Abstract
Since the beginning of humanity, many sectors have produced different chemicals. These chemicals are the main causes of environmental pollution and have become a global problem with irreversible effects in terms of health. Because of this, countries have set a target of “a pollution-free planet”. We need to determine target-specific strategies to both eliminate pollution and protect health. To date, traditional methods of monitoring in receiving aquatic environments have been used; however, they do not provide information on toxic levels of pollutants. For this reason, researchers have focused on “bio-indicator” or “bio-monitor” organisms. Since these organisms are in equilibrium with the aquatic environment, they can also be considered an integrated sampling tool and may indicate potential contamination. Danio rerio (zebrafish) is considered a promising model organism for single health studies in terms of its biological structure. This review aims to present Danio rerio’s characteristics, susceptibility to environmental pollutants, and risks associated with pollutants in the aquatic environment.
1. Introduction
Environmental change poses a devastating risk to human and environmental health. Environmental pollution is the most difficult challenge for all countries in the world, as it affects all living things and ecosystems under the concept of one health [1,2,3]. A rapid study of water conditions is necessary for monitoring, assessing, and addressing this global health hazard. Bio-indicators or biological monitors can monitor water quality changes in real-time. Zebrafish (Danio rerio) is an ideal bio-indicator for detecting environmental changes due to its biomedical equipment, widespread geographic distribution, and well-characterized specific properties against environmental pollutants [4,5]. Danio rerio, which is used to determine the toxicity (teratogenicity, cardiotoxicity, neurotoxicity, hepatotoxicity, and nephrotoxicity, etc.) effect of many chemicals, has also recently been used in the detection and research of diseases [6]. Considering the increasing use of zebrafish as an experimental animal against a wide variety of chemicals and pollutants in toxicity studies, it is also of great importance to improve laboratory conditions in terms of reproductive quality. Despite the zebrafish’s numerous advantages, this model organism has several limitations. As a model for monitoring the toxicity of environmental pollutants, zebrafish have some disadvantages, such as low sensitivity and inconvenient statistical experiments [3,4,5,6].
In general, a good animal model should have all or most of the following characteristics: (i) structural simplicity that also incorporates the basic cellular processes that more complex organisms have; (ii) easy accessibility for research; (iii) easy and economical to manufacture in the laboratory; (iv) prone to genetic changes; and (v) if possible, have a relatively small and stable genome [7]. The most popular fish species preferred in experimental processes are listed as Danio rerio, Carassius auratus, Oryzias latipes, Poecia Reticulata, Gasterosteus aculeatus, Oncorhynchus mykiss, Takifugu rubripes, and Xiphophorus hellerii. Research on zebrafish has become more popular in the last decade. Danio rerio’s high fertility rate (ability to fertilize approximately 200–300 eggs every 5–7 days), economic maintenance, and ease of genetic modification make Danio rerio an alternative and valuable vertebrate model compared to other species [8]. The use of zebrafish as a model for toxicity studies is carried out according to standards. These standards are the Zebrafish Toxicity Test, listed as British standard BS/EN/ISO 7346-3-1998, German standard DIN/EN/ISO 7346-3-1998, and Chinese standard GB/T13267-91, OECD n 203-236-473-487-489-490 [9,10]. Danio rerio (Cyprinidae family: freshwater teleost) is considered a model organism in many research areas, especially in health and pollution detection. In this study, we describe the criteria for the use of this fish species as a model for research in environmental toxicology and argue that Danio rerio is an ideal bio-indicator for detecting the toxicity of pollutants.
2. Methodology
A literature search related to zebrafish used as a model in ecotoxicity assessments was conducted in databases such as Web of Science, Science Direct, Scopus, and PubMed. The following keywords were used in the search process: “Zebrafish” and “Ecotoxicity”, “Danio rerio” and “Acute toxicity”, “Zebrafish development” and “Fish assay”, and “Zebrafish embryo test” and “Exposure”, etc. A total of 225 articles were reviewed in all databases. Studies that did not comply with the study’s purpose were not included in the evaluation. Of the 225 articles published until January 2023, 92 were thoroughly reviewed. Each article used in the study was compiled according to the following parameters: (i) publication year; (ii) the type of pollutant; (iii) exposure factors; (iv) toxicity; (v) stage of development; (vi) types of tests; and (vii) ecotoxic effects (Figure 1) [1,11,12].
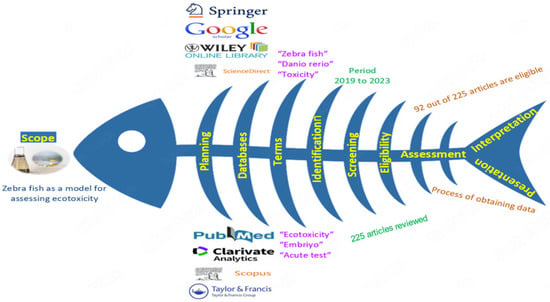
Figure 1.
