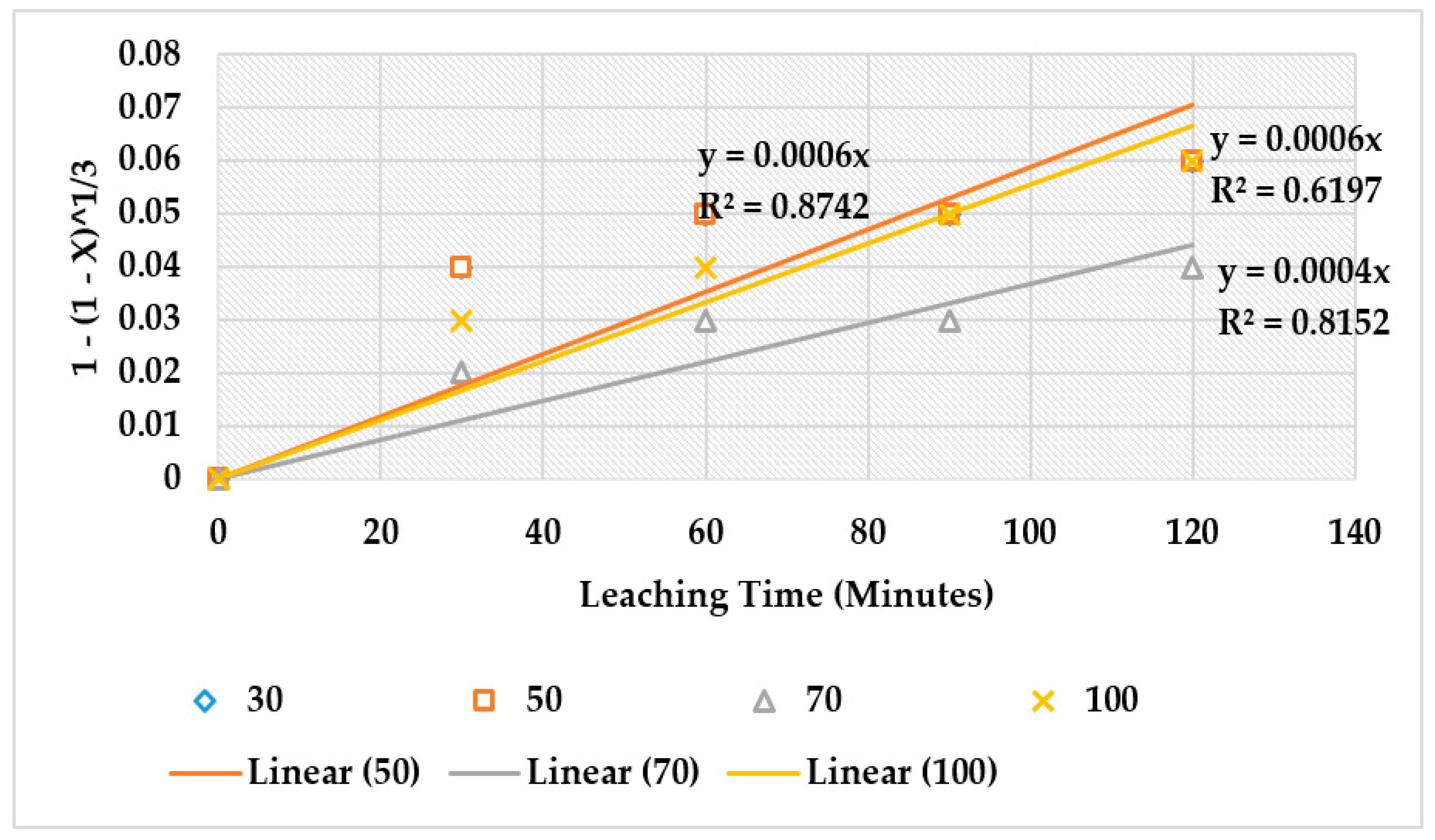
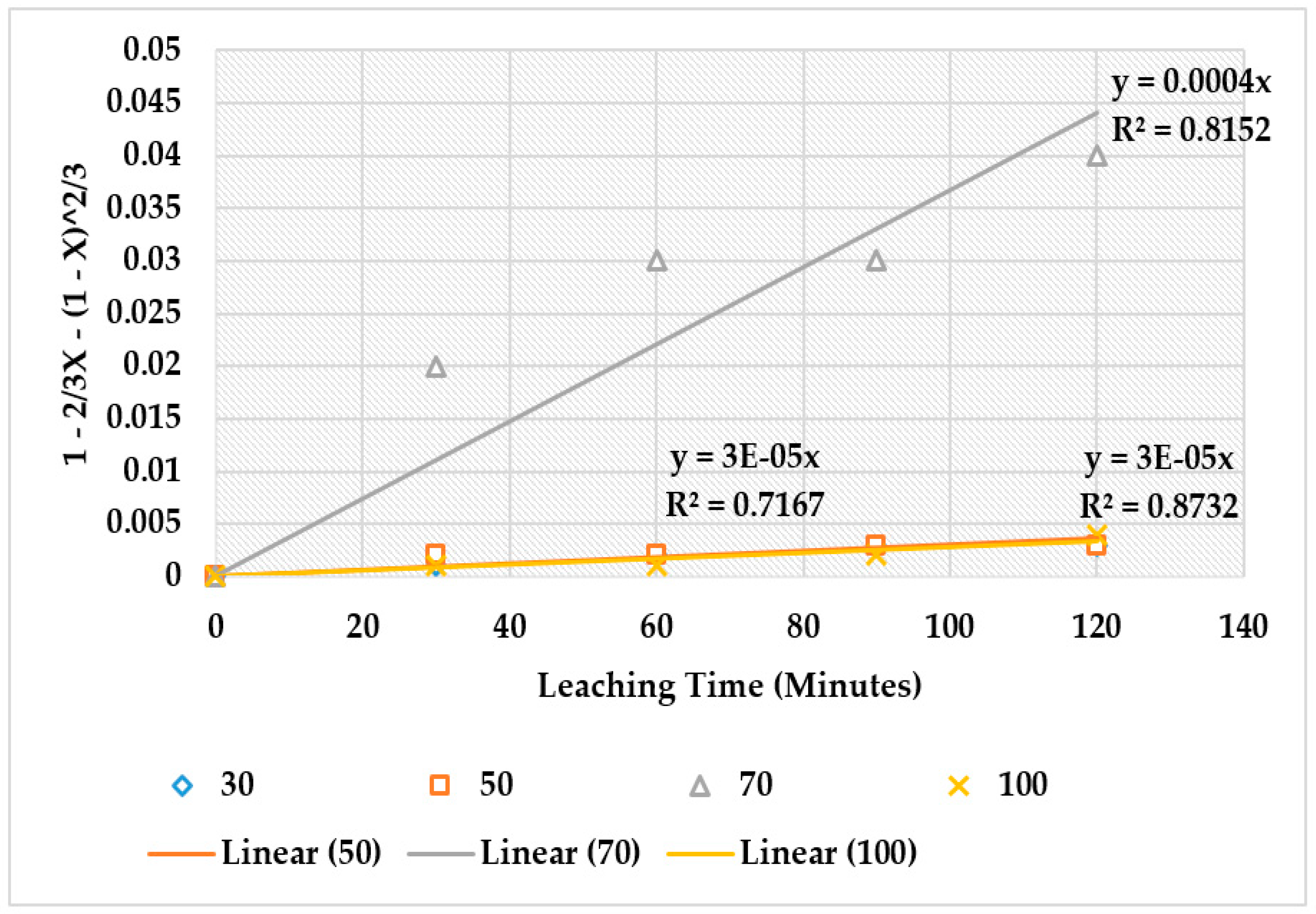
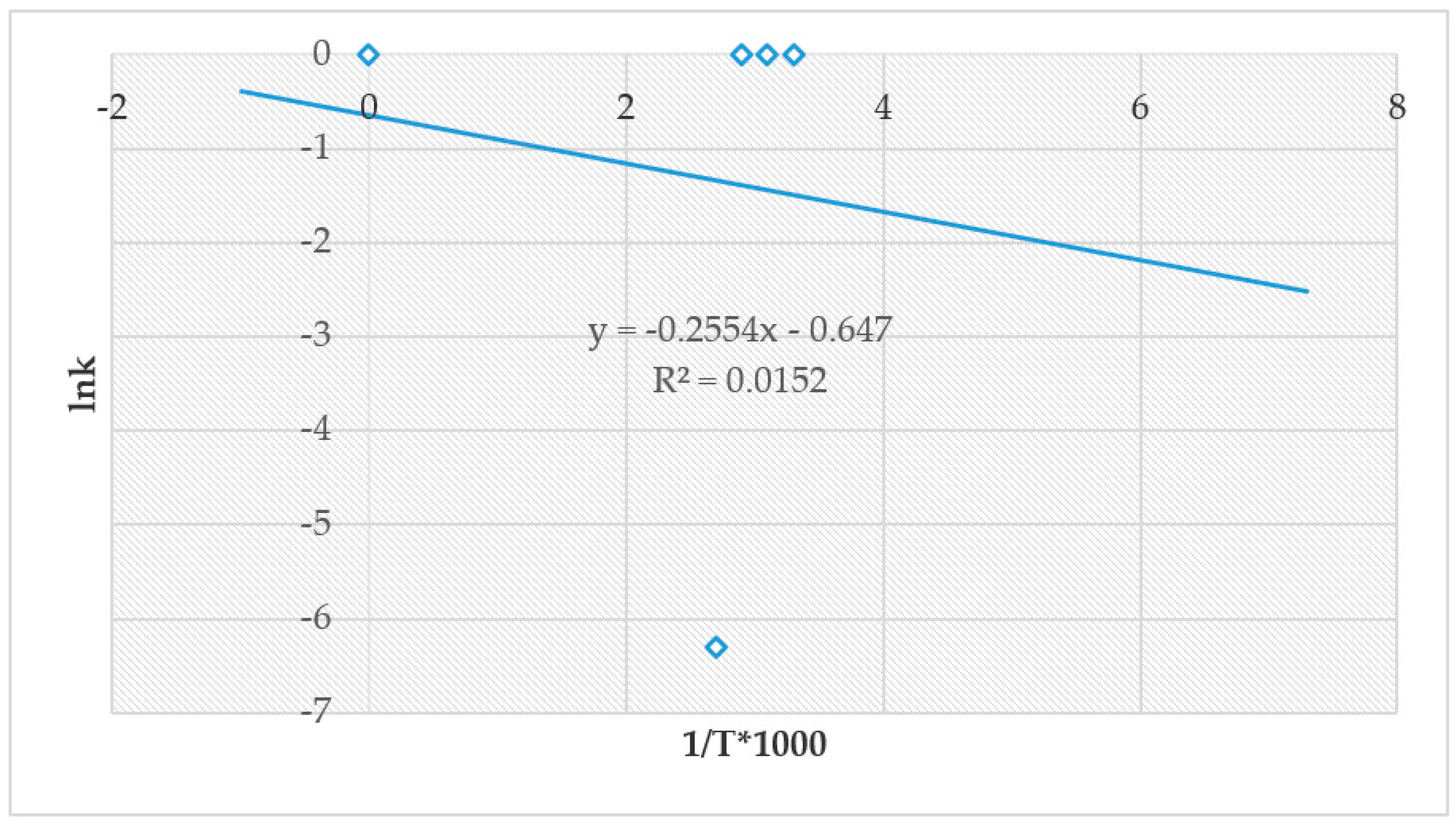
4.4. The Effect of Reaction Rate-Controlling Mechanisms on the Leaching of Calcium from Ironmaking Slag
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Cloete, M. Atlas on Geological Storage of CO2 in South Africa; Council for Geosciences: Pretoria, South Africa, 2010. [Google Scholar]
- Robie, R.A.; Hemingway, B.S.; Fischer, J.R. Thermodynamic Properties of Minerals and Related Substances at 298.15K and 1 bar (105 Pa) Pressure and at Higher Temperatures; Bulletin 1452; U.S. Geological Survey: Reston, VA, USA, 1978. [Google Scholar]
- Fehling, K.A.; Proctor, D.M.; Shay, E.C.; Wittenborn, J.L.; Green, J.J.; Avent, C.; Bigham, R.D.; Connolly, M.; Lee, B.; Shepker, T.O.; et al. Physical and chemical characteristics of blast furnace, basic oxygen furnace and electric arc furnace steel industry slags. Environ. Sci. Technol. 2000, 34, 1576–1582. [Google Scholar]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals, and Equilibria; Jones and Bartlett Publishers: Boston, UK; London, UK, 1990. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Othusitse, N.; Muzenda, E. Predictive models of leaching processes: A critical review. In Proceedings of the 7th International Conference on Latest Trends in Engineering and Technology, Himeji, Japan, 5–7 November 2012; pp. 136–141. [Google Scholar]
