Cryptosporidium Species in Fish: An Update †
Abstract
1. Introduction
2. Cryptosporidium Species in Piscine Hosts
3. Cryptosporidium Zoonotic Species in Fish
4. Pathology
5. Conclusions
Author Contributions
Conflicts of Interest
References
- FAO Identifies Top 10 Foodborne Parasites. Vet. Rec. 2014, 175, 58.
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef]
- Reid, A.; Lymbery, A.; Ng, J.; Tweedle, S.; Ryan, U. Identification of novel and zoonotic Cryptosporidium species in marine fish. Vet. Parasitol. 2010, 168, 190–195. [Google Scholar] [PubMed]
- Koinari, M.; Karl, S.; Ng-Hublin, J.; Lymbery, A.J.; Ryan, U.M. Identification of novel and zoonotic Cryptosporidium species in fish from Papua New Guinea. Vet. Parasitol. 2013, 198, 1–9. [Google Scholar] [CrossRef]
- Certad, G.; Dupouy-Camet, J.; Gantois, N.; Hammouma-Ghelboun, O.; Pottier, M.; Guyot, K.; Benamrouz, S.; Osman, M.; Delaire, B.; Creusy, C.; et al. Identification of Cryptosporidium species in fish from lake Geneva (Lac Léman) in France. PLoS ONE 2015, 10, e0133047. [Google Scholar] [CrossRef] [PubMed]
- Couso-Pérez, S.; Ares-Mazás, E.; Gómez-Couso, H. Identification of a novel piscine Cryptosporidium genotype and Cryptosporidium parvum in cultured rainbow trout (Oncorhynchus mykiss). Parasitol. Res. 2018, 117, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Pellitero, P.; Quiroga, M.I.; Sitjà-Bobadilla, A.; Redondo, M.J.; Palenzuela, O.; Padrós, F.; Vázquez, S.; Nieto, J.M. Cryptosporidium scophthalmi n. sp. (Apicomplexa: Cryptosporidiidae) from cultured turbot Scophthalmus maximus. Light and electron microscope description and histopathological study. Dis. Aquat. Organ. 2004, 62, 133–145. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Padrós, F.; Aguilera, C.; Alvarez-Pellitero, P. Epidemiology of Cryptosporidium molnari in Spanish gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.) cultures: From hatchery to market size. Appl. Environ. Microbiol. 2005, 71, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.M.; Hoerr, F.J.; Carlton, W.W.; Hinsman, E.J.; Ferguson, H.W. Enteric cryptosporidiosis in a naso tang, Naso lituratus Bloch and Schneider. J. Fish Dis. 1981, 4, 425–428. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P.; Sitjà-Bobadilla, A. Cryptosporidium molnari n. sp. (Apicomplexa: Cryptosporidiidae) infecting two marine fish species, Sparus aurata L. and Dicentrarchus labrax L. Int. J. Parasitol. 2002, 32, 1007–1021. [Google Scholar] [CrossRef]
- Palenzuela, O.; Alvarez-Pellitero, P.; Sitjá-Bobadilla, A. Molecular characterization of Cryptosporidium molnari reveals a distinct piscine clade. Appl. Environ. Microbiol. 2010, 76, 7646–7649. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Paparini, A.; Tong, K.; Yang, R.; Gibson-Kueh, S.; O’Hara, A.; Lymbery, A.; Xiao, L. Cryptosporidium huwi n. sp. (Apicomplexa: Eimeriidae) from the guppy (Poecilia reticulata). Exp. Parasitol. 2015, 150, 31–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paparini, A.; Yang, R.; Chen, L.; Tong, K.; Gibson-Kueh, S.; Lymbery, A.; Ryan, U.M. Cryptosporidium in fish: Alternative sequencing approaches and analyses at multiple loci to resolve mixed infections. Parasitol. 2017, 144, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Palermo, C.; Chen, L.; Edwards, A.; Paparini, A.; Tong, K.; Gibson-Kueh, S.; Lymbery, A.; Ryan, U. Genetic diversity of Cryptosporidium in fish at the 18S and actin loci and high levels of mixed infections. Vet. Parasitol. 2015, 214, 255–263. [Google Scholar] [CrossRef]
- Costa, J.F.; Saraiva, A. Cryptosporidium cf. scophthalmi JFC-2015 18S Ribosomal RNA Gene, Partial Sequence. 2015. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KR340588.1 (accessed on 15 May 2020).
