Occurrence and Activity of Roe Deer in Urban Forests of Warsaw †
Abstract
:1. Introduction
2. Experimental Section
3. Results
3.1. Occurrence of Roe Deer in Different Habitats of Warsaw
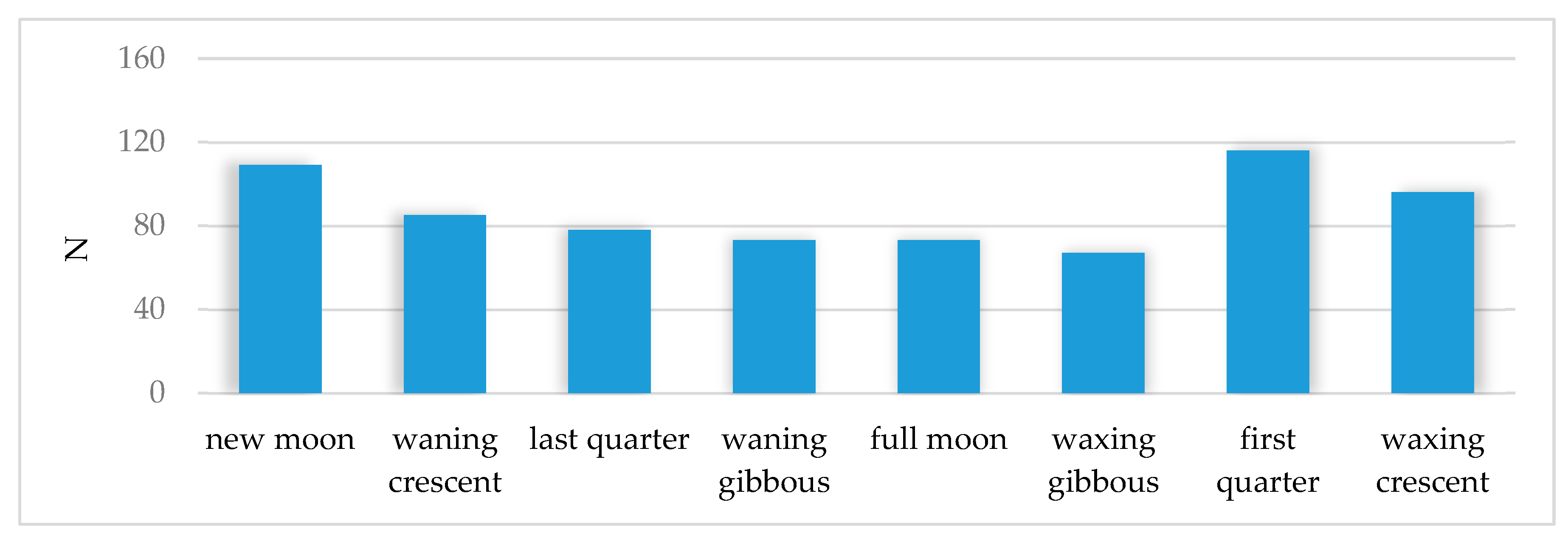
3.2. Activity of Roe Deer in Warsaw Urban Forests
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Hewison, A.J.M.; Vincent, J.P.; Joachim, J.; Angibault, J.M.; Cargnelutti, B.; Cibien, C. The effects of woodland fragmentation and human activity on roe deer distribution in agricultural landscapes. Can. J. Zool. 2001, 79, 679–689. [Google Scholar] [CrossRef]
- Acevedo, P.; Delibes-Mateos, M.; Escudero, M.A.; Vicente, J.; Marco, J.; Gortazar, C. Environmental constraints in the colonization sequence of roe deer (Capreolus capreolus Linnaeus, 1758) across the Iberian Mountains, Spain. J. Biogeogr. 2005, 32, 1671–1680. [Google Scholar] [CrossRef]
- Syphard, A.D.; Clarke, K.C.; Franklin, J.; Regan, H.M.; McGinnis, M. Forecasts of habitat loss and fragmentation due to urban growth are sensitive to source of input data. J. Environ. Manag. 2011, 92, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Scolozzi, R.; Geneletti, D. A multi-scale qualitative approach to assess the impact of urbanization on natural habitats and their connectivity. Environ. Impact Assess. Rev. 2012, 36, 9–22. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L.M. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Proffitt, K.M.; Grigg, J.L.; Hamlin, K.L.; Garrott, R.A. Contrasting effects of wolves and human hunters on elk behavioral responses to predation risk. J. Wildl. Manag. 2009, 73, 345–356. [Google Scholar] [CrossRef]
- Ciuti, S.; Northrup, J.M.; Muhly, T.B.; Simi, S.; Musiani, M.; Pitt, J.A.; Boyce, M.S. Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS ONE 2012, 7, e50611. [Google Scholar] [CrossRef]
- Suarez, A.V.; Holway, D.A.; Case, T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc. Natl. Acad. Sci. USA 2001, 98, 1095–1100. [Google Scholar] [CrossRef]
- Rich, C.; Longcore, T. Ecological Consequences of Artificial Night Lighting; Island Press: London, UK, 2006. [Google Scholar]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation—A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Warren, R. Deer overabundance in the USA: Recent advances in population control. Anim. Prod. Sci. 2011, 51, 259–266. [Google Scholar] [CrossRef]
- Boonstra, R.; Hik, D.; Singleton, G.; Tinnikov, A. The impact of predator- induced stress on the snowshoe hare cycle. Ecol. Monogr. 1998, 68, 371–394. [Google Scholar] [CrossRef]
- Zbyryt, A.; Bubnicki, J.W.; Kuijper, D.P.J.; Dehnhard, M.; Churski, M.; Schmidt, K. Do wild ungulates experience higher stress with humans than with large carnivores? Behav. Ecol. 2018, 29, 19–30. [Google Scholar] [CrossRef]
