3.1. Vegetation Diversity and Composition
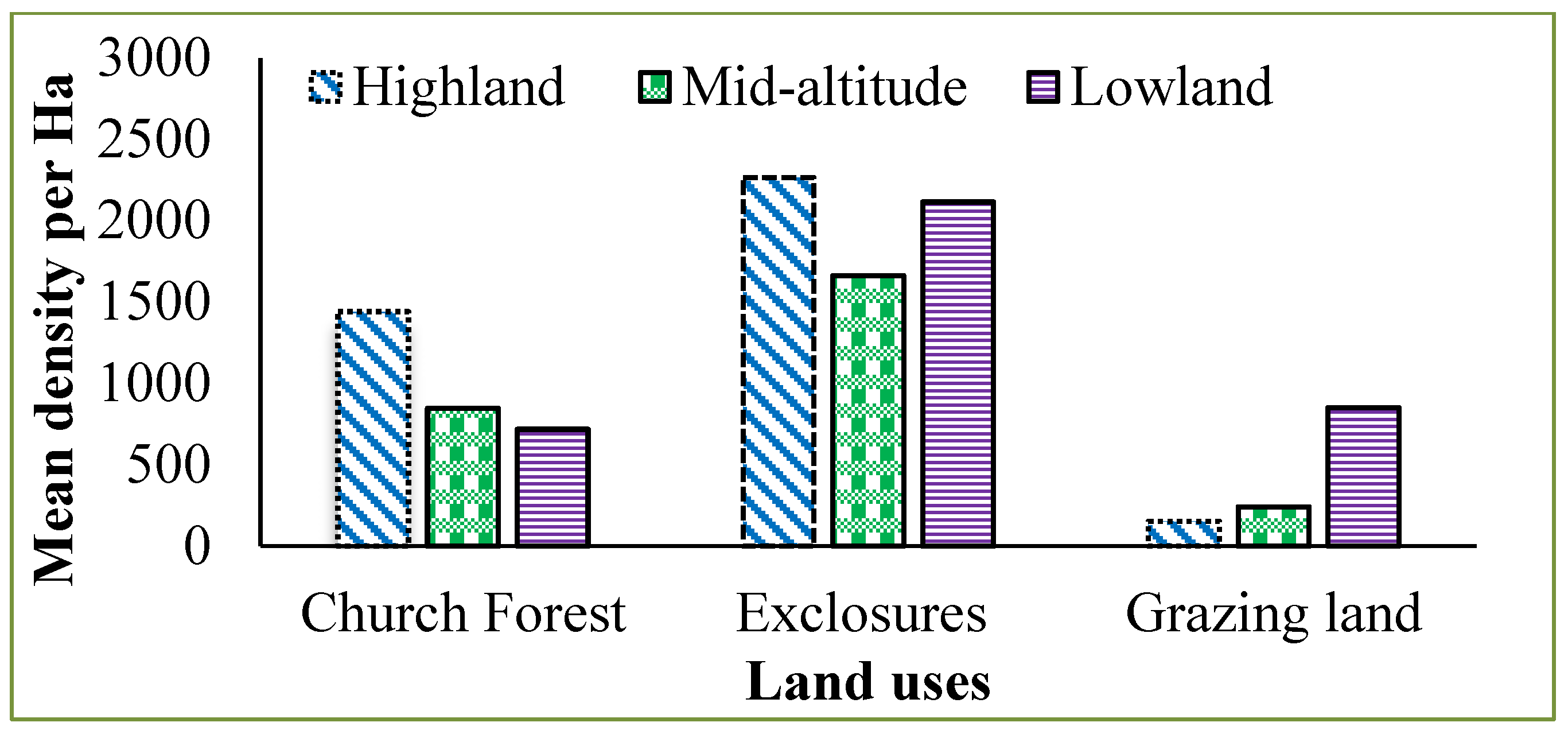
High woody species density (1660–2265 stem ha
−1) in exclosures was recorded, followed by church forests (717–1440 stem ha
−1) and grazing lands (152–850 stem ha
−1) (
Figure 2,
Appendix A Table A1). The exclosure has better stem density than church forest and grazing land. This is because of the open space, which provides favorable conditions for the regeneration of light-demanding species and means there is no competition for light due to upper strata vegetation; thus, scrub vegetation starts to grow. As a result, the number of stems increases in exclosures.
