Abstract
The article describes slurry and drainage water’s characteristics and shows the conditions of their formation in the technogenic system of the Komsomolsky Tin-ore District, Russian Far East. The investigation was conducted using environmental monitoring, and the physico-chemical modeling method. In a wide ambient temperature range (from minus 25 to 45 °C) the Eh-pH parameters of micropore solutions, which form technogenic (anthropogenic) waters at various host-rock—sulfide ratios (95:5, 50:50, 5:95), were determined. Depends on the primary ores and host rocks composition ionic and molecular composition of technogenic waters, as well as the association of crystallized hypergene minerals were established. The negative impact of slurry and drainage water on the hydrosphere and the health of the region’s population is shown. Following environmental monitoring, the content of dissolved metals exceeds background concentrations in slurry and drainage waters from hundreds to hundreds of thousand times. Modeling reveals, that from saturated technogenic waters, Fe, Cu, Zn, Pb, Al, Ca, Mg, K, and Na oxides and hydroxides, sulfates, carbonates, arsenates, phosphates, and silicate minerals are precipitates. The tendency of double growth for 24 types of digestive, respiratory, and nervous system diseases during a 20-years period has been noted, moreover, children morbidity rate exceeds that of adults.
1. Introduction
Komsomolsky Tin-ore District is located at the East of Khabarovsk krai, Russian Far East, left bank of the Amur River. The mining industry has been developing here for over 70 years and three types of ore mineralization have been revealed: tin, copper-copper, and tin-polymetallic. As a result of mining operations, three tailing dumps were formed in the district: Solnechnoye concentration mill (SCM), Central concentration mill (CCM), and Third. The fine mass of grey tailings consists of (in %): vein quartz—37.5, tourmaline—12.1, corneal and sedimentary rocks—45, and sulfides (chalcopyrite, pyrite, pyrrhotite, arsenopyrite, galena, sphalerite, chalcocite, covellite, and bornite)—3.8.
Sulfides in the enrichment tailings are in the crushed state, which leads to the increased access of weathering agents (water, oxygen, etc.) and activation of hypergenic processes. As a result of hypergenesis, sludge and drainage waters are formed, and the content of both sulfide ore elements and the host rocks there are higher than the background characteristics of natural waters of the district [1,2,3].
Environmental monitoring has been shown that the content of the considered chemical elements exceeds natural background conditions (times): in slurry waters Zn—from 253 to 385,000, Fe—1.2–24,253, Cu—8–26,230, Pb—630–1703, Al—2.1–915, Ca—1830–44,766, Mg—1542–100,285; in drainage waters Zn—470–38,200, Fe—41–921, Cu—416–768, Pb—2–1470, Al—3–253, Ca—17,066–78,133, Mg—6442–60,557. Therefore, if such waters get into hydrosphere, their dilution in thousands and hundreds of thousands of times is necessary, which is not always possible.
The purpose of this work was to study the conditions for the formation of sludge and drainage water at the tailings dumps of a considerable district in a wide temperature range from −25 to 45 °C using the physico-chemical modeling software complex Selektor, as well as to show their negative impact on the hydrosphere and the health of people living in the area. To achieve this goal, we wanted to: (1) determine the Eh-pH parameters of formation for technogenic waters microporous solutions in a wide temperature range from −25 to 45 °C, (2) show the possibility of technogenic minerals crystallization from solutions and identify the association or paragenesis of these minerals, (3) determine the ionic and molecular composition of sulfide ore elements in mine waters, (4) determine the content of sulfide ore elements in the simulated solutions, (5) show the negative impact of hypergenic processes on the hydrosphere and health of people living in these areas.
2. Materials and Methods
The computer physico-chemical modeling method in Selector software complex was used. The program is based on the convex programming mathematical approach, which allows an equilibrium to be established in heterogeneous systems by minimizing thermodynamic potentials (Gibbs energy) [4]. The formation of slurry and technogenic waters of tailings dumps was modeled as a result of sulfides ores and host rock oxidation with ratios 5:95, 50:50, 95:5. In total, 36 variants of physico-chemical models and 540 individual systems were analyzed.
Depending on the composition of primary ores and host rocks [5], the ionic and molecular composition of anthropogenic waters is determined, as well as the association of crystallizing hypergenic minerals.
3. Results and Discussion
Simulated microporous solutions that form slurry and drainage water have a wide range of Eh-pH parameters: from 0.76 to 1.15 V and 1.3–8.0. Modeling allowed us to reveal the hypergenic minerals that precipitated from saturated solutions in a wide ambient temperature range (Figure 1 and Figure 2). The following technogenic minerals are precipitate:
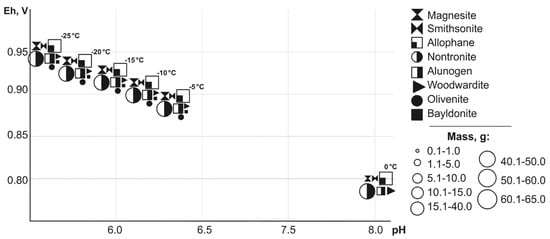
Figure 1.