Systematic review methodology.
3. Advantages and Disadvantages of Zebrafish in Ecotoxicity Tests
Zebrafish is a successful monitoring model applied in both medicine (drug production, disease diagnosis, treatment, etc.) and environmental pollution (determination of the effect level of pollutants) [13]. Zebrafish has been widely used in ecotoxicity studies in recent years due to its advantageous features, such as reproduction process and developmental stage [14]. The most important feature that distinguishes zebrafish from other model creatures is that it is used in both sexes, which has brought zebrafish to the forefront in ecotoxicity tests [1]. One of the main advantages of this model is that experimental studies can be carried out in the embryo process [13]. Additionally, embryos (laying from egg: 48–72 h (hpf); organ formation: 120 hpf; adulthood: 3 months) grow rapidly [15,16]. Rapid development is an excellent feature in toxicity studies with animal models as it allows instant monitoring of toxic exposure [17]. All genetic features of zebrafish are stored in a database (www.zfin.org, accessed on 1 March 2023). In addition, 70% of zebrafish genes are similar to human genes [8]. In recent years, zebrafish has been evaluated as an alternative ecotoxicity model to replace mice and other fish species. It has proven to be a bio-indicator that complies with the 3R (Reduce, Reuse, and Recycle) concept, as tests with zebrafish are economical, provide fast results, and the presence of a large number of embryos reduces the chemical requirements [18]. Among the disadvantages of zebrafish, it can be said that different reactions occur according to the sex type, and the embryo development pools are large.
4. Development and Distribution of Zebrafish
The zebrafish was described in 1882 by Francis Hamilton, who found it near the Ganges River in India [19]. After George Streisinger first used the zebrafish to study vertebrate development in the 1980s, it became one of the most important laboratory animals with unprecedented speed [8]. The zebrafish is native to most of the Indian subcontinent, from Pakistan in the west through India, Nepal, and Bangladesh to Myanmar in the east. Zebrafish can be found in a wide variety of habitats. The adult zebrafish is 2 to 4 cm long, and its body is characterized by zebra-like stripes (Figure 2).

Figure 2.
Adult zebrafish illustration.
In toxicological studies, especially in order to determine the toxic levels of environmental pollutants, parameters close to the natural environment of zebrafish should be provided in the laboratory environment. In this context, water quality parameters come to the fore (Table 1) [20,21,22,23]. The zebrafish is found in rivers, streams, canals, and rice fields in India, Pakistan, Bangladesh, Nepal, Myanmar, and Bhutan [22]. Wild zebrafish distribution includes Brazil, Colombia, Malaysia, Sri Lanka, Thailand, and the United States [21,23]. Native zebrafish is found in more than 3000 institutions in more than 100 countries and is a popular aquarium fish [22,24]. In research studies with zebrafish in the literature, China (26.7%), Brazil (14.8%), France (12.5%), Germany (8.5%), India (6.3%), and Italy (5.1%) take the lead [1]. The University of Oregon in the United States is home to the Zebrafish International Resource Center (ZIRC), the world’s first and largest zebrafish resource center located on the Zebrafish Information Network (ZFIN). According to data from the Federation of European Aquaculture Producers (FEAP), this type of fish is produced at a rate of 1.8% (https://feap.info/, accessed on 1 March 2023).

Table 1.
Physico-chemical properties of water for lab-raised zebrafish.
5. Zebrafish-Based Experimental Applications
In addition to fish species, experiment design is an important factor in ecotoxicity studies. The type of exposure (in vivo or in vitro), exposure route, exposure times, and stage of development are considered experimental parameters in data generation [13]. The majority of tests are performed in vivo (90.3%) compared to in vitro tests (9.7%) (Figure 3). Embryos (7.3%) and larvae (32.1%) are used less frequently than adults (55.8%) in ecotoxicity evaluation according to the development process (Figure 3). In terms of exposure route and duration, water (94.9%) and hour (51.1%) are evaluated at the highest rates, respectively (Figure 3). In Table 2, toxicity studies for which zebrafish are the models are given.
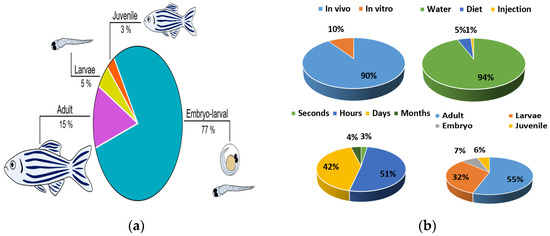
Figure 3.