- Murphy, B.G.; Bradway, D.; Walsh, T.; Sanders, G.E.; Snekvik, K. Gastric cryptosporidiosis in freshwater angelfish (Pterophyllum scalare). J. Vet. Diagn. Investig. 2009, 21, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Zanguee, N.; Lymbery, J.A.; Lau, J.; Suzuki, A.; Yang, R.; Ng, J.; Ryan, U. Identification of novel Cryptosporidium species in aquarium fish. Vet. Parasitol. 2010, 174, 43–48. [Google Scholar] [CrossRef][Green Version]
- Morine, M.; Yang, R.; Ng, J.; Kueh, S.; Lymbery, A.J.; Ryan, U.M. Additional novel Cryptosporidium genotypes in ornamental fishes. Vet. Parasitol. 2012, 190, 578–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, R.; Dorrestein, G.M.; Ryan, U. Molecular characterisation of a disseminated Cryptosporidium infection in a Koi carp (Cyprinus carpio). Vet. Parasitol. 2016, 226, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Certad, G.; Follet, J.; Gantois, N.; Hammouma-Ghelboun, O.; Guyot, K.; Benamrouz-Vanneste, S.; Fréalle, E.; Seesao, Y.; Delaire, B.; Creusy, C.; et al. Prevalence, molecular identification, and risk factors for Cryptosporidium infection in edible marine fish: A survey across sea areas surrounding France. Front. Microbiol. 2019, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals—A one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Efstratiou, A.; Ongerth, J.E.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2011–2016. Water Res. 2017, 114, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Nader, J.L.; Mathers, T.C.; Ward, B.J.; Pachebat, J.A.; Swain, M.T.; Robinson, G.; Chalmers, R.M.; Hunter, P.R.; van Oosterhout, C.; Tyler, K.M. Evolutionary genomics of anthroponosis in Cryptosporidium. Nat. Microbiol. 2019, 4, 826–836. [Google Scholar] [CrossRef]
- Couso-Pérez, S.; Ares-Mazás, E.; Gómez-Couso, H. First report of Cryptosporidium molnari-like genotype and Cryptosporidium parvum zoonotic subtypes (IIaA15G2R1 and IIaA18G3R1) in brown trout (Salmo trutta). J. Parasitol. 2019, 105, 170. [Google Scholar] [CrossRef]
- McOliver, C.C.; Lemerman, H.B.; Silbergeld, E.K.; Moore, R.D.; Graczyk, T.K. Risks of recreational exposure to waterborne pathogens among persons with HIV/AIDS in Baltimore, Maryland. Am. J. Public Health 2009, 99, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, T.K.; McOliver, C.; Silbergeld, E.K.; Tamang, L.; Roberts, J.D. Risk of handling as a route of exposure to infectious waterborne Cryptosporidium parvum oocysts via Atlantic blue crabs (Callinectes sapidus). Appl. Environ. Microbiol. 2007, 73, 4069–4070. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P.; Perez, A.; Quiroga, M.I.; Redondo, M.J.; Vázquez, S.; Riaza, A.; Palenzuela, O.; Sitjà-Bobadilla, A.; Nieto, J.M. Host and environmental risk factors associated with Cryptosporidium scophthalmi (Apicomplexa) infection in cultured turbot, Psetta maxima (L.) (Pisces, Teleostei). Vet. Parasitol. 2009, 165, 207–215. [Google Scholar] [CrossRef]
