The Impact of Possible Mercury Source-Point Contamination in the Coastal Area of Skiathos Island †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Study
2.2. Field Experiment
2.3. Laboratory Analysis
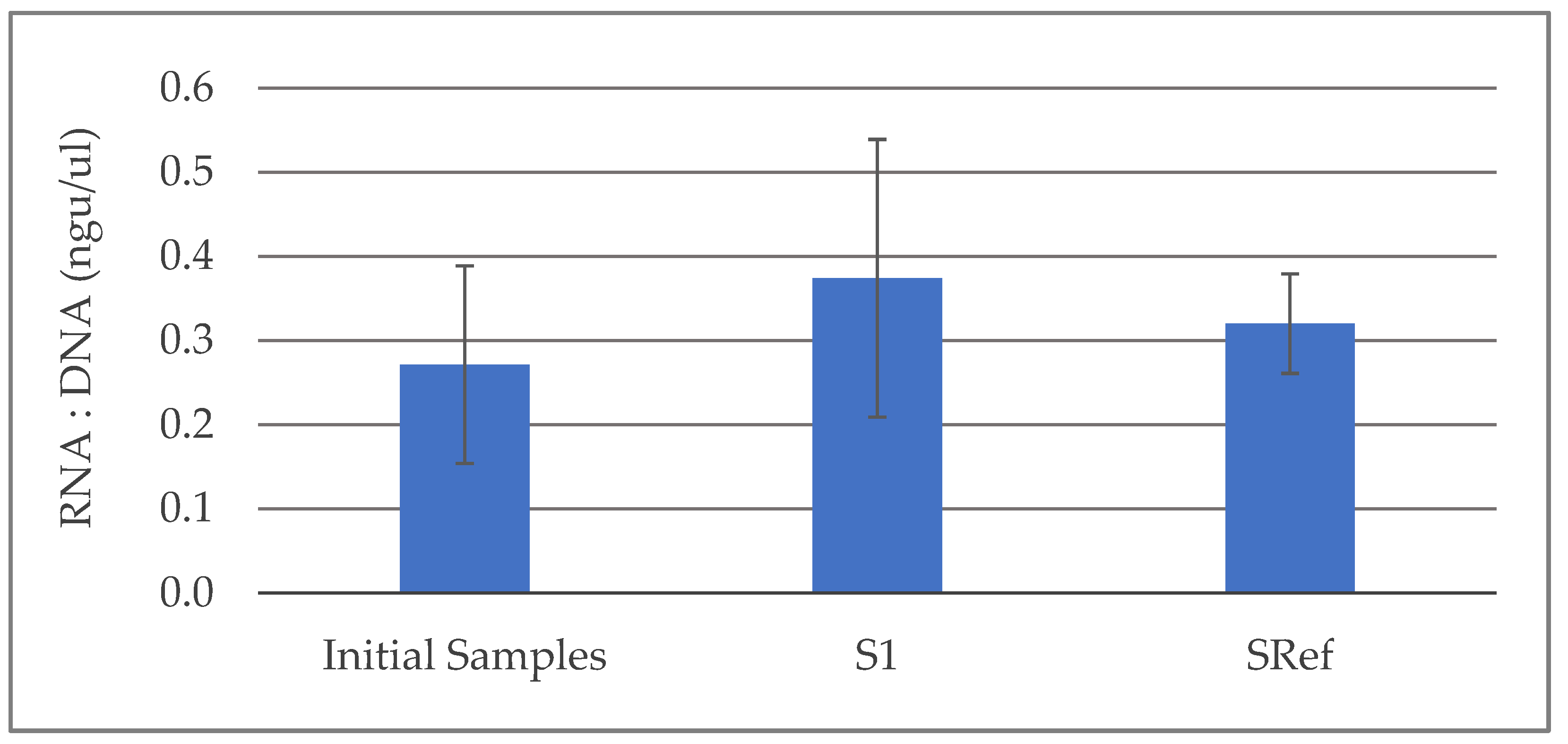
3. Results and Discussion
3.1. Laboratory Results
3.2. Quantity of Mercury through Mussels That Can Be Consumed on A Weekly Basis from the Coastal Area of Skiathos Island
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2016, 36, 609–662. [Google Scholar] [CrossRef]
- Adams, D.H.; Engel, M.E. Mercury, lead, and cadmium in μblue crabs, Callinectes sapidus, from the Atlantic coast of Florida, USA: A multipredator approach. Ecotoxicol. Environ. Saf. 2014, 102, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Arcagni, M.; Juncos, R.; Rizzo, A.; Pavlin, M.; Fajon, V.; Arribére, M.A.; Hovart, M.; Guevara, S.R. Species-and habitat-specific bioaccumulation of total mercury and methylmercury in the food web of a deep oligotrophic lake. Sci. Total Environ. 2018, 612, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Paloma de Almeida Rodrigues, P.; Ferrari, R.G.; dos Santos, L.N.; Junior, C.A.C. Mercury in aquatic fauna contamination: A systematic review on its dynamics and potential health risks. J. Environ. Sci. 2019, 84, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, C.; Kormas, K.; Strogyloudi, E.; Hatzianestis, I.; Neofitou, C.; Andral, B.; Galgani, F. Multiple biomarkers of pollution effects in caged mussels on the Greek coastline. Comp. Bioche. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Vlahogianni, T.; Dassenakis, M.; Scoullos, M.J.; Valavanidis, A. Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Mar. Pollut. Bull. 2007, 54, 1361–1371. [Google Scholar] [CrossRef]
- Tsangaris, C.; Hatzianestis, I.; Catsiki, V.A.; Kormas, K.A.; Strogyloudi, E.; Neofitou, C.; Andral, B.; Galgani, F. Active biomonitoring in Greek coastal waters: Application of the integrated biomarker response index in relation to contaminant levels in caged mussels. Sci. Total Environ. 2011, 412, 359–365. [Google Scholar] [CrossRef]
- Andersson, M.E.; Gårdfeldt, K.; Wängberg, I.; Sprovieri, F.; Pirrone, N.; Lindqvist, O. Seasonal and daily variation of mercury evasion at coastal and off shore sites from the Mediterranean Sea. Mar. Chem. 2007, 104, 214–226. [Google Scholar] [CrossRef]
- Cossa, D.; Martin, J.M.; Takayanagi, K.; Sanjuan, J. The distribution and cycling of mercury species in the western Mediterranean. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 721–740. [Google Scholar] [CrossRef]
- Llull, R.M.; Garí, M.; Canals, M.; Rey-Maquieira, T.; Grimalt, J.O. Mercury concentrations in lean fish from the Western Mediterranean Sea: Dietary exposure and risk assessment in the population of the Balearic Islands. Environ. Res. 2017, 158, 16–23. [Google Scholar] [CrossRef]