- Dickinson, C.F.; Heal, G.R. Solid-liquid diffusion-controlled rate equations. Thermochim. Acta 1999, 340–341, 89–103. [Google Scholar] [CrossRef]
- Radingoana, P.M. Leaching of Low-Grade Laterite ore from Waterval Mine in Mpumalanga through Electrochemical Process. Magister. Technologiae Dissertation, Tshwane University of Technology, Pretoria, South Africa, 2014. [Google Scholar]
- Thubakgale, C.K. Leaching and Recovery of Nickel and Cobalt from the Waterval Boven Laterite Ore. Magister. Technologiae Dissertation, Tshwane University of Technology, Pretoria, South Africa, 2013. [Google Scholar]
- Habashi, F. Kinetics of Metallurgical Process; Metallurgie Extractive Quebec: Quebec City, QC, Canada, 1999. [Google Scholar]
- Anand, S.; Das, S.C.; Das, R.P.; Jena, P.K. Leaching of manganese nodules at elevated temperature and pressure in the presence of oxygen. Hydrometallurgy 1988, 20, 155–167. [Google Scholar] [CrossRef]
- Romankiw, L.T.; De Bruyn, P.L. Kinetics of dissolution of zinc sulphide in aqueous sulphuric acid. In Unit Processes in Hydrometallurgy; Wadsworth, M., Davis, F.T., Eds.; Massachusetts Institute of Technology: Dallas, TX, USA, 1962. [Google Scholar]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Kotoane, M. Development and Optimisation of Selective Leaching Processes for the Extraction for Calcium from Steel Slag in View of Sequestering Carbon Dioxide. Magister. Technologiae Dissertation, Vaal University of Technology, Vanderbijlpark, South Africa, 2013. [Google Scholar]