- Baragahare, R.; Becker, J.A.; Landos, M.; Šlapeta, J.; Dennis, M.M. Gastric cryptosporidiosis in farmed Australian Murray cod, Maccullochella peelii peelii. Aquaculture 2011, 314, 1–6. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Alvarez-Pellitero, P. Experimental transmission of Cryptosporidium molnari (Apicomplexa: Coccidia) to gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.). Parasitol. Res. 2003, 91, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.; Ramos, M.F.; Barandela, T.; Sousa, J.A.; Rodrigues, P.N. Cryptosporidium sp. (Apicomplexa) from cultured turbot Psetta maxima. Bul. Eur. Assoc. Fish. Pathol. 2009, 29, 34–36. [Google Scholar]
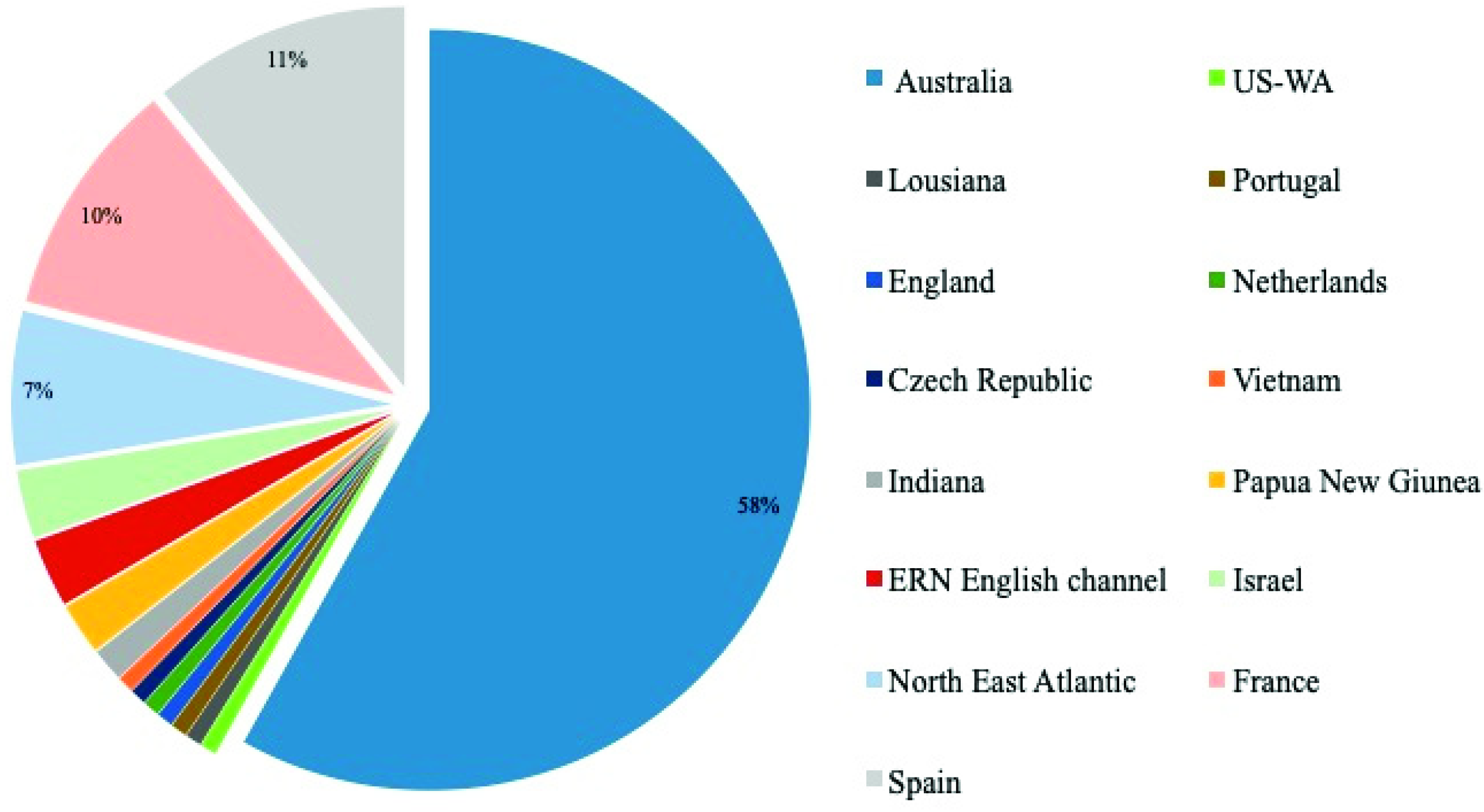


| Parasite Species | Host | Origin | Geog. Origin | References |
|---|---|---|---|---|
| C. nasorum | Naso tang Naso lituratus | M/O | Indiana USA | [10] |
| C. molnari | Gilthead sea bream Sparus aurata | M/C | Spain | [9,11,24] |
| European sea bass Dicentrarchus labrax | M/C | Spain | [9,11,24] | |
| Murray cod Maccullochella peelii | F/C | Australia | [25] | |
| Northern pike Esox lucius | F/W | France | [6] | |
| C. molnari-like | Bristle tooth tang Ctenochaetus tominiensis | M/O | Australia | [18] |
| Butter bream Monodactylus argenteus | M/O | Australia | [18] | |
| Madder seaperch Pseudodanthias dispar | M/O | Australia | [18] | |
| Golden algae eater Crossocheilus aymonieri | F/O | Australia | [18] | |
| Green chromis Chromis viridis | M/O | Australia | [18] | |
| Upside down cat fish Synodontis nigriventris | F/O | Australia | [18] | |
| Wedgetailed blue tang Paracanthurus hepatus | M/O | Australia | [18] | |
| Angelfish Pterophyllum altum | F/O | Australia | [15] | |
| Azure damsel Chrysiptera hemicyanea | M/O | Australia | [14,15] | |
| Goldfish Carassius auratus | F/O | Australia | [14,15] | |
| Guppy Poecilia reticulata | F/O | Australia | [15] | |
| Orange clownfish Amphiprion percula | M/O | Australia | [15] | |
| Oscar Astronotus ocellatus | F/O | Australia | [15] | |
| Peach anthias Pseudanthias dispar | M/O | Australia | [14,15] | |
| Red-striped angelfish Centropyge eibli | M/O | Australia | [14,15] | |
| Yellow-headed jawfish Opistognathus aurifrons | M/O | Australia | [14,15] | |
| Cod Gadus morhua | M/W | NEA | [21] | |
| Brown trout Salmo trutta | F/W | Spain | [26] | |
| C. scophthalmi | Turbot Scophthalmus maximus | M/C | Spain | [8,16] |
| Turbot Psetta maxima | M/C | Spain | [27] | |
| C. huwi | Guppy Poecilia reticulata | F/O | Australia | [13,14,15] |
| Golden tiger barb Puntigrus tetrazona | F/O | Australia | [13,14,15] | |
| Neon tetra Paracheirodon innesi | F/O | Australia | [13,14,15,18] |
| Genotypes | Host | Origin | Geog. Origin | References |
|---|---|---|---|---|
| PG 2 | Neon tetra Paracheirodon innesi | F/O | Australia | [18] |
| Oscar Astronotus ocellatus | F/O | [13,14,15,18] | ||
| Mullet Mugil cephalus | M/W | [15] | ||
| PG 3 | Mullet Mugil cephalus | M/W | Australia | [15] |
| PG 3-like | Goldfish Carassius auratus | F/O | [15] | |