- Cossa, D.; Coquery, M. The Mediterranean mercury anomaly, a geochemical or a biological issue. In The Mediterranean Sea; Springer: Berlin/Heidelberg, Germany, 2005; pp. 177–208. [Google Scholar]
- EFSA. Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption; WHO Food Additives Series; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2011; p. 53.
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). Safety evaluation of certain food additives and contaminants. Methylmercury. WHO Food Addit. Ser. 2011, 63, 605–684. [Google Scholar]
- GMO (EFSA Panel on Genetically Modified Organisms). Guidance for renewal applications of genetically modified food and feed authorised under Regulation (EC) No 1829/2003. EFSA J. 2015, 13, 4129. [Google Scholar]
- Costa, V.; Magalhães, M.; Menezes, G.; Pinho, M.; Santos, R.; Monteiro, L. The influence of biological and ecological factors in the specific variability of mercury bioaccumulation in marine fish species from the Azores archipelago. Mar. Environ. Res. 2008, 66, 46. [Google Scholar]
- Mallory, M.L.; O’Driscoll, N.J.; Klapstein, S.; Varela, J.L.; Ceapa, C.; Stokesbury, M.J. Methylmercury in tissues of Atlantic sturgeon (Acipenser oxyrhynchus) from the Saint John River, New Brunswick, Canada. Mar. Pollut. Bull. 2018, 126, 250–254. [Google Scholar]
- Taylor, D.L.; Calabrese, N.M. Mercury content of blue crabs (Callinectes sapidus) from southern New England coastal habitats: Contamination in an emergent fishery and risks tο human consumers. Mar. Pollut. Bull. 2018, 126, 166–178. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 466/2001 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Commun. 2001, 77, 1–3. [Google Scholar]
- Condini, M.V.; Hoeinghaus, D.J.; Roberts, A.P.; Soulen, B.K.; Garcia, A.M. Mercury concentrations in dusky grouper Epinephelus marginatus in littoral and neritic habitats along the southern Brazilian coast. Mar. Pollut. Bull. 2017, 115, 266–272. [Google Scholar] [CrossRef]
- Mellios, N.; Kofinas, D.; Papageorgiou, E.; Laspidou, C. A multivariate analysis of the daily water demand of Skiathos Island, Greece, implementing the artificial neuro-fuzzy inference system (ANFIS). In Proceedings of the E-proceedings of the 36th IAHR World Congress, The Hague, The Netherlands, 28 June–3 July 2015; Volume 28, pp. 1–8. [Google Scholar]
- Kofinas, D.; Ulanczyk, R.; Laspidou, C.S. Simulation of a Water Distribution Network with Key Performance Indicators for Spatio-Temporal Analysis and Operation of Highly Stressed Water Infrastructure. Water 2010, 12, 1149. [Google Scholar] [CrossRef]
- Spyropoulou, A.; Lazarou, Y.G.; Laspidou, C. Mercury Speciation in the Water Distribution System of Skiathos Island, Greece. Proceedings 2018, 2, 668. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004.