- Yogo, K.; Teng, Y.; Yashima, T.; Yamada, K. Development of a new CO2 fixation/utilisation process (1): Recovery of calcium from steelmaking slag and chemical fixation of carbon dioxide by carbonation reaction. Greenhouse Gas Control Technol. 2005, 7, 2427–2430. [Google Scholar]
- Witkamp, J.J.; Huijgen, G.J.; Comans, R.N.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar]
- Hall, C.; Large, D.J.; Adderley, B.; West, H.M. School of Chemical and Environmental Engineering; University of Nottingham: Nottinham, UK, 2014. [Google Scholar]
- Mattilla, H.-P.; Grigaliunaite, I.; Zevenhoven, R. Chemical kinetics modelling and process parameter sensitivity for precipitated calcium carbonate production from steelmaking slags. In Thermal and Flow Engineering Laboratory; Abo Akademi University: Turku, Finland, 2012. [Google Scholar]
- Eloneva, S.; Salminen, J.; Fogelholm, C.; Zevenhoven, R. Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar]
- Bilen, M.; Altiner, M.; Yildirim, M. Evaluation of steelmaking slag for CO2 fixation by leaching-carbonation process. Part. Sci. Technol. 2017, 36, 368–377. [Google Scholar] [CrossRef]
- Stange, W. The process design of gold leaching and carbon-in-pulp circuits. J. S. Afr. Inst. Min. Metall. 1999, 99, 13–25. [Google Scholar]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless-steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef]
