Clay-Based Sorbents for Environmental Protection from Inorganic Pollutants †
Abstract
:1. Introduction
2. Materials and Methods
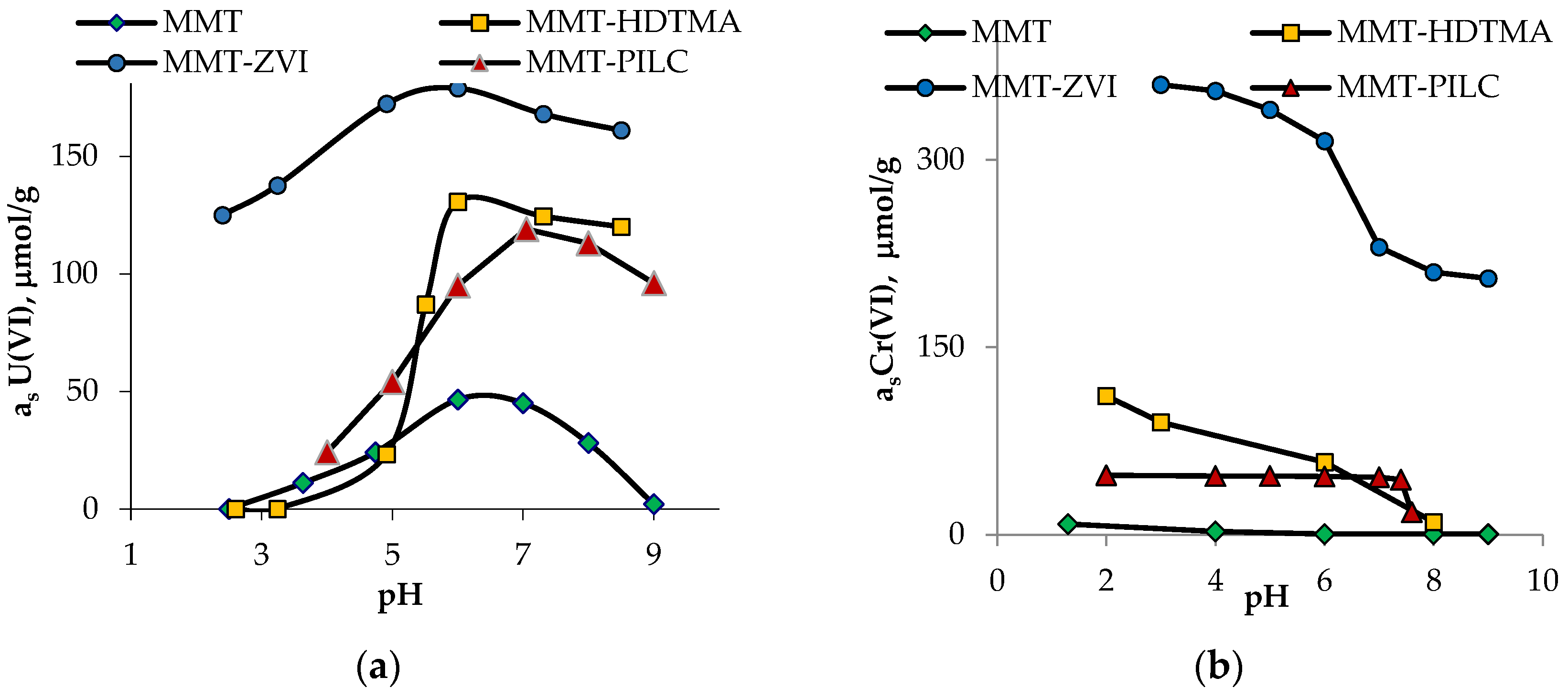
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bockris, J.M. Environmental Chemistry; Springer: Boston, MA, USA, 1977. [Google Scholar]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Babak, M.I.; Koshyk, Y.I.; Avdeev, O.K.; Bezrodnyi, S.A.; Savelyev, Y.Y.; Kucha, P.M. Mining and Processing of Uranium Ores in Ukraine; Chernov, A.P., Ed.; ADEF-Ukraine: Kyiv, Ukraine, 2001; p. 238. (In Ukrainian) [Google Scholar]
- Sapozhnikov, Y.A.; Aliyev, R.A.; Kalmykov, S.N. Environmental Radioactivity; BINOM: Moscow, Russia, 2006; p. 286. (In Russian) [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Zachara, J.M.; Cowan, C.E.; Schmidt, R.L.; Ainsworth, C.C. Chromate Adsorption by Kaolinite. Clays Clay Miner. 1988, 36, 317–326. [Google Scholar] [CrossRef]
- Yuan, G.D.; Theng, B.K.G.; Churchman, G.J.; Gates, W.P. Clays and Clay Minerals for Pollution Control. In Handbook of Clay Sciences; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 587–644. [Google Scholar]
- Majdan, M.; Pikus, S.; Gajowiak, A.; Gładysz-Płaska, A.; Krzyżanowska, H.; Żuk, J.; Bujacka, M. Characterization of uranium(VI) sorption by organobentonit. Appl. Surf. Sci. 2010, 256, 5416–5421. [Google Scholar] [CrossRef]
- Kovalchuk, I.A.; Laguta, A.M.; Kornilovych, B.Y.; Tobilko, V.Y. Organophilized layered silicates for removing uranium (VI) compounds from mineralized waters. Chem. Phys. Technol. Surf. 2020, 2, 215–227. [Google Scholar]
- Houhoune, F.; Nibou, D.; Chegrouche, S.; Menacer, S. Behaviour of modified hexadecyltrimethylammonium bromide toward uranium species. J. Environ. Chem. Eng. 2016, 4, 3459–3467. [Google Scholar] [CrossRef]
- Gajowiak, A.; Gładysz-Płaska, A.; Sternik, D.; Pikus, S.; Sabah, E.; Majdan, M. Sorption of uranyl ions on organosepiolite. Chem. Eng. J. 2013, 219, 459–468. [Google Scholar] [CrossRef]
- Krishna, B.S.; Murty, D.S.R.; Prakash, B.S.J. Surfactant-modified clay as adsorbent for chromate. Appl. Clay Sci. 2001, 20, 65–71. [Google Scholar] [CrossRef]
- Li, Z. Oxyanion sorption and surface anion exchange by surfactant-modified clay minerals. J. Environ. Qual. 1999, 28, 1457–1463. [Google Scholar] [CrossRef]
- Li, Z.H.; Bowman, R.S. Retention of inorganic oxyanions by organo-kaolinite. Water Res. 2001, 35, 3771–3776. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Korili, S.A.; Trujillano, R.; Vicente, M.A. Pillared Clays and Related Catalysts; Springer: New York, NY, USA, 2010; p. 522. [Google Scholar]
- Vicente, M.A.; Gil, A.; Bergaya, F. Pillared Clays and Clay Minerals. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 523–557. [Google Scholar]