Therefore, excluding open grazing land from livestock and human interference is a better strategy for natural regeneration. The better natural regeneration facilitates forest restoration. This idea is similar to that of [
22] in Tigray and northern Ethiopia; [
23] in northeast Ethiopia and South Wollo; and [
24] in central Ethiopia and north Shoa, who found that area exclosures increase the vegetation density. All above scholars approved that excluding livestock from open grazing land in an area exclosure increases natural regeneration, leading to natural forest restoration. Overgrazing (browsing and trampling) destroys the newly emerged seedlings and saplings. There is a similar argument [
25] in a review of works on effects of area exclosures in different parts of Ethiopia, in which exclosure recovers vegetation better than open grazing lands over 5 to 10 years of the exclosure. Furthermore, many studies [
26,
27,
28] argued without reservation that wood density, diversity, and regeneration of vegetation recovered after the area was excluded from anthropogenic disturbances.
The highest number of species (7–16) was recorded in exclosure forest, followed by church forest (11–12) and open grazing land (3–5). The number of species was low at mid-altitude but similar at lowland and highland areas (
Figure 3).
Species diversity was high (0.78–2.27) in church forests, followed by exclosures (1.1–1.73) and open grazing lands (0.48–0.8). The dominance and evenness index is highest in church forests, followed by exclosures and open grazing lands (
Table 2).
Exclosures have the highest species richness compared with church forests and grazing lands. This is because in exclosures there is open space, and low trampling and other disturbances. Therefore, the dormant species from the soil seed bank start to regenerate. On the other hand, there is seed dispersal by wind and wild animals from near-natural forests or church forests.
According to [
11] species diversity index, the church forests have a good range (2 to 2.4) and exclosures have a medium-range (1 to 1.5), but open grazing lands are below the minimum range (<1) of species diversity index (
Table 2). [
29] evenness index ranged from zero to one, where close to one means all species evenly distributed, while close to zero means few dominant species control the community. In exclosure and church forest species, evenness is close to one, which means the species have a chance for special distribution; however, in open grazing lands, evenness indexes are close to zero, at which few highly stress-resistant species are dominant.
In church forests, light-demanding species have no chance to germinate because of the close space of the upper canopy. In exclosures, there is enough open space; thus, light-demanders start to germinate. Therefore, exclosures have optimum species diversity, which may increase with the age of exclosures, but the upper canopy will still be closed. In open grazing lands, there are few dominant species presented that resist grazing stress. Thus, species diversity is very low. Similarly, [
