Abstract
An adapted sustainable management program was used to evaluate lettuce tolerance to salt stress using autochthonous biostimulants (arbuscular mycorrhizal fungi (AMF), plant growth-promoting rhizobacteria (PGPR), and compost). Salinity harmed plant growth, root colonization, and physiology. However, biostimulants application, especially AMF and PGPR treatments, significantly improved lettuce growth and salinity tolerance (120% and 50%, respectively, for biomass; 60% and 20%, respectively, for stomatal conductance; and 1.5% and 1.3%, respectively, for chlorophyll fluorescence) compared to non-inoculated and compost-free controls under stressed conditions.
1. Introduction
Salt-affected soils are constantly increasing worldwide, especially in arid and semi-arid areas. Salinity-related impacts include low agricultural productivity, low economic returns, and soil erosion; these threaten global food productivity and challenge the sustainability of cultivating crops under saline conditions. New directions towards natural biological resources (e.g., AMF, PGPR, and organic amendments) are promising and environmentally friendly strategies to improve the growth and development of plants under abiotic stress. Our objectives are the following: (1) mitigate the harmful effects of salinity by using biostimulants with indigenous plant-associated microbiomes and compost; and (2) understand the mechanisms underlying plant-microbe-compost interactions that confer salt stress tolerance.
2. Materials and Methods
2.1. Biological Materials and Cultivation Conditions
Four bacterial strains (Z1, Z2, Z4, and ER21) were used in this experiment, which were isolated from arid and semi-arid regions of southeast Marrakesh, Morocco. The bacterial suspension inoculation was carried out near the lettuce (lactuca sativa L.) roots. Similarly, the native fungal complex of AMF was isolated from the same region. This AMF consortium is based on a mixture of native species [1]. Inoculation of lettuce plants was performed by adding a quantity of the inoculum next to the root system of lettuce seedlings. The compost used underwent composting processes for 3 months, of which no other inorganic products were added. At the three leaves stage, lettuce seedlings were transplanted into plastic bags filled with sterilized soil. Plants were grown in a controlled greenhouse at 25.5 C (16/8 h light/dark) with supplemented light and average relative humidity of 68.5%.
2.2. Treatments and Study Design
The experimental design consisted of 12 treatments (10 replicates each): control, seedlings treated with AMF (M), PGPR (R), or compost (C) grown under 0, 50, and 100 mM NaCl. The salt stress was applied 15 days after transplantation. In order to avoid osmotic shock to the plant, NaCl doses were applied progressively.
2.3. Mycorrhization Parameters
After harvesting, root samples were washed with distilled water and treated according to the Phillips and Hayman method [2]. Mycorrhizal structures’ rate of root infection was assessed by microscopic observation (ZEISS, Model Axioskop 40) according to the technique described by Trouvelot et al. [3]. The mycorrhization frequency (MF) and intensity (MI) were calculated using the following equations:
2.4. Growth Assessment
The growth performance of lettuce plants was assessed by measuring the total dry matter (TDM; obtained after drying the samples at 80 °C until the weight remained constant).
2.5. Photosynthetic Efficiency and Gas Exchanges
Chlorophyll fluorescence was measured by a fluorometer (OPTISCIENCE, OS30p). Dark adaptation was made on the upper side of the leaf by obscuring for 30 min. This parameter was measured by transmission at 650 nm on a leaf area of 12.5 mm2. The fluorescence signal was recorded at an acquisition speed of 10 ms for a second. Stomatal conductance (gs) was measured using a porometer system (Leaf Porometer LP1989, Decagon Device, Inc., Washington, DC, USA).
3. Results
3.1. Mycorrhizal Symbiosis
Our results showed that no mycorrhizal structure was observed in the roots of non-treatment controls nor PGPR- and compost-treated plants. AMF infection frequency decreased significantly under 100 mM NaCl (Figure 1A). Mycorrhizal lettuce roots showed the lowest root colonization intensity under salt stress conditions (Figure 1B).
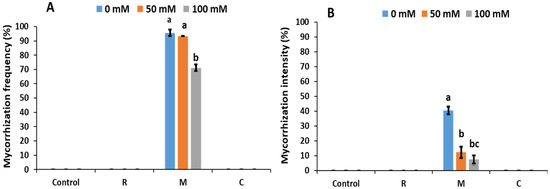
Figure 1.
Influence of salinity levels (0, 50, and 100 mM NaCl) on (A) mycorrhization frequency and (B) intensity in lettuce control plants (non-amended, non-inoculated), and plants inoculated with plant growth promoting rhizobacteria (R), or arbuscular mycorrhizal fungi (M), or amended with composts (C). Data are mean ± SE of 10 biological replicates. Means followed by the same letters are not significantly different at p < 0.05 (Tukey’s HSD).