Dependence of mineral masses on Eh-pH parameters (T = -25 to 0 °C).
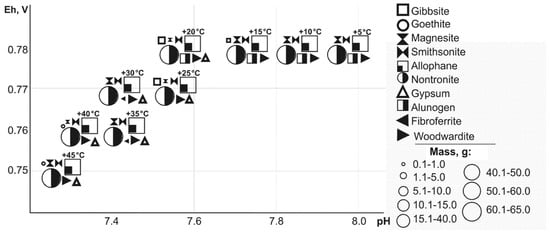
Figure 2.
Dependence of mineral masses on Eh-pH parameters (T = 5 to 45 °C).
- oxides and hydroxides: Fe and Al—goethite, gibbsite;
- sulfates Cu—chalcanthite, ktenacite, antlerite, Cu and Al—woodwardite, Pb—anglesite. Pb and Fe—plumbojarosite, Fe—fibroferrite, K and Fe—jarosite, Ca—gypsum, Mg—starkeyite, Al—alunogen;
- carbonates: Zn—smithsonite, Fe—siderite, Mg—magnesite;
- arsenates: Cu—Cu and Pb—bayldonite, clinoclase, Pb—mimetite, Cu and Pb—duftite, bayldonite, Fe—scorodite, pittitcite;
- silicates: Na and Fe—nontronite, Al—allophane.
For all the considered minerals the temperature range of formation, as well as associations are estimated. The masses of minerals are varied from hundredths to 150 g.
The solutions obtained as a result of modeling contain all the elements of sulfide ores and host rocks: Cu, Zn, Pb, Fe, As, S, Al, Ca, Mg, K, and Na. Their concentration in the ion and molecular form reaches tens of grams, and under cryogenic conditions, it is one order of magnitude and two orders of magnitude higher due to the reduction of solutions volume during ice crystallization.
The total composition of ions and molecules in the simulated solutions is presented by:
- sulfide ore elements: Cu+, Cu2+, CuO, CuOH+, CuHCO3+, CuCO3, Cu(CO3)22−, CuSO4, HCuO2−, Pb2+, PbO, PbOH+, PbCO3, Pb(CO3)22−, PbHCO3+, PbSO4, Pb(SO4)2−, HPbO2−, Zn2+, ZnO, ZnO22−, ZnOH+, ZnCO3, Zn(CO3)22−, ZnHCO3+, ZnSO4, Zn(SO4) 22−, HZnO2−, HFeO2, As3+, As5+, AsO43−, H2AsO4−, HAsO42−, H3AsO4, SO42− HSO4−;
- host rocks elements: Al3+, AlO+, AlO2−, Al(OH)2+, HAlO2, Ca2+, CaOH+, Ca(HCO3)+, CaCO3, CaSO4, CaHSiO3+, K+, KOH, KSO4−, KHSO4, Na+, NaOH, NaAsO42−, NaHSiO3, NaSO4−, Mg2+, Mg(HCO3)+, MgCO3, MgHSiO3+, HSiO3−, SiO2.
The comparison of modeling results (case of the Third tailings dump oxidation at 25 °C based on tailings drilling composition) was verified by the hydrochemical analysis results of the considered tailings slurry waters sampled at this temperature. Thus, we can compare the contents of Cu, Fe, Pb, and Zn in modeling solutions of this tailings dump both with their natural background concentrations in rivers and maximum permissible concentrations for fishery needs and for drinking consumption. It shows the excess of background and maximum permissible concentrations (in times): As, fishery needs—18, drinking consumption—89, background—1481; Cu, fishery needs—6470, drinking consumption–6,5, background—12,940, Fe, fishery needs—118, drinking consumption—39, background—787; Pb, fishery needs–31, drinking consumption– 19, background—1850; Zn, fishery needs–528, drinking consumption–5, background—17,600.
The obtained data show that solutions of the Third tailing dump must be diluted tens, hundreds, and even thousands of times to reach background and maximum permissible concentrations. It should also be noted that sludge and drainage waters of this tailings dump are discharged into the Levaya Silinka River (drinking water intake of the Gorny settlement), then it flows into the Amur River, and enters the Sea of Okhotsk.
It is well known that both lack and excess of vital elements lead to numerous diseases of people living in mining areas [6,7]. The toxic effect of the elements on humans depends on their chemical nature, concentrations, and composition of ions and compounds, as well as individual features of the organism [8], so it was important to establish forms of migration for elements.