- Creel, S.; Christianson, D.; Liley, S.; Winnie, J.A., Jr. Predation risk affects reproductive physiology and demography of elk. Science 2007, 315, 960. [Google Scholar] [CrossRef] [PubMed]
- Hetem, R.S.; Strauss, W.M.; Fick, L.G.; Maloney, S.K.; Meyer, L.C.R.; Shobrak, M.; Fuller, A.; Mitchell, D. Does size matter? Comparison of body temperature and activity of free-living Arabian oryx (Oryx leucoryx) and the smaller Arabian sand gazelle (Gazella subgutturosa marica) in the Saudi desert. J. Comp. Physiol. B 2012, 182, 437–449. [Google Scholar] [CrossRef]
- Phillips, A.J.K.; Fulcher, B.D.; Robinson, P.A.; Klerman, E.B. Mammalian rest/activity patterns explained by physiologically based modeling. PLoS Comput. Biol. 2013, 9, e1003213. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Bonnot, N.; Morellet, N.; Verheyden, H. Habitat use under predation risk: Hunting, roads and human dwellings influence the spatial behavior of roe deer. Eur. J. Wildl. Res. 2013, 59, 185–193. [Google Scholar] [CrossRef]
- Bonnot, N.; Morellet, N.; Hewison, A.J.M.; Martin, J.-L.; Benhamou, S.; Chamaillé-Jammes, S. Black-tailed deer (Odocoileus hemionus sitkensis) adjust habitat selection and activity rhythm to the absence of predators. Can. J. Zool. 2016, 94, 385–394. [Google Scholar] [CrossRef]
- Bragina, E.V.; Ives, A.R.; Pidgeon, A.M.; Balčiauskas, L.; Csányi, S.; Khoyetskyy, P.; Kysucká, K.; Lieskovsky, J.; Ozolins, J.; Randveer, T.; et al. Wildlife population changes across Eastern Europe after the collapse of socialism. Front. Ecol. Environ. 2018, 16, 77–81. [Google Scholar] [CrossRef]
- Putman, R.J. Foraging by roe deer in agricultural areas and impact on arable crops. J. Appl. Ecol. 1986, 23, 91–99. [Google Scholar] [CrossRef]
- McLoughlin, P.D.; Gaillard, J.-M.; Boyce, M.S.; Bonenfant, C.; Messier, F.; Duncan, P.; Delorme, D.; Van Moorter, B.; Saïd, S.; Klein, F. Lifetime reproductive success and composition of the home range in a large herbivore. Ecology 2007, 88, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Panzacchi, M.; Linnell, J.D.C.; Odden, M.; Odden, J.; Andersen, R. Habitat and roe deer fawn vulnerability to red fox predation. J. Anim. Ecol. 2009, 78, 1124–1133. [Google Scholar] [CrossRef]
- McCarthy, A.; Baker, A.; Rotherham, I. Urban-fringe deer management issues-a South Yorkshire case study. Br. Wildl. 1996, 8, 12–19. [Google Scholar]
- Kilpatrick, H.; Spohr, S. Spatial and temporal use of a suburban landscape by female white-tailed deer. Wildl. Soc. Bull. 2000, 28, 1023–1029. [Google Scholar]
- Mattila, M.; Hadjigeorgiou, I. Conservation and management of fallow deer (Dama dama dama L.) on Lemnos Island, Greece. Turk. J. Vet. Anim. Sci. 2015, 39, 560–567. [Google Scholar] [CrossRef]
- Loro, M.; Ortega, E.; Arce, R.M.; Geneletti, D. Assessing landscape resistance to roe deer dispersal using fuzzy set theory and multicriteria analysis: A case study in Central Spain. Landsc. Ecol. Eng. 2016, 12, 41–60. [Google Scholar] [CrossRef]