(a) Danio rerio development; (b) type of studies in vivo and in vitro, zebrafish exposure route, exposure time, and developmental stages (Adapted from Refs. [1,11]).

Table 2.
Different toxicity studies in the literature.
6. Results
The zebrafish’s small size, resistance to biotic and abiotic conditions, rapid development process, short reproduction period, compatibility with laboratory conditions, easy supply, economy, high fertility, ability to manipulate embryos, and genetic similarity with humans increase the importance of this species for toxicity studies. Thanks to these advantages, zebrafish can be used instead of other living models in scientific research and can support the 3R rule. As a result of studies researched in international databases, it is thought that different uses of this species will be widespread, and it will be a key model organism in the future.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, resources, writing—original draft preparation, writing—review and editing, visualization, H.Ç., T.B., İ.Ş. and Ş.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was carried out in Aksaray University Central Library and Engineering Faculty Environmental Engineering Department.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Canedo, A.; Rocha, T.L. Zebrafish (Danio rerio) using as model for genotoxicity and DNA repair assessments: Historical review, current status and trends. Sci. Total Environ. 2021, 762, 144084. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Khan, M.D.Z.A.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging pollutants in aquatic environment: Source, effect, and challenges in biomonitoring and bioremediation—A review. Pollution 2020, 6, 99–113. [Google Scholar]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Kelly, J.R.; Shelton, S.G.; Daniel, D.K.; Bhat, A.; Mondal, R.; Nipple, F.; Amro, H.; Bower, M.E.; Isaac, G.; McHaney, G.; et al. Wild zebrafish sentinels: Biological monitoring of site differences using behavior and morphology. Toxics 2021, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Beffagna, G. Zebrafish as a smart model to understand regeneration after heart injury: How fish could help humans. Front. Cardiovasc. Med. 2019, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Strungaru, S.A.; Plavan, G.; Ciobica, A.; Nicoara, M.; Robea, M.A.; Solcan, C.; Petrovici, A. Toxicity and chronic effects of deltamethrin exposure on zebrafish (Danio rerio) as a reference model for freshwater fish community. Ecotoxicol. Environ. Saf. 2019, 171, 854–862. [Google Scholar] [CrossRef]
- Shen, Q.; Truong, L.; Simonich, M.T.; Huang, C.; Tanguay, R.L.; Dong, Q. Rapid well-plate assays for motor and social behaviors in larval zebrafish. Behav. Brain Res. 2020, 391, 112625. [Google Scholar] [CrossRef]
- Russo, I.; Sartor, E.; Fagotto, L.; Colombo, A.; Tiso, N.; Alaibac, M. The Zebrafish model in dermatology: An update for clinicians. Discov. Oncol. 2022, 13, 48. [Google Scholar] [CrossRef]
- Chatterjee, N.; Lee, H.; Kim, J.; Kim, D.; Lee, S.; Choi, J. Critical window of exposure of CMIT/MIT with respect to developmental effects on zebrafish embryos: Multi-level endpoint and proteomics analysis. Environ. Pollut. 2021, 268, 115784. [Google Scholar] [CrossRef]
- Kelly, J.R.; Benson, S.A. Inconsistent ethical regulation of larval zebrafish in research. J. Fish Biol. 2020, 97, 324–327. [Google Scholar] [CrossRef]
- Saiki, P.; Mello-Andrade, F.; Gomes, T.; Tocha, T.L. Sediment toxicity assessment using zebrafish (Danio rerio) as a model system: Historical review, research gaps and trends. Sci. Total Environ. 2021, 793, 148633. [Google Scholar] [CrossRef]
- Canedo, A.; de Jesus, L.W.O.; Bailao, E.F.L.C.; Rocha, T.L. Micronucleus test and nuclear abnormality assay in zebrafish (Danio rerio): Past, present, and future trends. Environ. Pollut. 2021, 290, 118019. [Google Scholar] [CrossRef]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv. Drug Deliv. Ver. 2019, 151–152, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar]
- Li, X.; Xiong, D.; Ju, Z.; Xiong, Y.; Ding, G.; Liao, G. Phenotypic and transcriptomic consequences in zebrafish early-life stages following exposure to crude oil and chemical dispersant at sublethal concentrations. Sci. Total Environ. 2021, 763, 143053. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish models of neurodevelopmental disorders: Limitations and benefits of current tools and techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef] [PubMed]