3.2. Growth Assessment, Photosynthetic Efficiency and Gas Exchanges
The application of biostimulants improved TDW compared to untreated control, independently of salt levels (Figure 2A). Total biomass was significantly affected by salt stress. The 100-millimolar NaCl treatment reduced this parameter substantially. R and M treatments improved the biomass under moderate salt stress (50 mM NaCl), while only M enhanced this trait under 100 mM NaCl compared to the control plants.
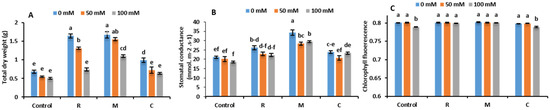
Figure 2.
(A) total dry weight, (B) stomatal conductance and (C) chlorophyll fluorescence of lettuce plants grown without (0 mM NaCl) or with (50 and 100 mM NaCl) salt stress, and subjected to different biostimulant treatments. Control: untreated plants, R: inoculated with PGPR, M: inoculated with arbuscular mycorrhizal fungi, C: amended with compost. Data are mean ± SE of 10 biological replicates. Means followed by the same letters are not significantly different at p < 0.05 (Tukey’s HSD).
The overall results revealed that salinity caused a significant decline in stomatal conductance (gs) in all treatments and chlorophyll fluorescence (Fv/Fm), both in controls and compost-treated plants under 100 mM NaCl. Application of biostimulants, especially M and R, significantly improved gs, independently of the salinity (Figure 2B). Under 100 mM NaCl, plants inoculated with R or M yielded an improvement in Fv/Fm compared to untreated plants (Figure 2C).
4. Discussion
The ability of plants to tolerate salinity stress is usually evaluated in terms of biomass produced, and several studies have highlighted that biofertilizers impart salinity tolerance in host plants by virtue of higher biomass compared to untreated plants. Our results showed that M and R were more effective, under both normal and salt stress conditions, despite the reduction of AMF root colonization (Figure 1). This reduction might be due to the adverse effects of salinity on both the host plant and fungal growth, and/or on establishing arbuscular mycorrhiza. Our results align with the study of Santander et al. [4], which showed that mycorrhizal infection and intensity decrease when host plants are exposed to salt stress.
Native AMF or PGPR strains were very effective in helping lettuce attenuate salt stress’ detrimental effects on growth and photosynthetic machinery. In the absence of biofertilizers, lettuce biomass production was negatively affected by increasing NaCl stress levels (Figure 2). This reduction in untreated plants is a result of both the osmotic phase during which growth inhibition is caused by the difficulty for the plant to absorb water, and an ionic phase due to the toxic effect of the salt within the plant, observed with the higher Na+ and Cl-. Interestingly, we found that AMF, or PGPR, or organic amendments significantly promoted lettuce biomass under both control and saline conditions as compared to the untreated controls. This could be justified by the stimulation of phytohormones that accelerate cell division processes [5], improving nutrient acquisition (especially P) and ionic homeostasis, plant growth, photosynthesis machinery, osmoregulation, water status, alteration in root architecture, and/or protection against ROS-induced oxidative stress [6]. Salt-treated plants’ lowered leaf area, coupled with a decrease in stomatal and mesophyll conductance, limits CO2 availability and assimilation, which results ultimately in decreased CO2 supply to RuBisCO, and/or accumulation of excess energy; this in turn leads to increased accumulation of electrons in the thylakoid membranes. Our data showed that AMF and PGPR bolster these mechanisms and alleviate the negative effects of salinity on plant photosynthetic capacity by increasing the gs and net photosynthetic rate, resulting in more quantum yield (Fv/Fm) compared to untreated control plants.
5. Conclusions
Inoculation with selected AMF and PGPR, or amendment with organic compost, can improve the performance of lettuce under salt stress conditions. Data imply that AMF, PGPR, or compost deploy an array of biochemical and physiological mechanisms that act in a concerted manner to provide more salinity tolerance to the host plant. Altogether, the use of biostimulants is considered to be an efficient approach for bio-amelioration of salinity stress.
Author Contributions
A.M. and M.B. designed and supervised the research. R.O., R.B.-L. and A.S. performed the experiments and carried out the analyses. R.O., R.B.-L., M.A. and A.B. performed the data analysis, interpretation and contributed to analytic tools. A.M., M.B. and K.O. contributed to the conception and design of the work. R.O., R.B.-L. and M.A. wrote the manuscript. A.M., M.A. and A.B. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the FOSC project (Sus-Agri-CC) from the European Union’s Horizon 2020 research and innovation program under grant agreement N°862555 and Grant-in-Aid for Early-Career Scientists to MB (JSPS KAKENHI Grant Number 20K15425).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. Mycorhizes Physiol. Génétique 1986, 217–220. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Cartes, P.; Vidal, G.; Cornejo, P. Aquaporins and cation transporters are differentially regulated by two arbuscular mycorrhizal fungi strains in lettuce cultivars growing under salinity conditions. Plant Physiol. Biochem. 2021, 158, 396–409. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ben-Laouane, R.; Baslam, M.; Ait-El-mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S.; et al. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).