- Humphrey, C.A.; Codi King, S.; Klumpp, D.W. A multi-biomarker approach in barramundi (Lates calcarifer) to measure exposure to contaminants in estuaries of tropical North Queensland. Mar. Pollut. Bull. 2007, 54, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; de Souza, S.S.; de Oliveira Souza, V.C.; Barbosa, F., Jr. Methylmercury and inorganic mercury determination in blood by using liquid chromatography with inductively coupled plasma mass spectrometry and a fast sample preparation procedure. Talanta 2010, 80, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.S.; Yu, R.Q.; Barkay, T.; Hamilton, T.L.; Baxter, B.K.; Naftaz, D.L.; Marvin-DiPasquale, M. Effect of salinity on mercury methylating benthic microbes and their activities in great salt Lake, Utah. Sci. Total Environ. 2017, 581–582, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Lissau, I.; Overpeck, M.D.; Ruan, W.J.; Due, P.; Holstein, B.E.; Hediger, M.L. Body mass index and overweight in adolescents in 13 European countries, Israel, and the United States. Arch. Pediatr. Adolesc. Med. 2004, 158, 27–33. [Google Scholar] [CrossRef]
- WHO. Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for length, weight-for-height and body mass index-for-age. In Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Onis, M.D.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bullet. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]

| Mean Indicative Body Weight per Target Group (WHO) | Quantity of Mercury That Can Be Consumed Weekly | Provisional Tolerable Weekly Intake (PWTI) for MeHg (μg/gr) | Source | ||
|---|---|---|---|---|---|
| Adult (men) | 80 kgr | 945 gr | 0.0013 μg/gr b.w | EFSA 2012, (last update 2018) | |
| Adult (women) | 60 kgr | 709 gr | |||
| Adolescents (12–18 years) | boys | 26.07–38.28 kgr | 308–452 gr | ||
| girls | 27.45–34.63 kgr | 324–409 gr | |||
| Children (5–11 years) | boys | 17.08–24.31 kgr | 202–287 gr | ||
| girls | 17.54–25 kgr | 207–296 gr | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyropoulou, A.; Laspidou, C.; Kormas, K.; Lazarou, Y.G. The Impact of Possible Mercury Source-Point Contamination in the Coastal Area of Skiathos Island. Environ. Sci. Proc. 2020, 2, 50. https://doi.org/10.3390/environsciproc2020002050
Spyropoulou A, Laspidou C, Kormas K, Lazarou YG. The Impact of Possible Mercury Source-Point Contamination in the Coastal Area of Skiathos Island. Environmental Sciences Proceedings. 2020; 2(1):50. https://doi.org/10.3390/environsciproc2020002050
Chicago/Turabian StyleSpyropoulou, Alexandra, Chrysi Laspidou, Kostantinos Kormas, and Yannis G. Lazarou. 2020. "The Impact of Possible Mercury Source-Point Contamination in the Coastal Area of Skiathos Island" Environmental Sciences Proceedings 2, no. 1: 50. https://doi.org/10.3390/environsciproc2020002050
APA StyleSpyropoulou, A., Laspidou, C., Kormas, K., & Lazarou, Y. G. (2020). The Impact of Possible Mercury Source-Point Contamination in the Coastal Area of Skiathos Island. Environmental Sciences Proceedings, 2(1), 50. https://doi.org/10.3390/environsciproc2020002050