- Didyk-Mucha, A.; Pawlowska, A.; Sadowski, Z. Application of the Shrinking Core Model for Dissolution of Serpentine in an Acid Solution; Department of Chemical Engineering, Wroclaw University of Technology: Wybrzeze, Poland, 2016. [Google Scholar]
- Sun, Y.; Yao, M.-S.; Zhang, J.-P.; Yang, G. Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem. Eng. J. 2011, 173, 437–445. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohitlhetse, I.; Thubakgale, K.; Mendonidis, P.; Manono, M. Investigating the Effect of Reaction Temperature on the Extraction of Calcium from Ironmaking Slag: A Kinetics Study. Environ. Sci. Proc. 2021, 6, 31. https://doi.org/10.3390/iecms2021-09366
Kohitlhetse I, Thubakgale K, Mendonidis P, Manono M. Investigating the Effect of Reaction Temperature on the Extraction of Calcium from Ironmaking Slag: A Kinetics Study. Environmental Sciences Proceedings. 2021; 6(1):31. https://doi.org/10.3390/iecms2021-09366
Chicago/Turabian StyleKohitlhetse, Itumeleng, Kentse Thubakgale, Peter Mendonidis, and Malibongwe Manono. 2021. "Investigating the Effect of Reaction Temperature on the Extraction of Calcium from Ironmaking Slag: A Kinetics Study" Environmental Sciences Proceedings 6, no. 1: 31. https://doi.org/10.3390/iecms2021-09366
APA StyleKohitlhetse, I., Thubakgale, K., Mendonidis, P., & Manono, M. (2021). Investigating the Effect of Reaction Temperature on the Extraction of Calcium from Ironmaking Slag: A Kinetics Study. Environmental Sciences Proceedings, 6(1), 31. https://doi.org/10.3390/iecms2021-09366