- Zhou, J.; Wu, P.; Dang, Z.; Zhu, N.; Li, P.; Wu, J.; Wang, X. Polymeric Fe/Zr pillared montmorillonite for the removal of Cr(VI) from aqueous solutions. Chem. Eng. J. 2010, 162, 1035–1044. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Shang, C.; Shao, J.; Zhang, J.; Huang, H. The application of iron-based technologies in uranium remediation: A review. Sci. Total Environ. 2017, 575, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Shao, X.; Li, Y.; Li, J.; Dong, H.; Cheng, W.; Gao, X.; Huang, Y. Enhanced Removal of Uranium(VI) by Nanoscale Zerovalent Iron Supported on Na–Bentonite and an Investigation of Mechanism. Phys. Chem. 2014, 118, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.N.; Zhang, X.; Chen, Z.L. Removal of Chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res. 2011, 45, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–727. [Google Scholar] [CrossRef] [PubMed]
- Carrado, K.A. Introduction: Clay Structure, Surface Acidity, and Catalysis. In Handbook of Layered Materials; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Guerra, D.L.; Airoldi, C.; Lemos, V.P.; Angelica, R.S. Adsorptive, thermodynamic and kinetic performances of Al/Ti and Al/Zr-pillared clays from the Brazilian Amazon region for zinc cation removal. J. Hazard. Mater. 2008, 155, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Pylypenko, I.V.; Kovalchuk, I.A.; Veremeyenko, V.V.; Spasyonova, L.M. Sorption of cobalt, chromium and uranium ions by Fe/Ti-pillared montmorillonite. Easter Eur. J. Enterp. Technol. 2014, 4, 57–61. [Google Scholar]
- Kornilovych, B.; Kovalchuk, I.; Tobilko, V.; Ubaldini, S. Uranium Removal from Groundwater and Wastewater Using Clay-Supported Nanoscale Zero-Valent Iron. Metals 2020, 10, 1421. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. Res. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1997; 600p. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalchuk, I. Clay-Based Sorbents for Environmental Protection from Inorganic Pollutants. Environ. Sci. Proc. 2023, 25, 34. https://doi.org/10.3390/ECWS-7-14247
Kovalchuk I. Clay-Based Sorbents for Environmental Protection from Inorganic Pollutants. Environmental Sciences Proceedings. 2023; 25(1):34. https://doi.org/10.3390/ECWS-7-14247
Chicago/Turabian StyleKovalchuk, Iryna. 2023. "Clay-Based Sorbents for Environmental Protection from Inorganic Pollutants" Environmental Sciences Proceedings 25, no. 1: 34. https://doi.org/10.3390/ECWS-7-14247
APA StyleKovalchuk, I. (2023). Clay-Based Sorbents for Environmental Protection from Inorganic Pollutants. Environmental Sciences Proceedings, 25(1), 34. https://doi.org/10.3390/ECWS-7-14247