An analysis of morbidity in the population carried out by the author in the Komsomolsky district from 1991 to 2001 showed the following results. The most common diseases include digestive organs, which in that period were sick up to 20% of children and adults; respiratory organs—up to 70% of children, and 20% of adults; and nervous system—up to 17% of children, and 10 % of adults. During the period under review, there was a trend of doubling of almost all types of diseases, both in adults and children, and the morbidity of the child population for almost all 24 analyzed diseases is much higher than for adults. It should be noted that during this period, ore extraction and processing decreased significantly, and the population of the district decreased by 18% [9,10].
4. Conclusions
We have found that minerals play a major role in the mobility and spreading of inorganic contaminants into the environment, including surface and groundwater, as they participate in the processes associated with the change of primary phases (hypogenic) and in the formation of secondary phases (hypergenic and technogenic minerals).
The relationship between minerals and pollutants in the form of ions and molecules from sludge and drainage waters is an important issue of ecological mineralogy and geochemistry of natural waters. The main objective of such type studies is the development of models capable to link the obtained data with macroscopic observations at tailings dumps, but the latter is not always possible due to the fine dispersion of the tailings and the laborious selection of anthropogenic minerals.
Highly concentrated solutions, which get into the surface and ground waters before and after mineral deposition 24 h a day and all the year-round for decades, contaminate them. Over time, this may lead to a worsening of the background water characteristics of Komsomolsk Tin Ore District. To understand these processes, the results of the analysis of hydrochemical samples of slurry and drainage water were demonstrated, as well as their characteristics were revealed using physico-chemical modeling. The conducted researches help to show the negative impact of hypergenic and technogenic processes, as well as of discharging technogenic waters on the river network. The consumption of such water by the population, as it was noted, leads to high morbidity in the mining areas of the Russian Far East.
Using modern methods of analysis and mathematical modeling it is possible to estimate the elemental composition of waters, trace their chemical forms, and consider the transformation of elements depending on natural environmental physical conditions of this process (such as temperature). Therefore, the application of modeling makes it possible to assess the temporal evolution of the water system. The obtained results can be useful for monitoring measures and reclamation activities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
References
- Zvereva, V.P.; Krupskaya, L.T. Anthropogenic waters in the Komsomolsk, Kavalerovskii, and Dalnegorsk mining areas of the Far East and their impact on the hydrosphere. Russ. J. Gen. Chem. 2012, 82, 2244–2252. [Google Scholar] [CrossRef]
- Zvereva, V.P.; Krupskaya, L.T.; Salyukova, E.N. Estimation of effect of technogenic discharges on hydrosphere in Dalnegorsk district of the Far East. Appl. Mech. Mater. Trans. Tech. Publ. 2013, 825–832. [Google Scholar] [CrossRef]
- Zvereva, V.P. Impact of technogenic wastewaters of kavalerovskii and dalnegorskii mining districts on the hydrosphere of primorsky krai. Russ. J. Gen. Chem. 2019, 89, 2808–2817. [Google Scholar] [CrossRef]
- Karpov, I.K.; Chudnenko, K.V.; Bychinskii, V.A.; Kulik, D.A.; Avchenko, O.V. Minimization of Gibbs free energy in geochemical systems by convex programming. Geochem. Int. 2001, 39, 1108–1119. [Google Scholar]
- Geology, Mineralogy and Geochemistry of Komsomol’skiy District; Nauka: Moscow, Russia, 1971; 335p. (In Russian)
- Cheremisinoff, N.P. Handbook of Industrial Toxicology and Hazardous Materials; CRC Press: Boca Raton, FL, USA, 1999; 925p. [Google Scholar]
- Elder, J.F. Metal Biogeochemistry in Surface-Water Systems Review of Principles and Concepts; US Geological Survey Circular 1013; United States Government Printing Office: Denver, CO, USA, 1988; 55p. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Megorskiy, V.V.; Gashkina, N.A.; Kudryavtseva, L.P. Metals in surface water of Ukraine: The migration forms, features of distribution between the abiotic components of aquatic ecosystems, and potential bioavailability. Ecotoxicological approach to water quality assessment. Water Resour. 2010, 37, 199–208. [Google Scholar] [CrossRef]
- Zvereva, V.P. Ecological aspect of the tin deposit technogenic systems in the Far East. Mineral. Tehnog. 2005, 6, 265–274. (in Russian). [Google Scholar]
- Zvereva, V.P. Environmental Consequences of Hypergene Processes on Tin Ore Deposits of the Far East; Dal’nauka: Vladivostok, Russia, 2008; 165p. (In Russian) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).