- Brazaitis, G.; Pėtelis, K.; Žalkauskas, R.; Belova, O.; Danusevičius, D.; Marozas, V.; Narauskaitė, G. Landscape effect for the Cervidaes Cervidae in human-dominated fragmented forests. Eur. J. For. Res. 2014, 133, 857–869. [Google Scholar] [CrossRef]
- Ciach, M.; Fröhlich, A. Ungulates in the city: Light pollution and open habitats predict the probability of roe deer occurring in an urban environment. Urban. Ecosyst. 2019, 22, 513–523. [Google Scholar] [CrossRef]
- Wevers, J.; Fattebert, J.; Casaer, J.; Artois, T.; Beenaerts, N. Trading fear for food in the Anthropocene: How ungulates cope with human disturbance in a multi-use, suburban ecosystem. Sci. Total Environ. 2020, 741, 140369. [Google Scholar] [CrossRef]
- Prough, L.R.; Golden, C.D. Does moonlight increase predation risk? Meta-analysis reveals divergent responses of nocturnal mammals to lunar cycles. J. Anim. Ecol. 2014, 83, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Lone, K.; Loe, L.E.; Gobakken, T.; Linnell, J.D.C.; Odden, J.; Remmen, J.; Mysterud, A. Living and dying in a multi-predator landscape of fear: Roe deer are squeezed by contrasting pattern of predation risk imposed by lynx and humans. Oikos 2014, 123, 641–651. [Google Scholar] [CrossRef]
- Łopucki, R.; Klich, D.; Kitowski, I. Are small carnivores urban avoiders or adapters: Can they be used as indicators of well-planned green areas? Ecol. Indic. 2019, 101, 1026–1031. [Google Scholar] [CrossRef]
- Jasińska, K.; Goszczyński, J. The occurrence of mammals in Warszawa cemeteries. In Urban Fauna. Studies of Animal Biology, Ecology and Conservation in European Cities; Indykiewicz, P., Jerzak, L., Böhner, J., Kavanagh, B., Eds.; University of Technology and Life Sciences in Bydgoszcz: Bydgoszcz, Poland, 2011; pp. 533–541. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, K.D.; Jackowiak, M.; Gryz, J.; Bijak, S.; Szyc, K.; Krauze-Gryz, D. Occurrence and Activity of Roe Deer in Urban Forests of Warsaw. Environ. Sci. Proc. 2021, 3, 35. https://doi.org/10.3390/IECF2020-07913
Jasińska KD, Jackowiak M, Gryz J, Bijak S, Szyc K, Krauze-Gryz D. Occurrence and Activity of Roe Deer in Urban Forests of Warsaw. Environmental Sciences Proceedings. 2021; 3(1):35. https://doi.org/10.3390/IECF2020-07913
Chicago/Turabian StyleJasińska, Karolina D., Mateusz Jackowiak, Jakub Gryz, Szymon Bijak, Katarzyna Szyc, and Dagny Krauze-Gryz. 2021. "Occurrence and Activity of Roe Deer in Urban Forests of Warsaw" Environmental Sciences Proceedings 3, no. 1: 35. https://doi.org/10.3390/IECF2020-07913
APA StyleJasińska, K. D., Jackowiak, M., Gryz, J., Bijak, S., Szyc, K., & Krauze-Gryz, D. (2021). Occurrence and Activity of Roe Deer in Urban Forests of Warsaw. Environmental Sciences Proceedings, 3(1), 35. https://doi.org/10.3390/IECF2020-07913