- Le Pabic, P.; Dranow, D.B.; Hoyle, D.J.; Schilling, T.F. Zebrafish endochondral growth zones as they relate to human bone size, shape and disease. Front. Endocrinol. 2022, 13, 1060187. [Google Scholar] [CrossRef]
- Chien, L.C.; Wu, Y.H.; Ho, T.N.; Huang, Y.Y.; Hsu, T. Heat stress modulates nucleotide excision repair capacity in zebrafish (Danio rerio) early and mid-early embryos via distinct mechanisms. Chemosphere 2020, 238, 124653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, Q.; Di Paolo, C.; Shao, Y.; Hollert, H.; Seiler, T.B. Behavioral profile alterations in zebrafish larvae exposed to environmentally relevant concentrations of eight priority pharmaceuticals. Sci. Total Environ. 2019, 664, 89–98. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Li, S.; Yeo, K.S.; Levee, T.M.; Howe, C.J.; Her, Z.P.; Zhu, S. Zebrafish as a neuroblastoma model: Progress made, promise for the future. Cells 2021, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, L.; de Girolamo, P. Fish as model systems. In Laboratory Fish in Biomedical Research Biology, Husbandry and Research Applications for Zebrafish, Medaka, Killifish, Cavefish, Stickleback, Goldfish and Danionella Translucida, 1st ed.; D’Angelo, L., de Girolamo, P., Eds.; Andre Gerhard Wolff: London, UK, 2022; pp. xix–xxiv. [Google Scholar]
- Lee, C.J.; Paull, G.C.; Tyler, C.R. Improving zebrafish laboratory welfare and scientific research through understanding their natural history. Biol. Rev. 2022, 97, 1038–1056. [Google Scholar] [CrossRef] [PubMed]
- Trigueiro, N.; Canedo, A.; Braga, D.; Luchiari, A.C.; Rocha, T.L. Zebrafish as an emerging model system in the global South: Two decades of research in Brazil. Zebrafish 2020, 17, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Penagos, C.D.; Zamora-Briseno, J.A.; Amendola-Pimenta, M.; Elizalde-Contreras, J.M.; Arcega-Cabrera, F.; Cruz-Quintana, Y.; Santana-Pineros, A.M.; Canizarez-Martinez, M.A.; Perez-Vega, J.A.; Ruiz-May, E.; et al. Pollution and children’s health. Toxicol. Appl. Pharmacol. 2022, 445, 116033. [Google Scholar]
- Barros, S.; Ribeiro, M.; Coimbra, A.M.; Pinheiro, M.; Morais, H.; Alves, N.; Montes, R.; Rodil, R.; Quintana, J.B.; Santos, M.M.; et al. Metformin disrupts Danio rerio metabolism at environmentally relevant concentrations: A full life-cycle study. Sci. Total Environ. 2022, 846, 157361. [Google Scholar] [CrossRef] [PubMed]
- Jijie, R.; Solcan, G.; Nicoara, M.; Micu, D.; Strungaru, S.A. Antagonistic effects in zebrafish (Danio rerio) behavior and oxidative stress induced by toxic metals and deltamethrin acute exposure. Sci. Total Environ. 2020, 698, 134299. [Google Scholar] [CrossRef]
- Licitra, R.; Marchese, M.; Naef, V.; Ogi, A.; Martinelli, M.; Kiferle, C.; Fronte, B.; Santorelli, F.M. A review on the bioactivity of cannabinoids on zebrafish models: Emphasis on neurodevelopment. Biomedicines 2022, 10, 1820. [Google Scholar] [CrossRef]
- Boulanger, E.; Barst, B.D.; Alloy, M.M.; Blais, S.; Houde, M.; Head, J.A. Assessment of environmentally contaminated sediment using a contact assay with early life stage zebrafish (Danio rerio). Sci. Total Environ. 2019, 659, 950–962. [Google Scholar] [CrossRef]
- Kataba, A.; Botha, T.L.; Nakayama, S.M.M.; Yohannes, Y.B.; Ikenaka, Y.; Wepener, V.; Ishizuka, M. Environmentally relevant lead (Pb) water concentration induce toxicity in zebrafish (Danio rerio) larvae. Comp. Biochem. Physiol. 2022, 252, 109215. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, Y.; Ramesh, M.; Li, B.; Poopal, R.K. Assessment of eco-toxic effects of commonly used water disinfectant on zebrafish (Danio rerio) swimming behaviour and recovery responses: An early-warning biomarker approach. Environ. Sci. Pollut. Res. 2022, 29, 41849–41862. [Google Scholar] [CrossRef]
- Hu, G.; Wang, H.; Wan, Y.; Zhou, L.; Wang, Q.; Wang, M. Combined toxicities of cadmium and five agrochemicals to the larval zebrafish (Danio rerio). Sci. Rep. 2022, 12, 16045. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, H.; Gök, O. Effect of triclosan exposure on mortality and behavioral changes of Poecilia reticulata and Danio rerio. Hum. Ecol. Risk Assess. 2018, 24, 1327–1341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).