| PG 4 | Golden algae eater Crossocheilus aymonieri | F/O | Australia | [18] |
| Kupang damsel Chrysiptera hemicyanea | M/O | [18] | ||
| Oscar Astronotus ocellatus | F/O | [18] | ||
| Neon tetra Paracheirodon innesi | F/O | [19] | ||
| Azure damsel Chrysiptera hemicyanea | F/O | [15] | ||
| Black ghost knife fish Apteronotus albifrons | F/O | [15] | ||
| Kribensis Pelvicachromis pulcher | F/O | [15] | ||
| PG 5 | Angel fish Pterophyllum scalare | F/O | Australia | [15,18] |
| Pterophyllum altum | ||||
| Butter bream Monodactylus argenteus | M/O | [15,18] | ||
| Golden algae eater Crossocheilus aymonieri | F/O | [18] | ||
| Black ghost knife fish Apteronotus albifrons | F/O | [15] | ||
| Blue tang Paracanthurus hepatus | M/O | [14,15] | ||
| Goldfish Carassius auratus | F/O | [15] | ||
| Guppy Poecilia reticulata | F/O | [15] | ||
| Mullet Mugil cephalus | M/W | [15] | ||
| Platyfish Xiphophorus maculatus | F/O | [15] | ||
| PG 6 | Guppy Poecilia reticulata | F/O | Australia | [18] |
| PG 6-like | Gold gourami Trichogaster trichopterus | F/O | [19] | |
| PG 7 | Red eye tetra Moenkhausia sanctaefilomenae | F/O | Australia | [19] |
| Neon tetra Paracheirodon innesi | F/O | [15] | ||
| PG 7-like | Neon tetra Paracheirodon innesi | F/O | [15] | |
| PG8 | Oblong silver biddy Gerres oblongus | M/W,M/O | Australia | [5,15] |
| G9 | Rainbow trout Oncorhynchus mykiss | F/C | Spain | [7] |
| NG | Azure damsel Chrysiptera hemicyanea | F/O | Australia | [14,15] |
| Sea mullet Mugil cephalus | M/W | [4] | ||
| Orange clownfish Amphiprion percula | M/O | [14,15] | ||
| Oscar Astronotus ocellatus | F/O | [15] | ||
| Platyfish Xiphophorus maculatus | F/O | [15] | ||
| Koi carp Cyprinus carpio | F/O | Netherlands | [20] | |
| NGC1 | Saithe Pollachius virens | M/W | NEA, EEC, NS | [21] |
| Blue ling Molva dypterygia | M/W | NEA | [21] | |
| NGC2 | Whiting Merlangius merlangus | M/W | NEA | [21] |
| Ling Molva molva | M/W | NEA | [21] | |
| NGC3 | Ling Molva molva | M/W | NEA | [21] |
| NGC4 | Blue ling Molva dypterygia | M/W | NEA | [21] |
| NGC5 | Saithe Pollachius virens | M/W | NEA, EEC, NS | [21] |
| Hake Merluccius merluccius | M/W | NEA | [21] | |
| NGC7 | Mackerel Scomber scombrus | M/W | EEC | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
E., G.; P., K. Cryptosporidium Species in Fish: An Update. Environ. Sci. Proc. 2020, 2, 13. https://doi.org/10.3390/environsciproc2020002013
E. G, P. K. Cryptosporidium Species in Fish: An Update. Environmental Sciences Proceedings. 2020; 2(1):13. https://doi.org/10.3390/environsciproc2020002013
Chicago/Turabian StyleE., Golomazou, and Karanis P. 2020. "Cryptosporidium Species in Fish: An Update" Environmental Sciences Proceedings 2, no. 1: 13. https://doi.org/10.3390/environsciproc2020002013
APA StyleE., G., & P., K. (2020). Cryptosporidium Species in Fish: An Update. Environmental Sciences Proceedings, 2(1), 13. https://doi.org/10.3390/environsciproc2020002013