29] conclude that in the northern highlands of Ethiopia, species diversity increases in open grazing land from 0.5 to 1.8 after exclosures. This idea is also supported by [
30] in central and northern highlands, who showed that exclosures have twice the species diversity of open grazing land. Therefore, the species at dormancy in open grazing land regenerate due to seed dispersal by wildlife after the area is enclosed. Thus, enclosed open grazing land increase the species diversity and species richness. Many studies approved [
26,
27,
31,
32,
33,
34,
35] that species diversity and species richness increased after the degraded forest area was excluded for overexploitation.
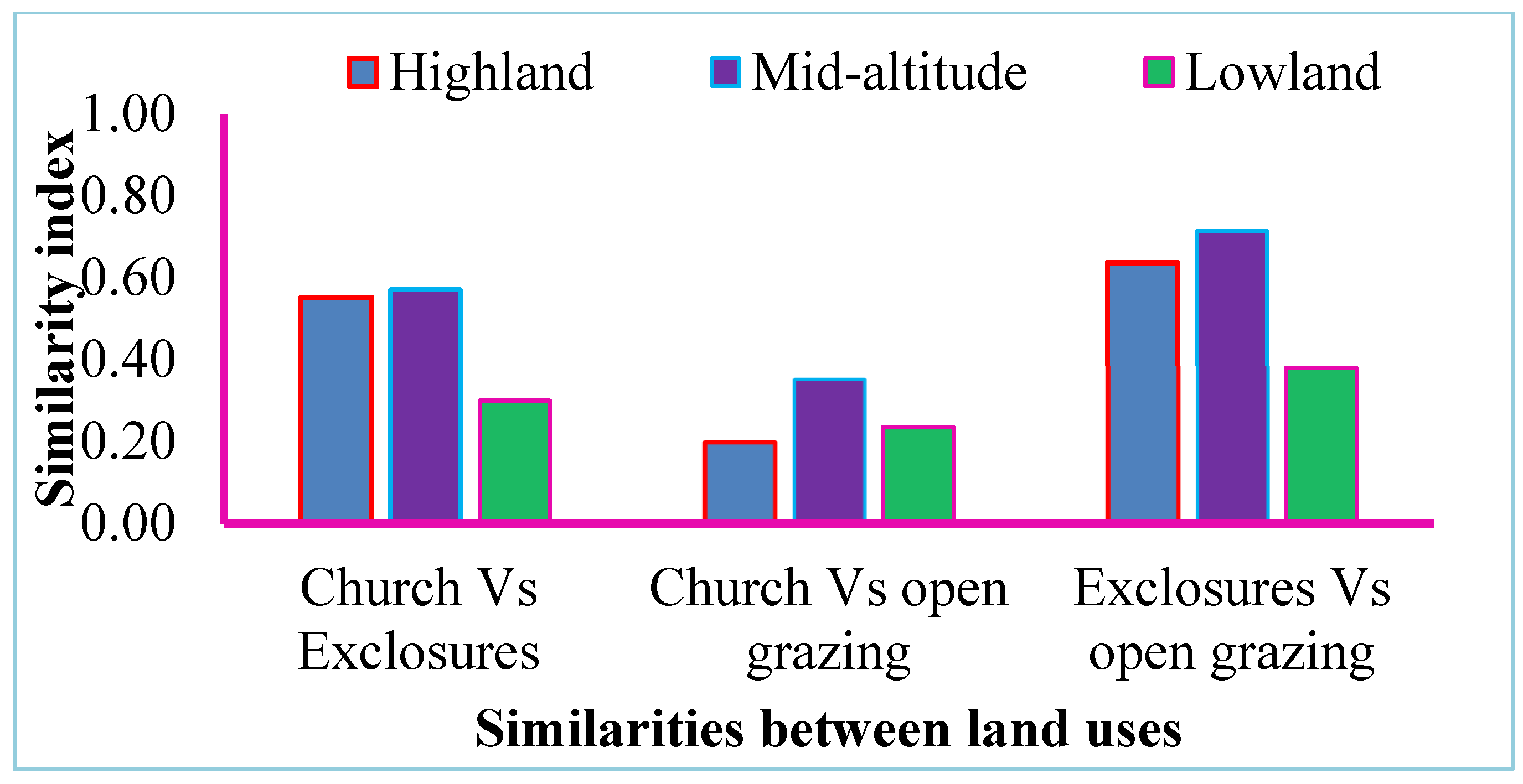
The highest species similarity is between exclosures and open grazing lands (0.4–0.8), followed by church forests and exclosures (0.3–0.6). High species similarity was also recorded at mid-altitude areas (0.6–0.8), followed by highland (0.24–0.6) and lowland areas (0.24–0.4) (
Figure 4).
The similarity index ranges from 0 to 1; close to one means there is high similarity and close to zero means there is low similarity [
12]. The similarity values of church forests vs. exclosures and exclosures vs. open grazing lands at mid-altitude and highland areas are close to one. However, in lowland areas, the similarity between church forests and exclosures is close to zero. The enclosed forest has species similarity midway between church and open grazing land, as the trajectory from degraded grazing land area references adjacent church forest in highland and mid-altitude areas but not in lowland areas. After exclosure, the species become regenerated and have a trajectory to the nearest protected forest from the seed bank and the composition close to the church forest, leading to dissimilarity with grazing lands. This is because, in grazing lands, the forest becomes continuously degraded and its composition is continuously lost. This idea is similarly argued in the literature [
27,
36,
37,
38], where the similarity of species composition is closely related to disturbances, management, and close latitudinal location; specifically, the similarity of species composition of area exclosures correlates with the nearest reference site.
3.2. Population Structure and Regeneration Status
In highland areas,
Junipers procera and
Olea europea are the dominant species in churches with inverted J-shaped population structures, while in exclosures,
Dodonia angostifolia and
Rhus glotinos are the dominant species having hump-shaped (unimodal) population structures. Similarly, the overall church forest in the highland area has an inverted J-shaped population structure (
Table 3). There are no dominant species in grazing land. However, their overall population structure shows a J-shaped structure, having only a few big trees with no seedling and sapling population.
The lower DBH population of the species is found where there is open space, while in the dense forest inside the church forest there is low regeneration and only big trees are present. The seedlings and saplings of these species are found at the border where there is open space. These species are light-demanders; therefore, the regeneration is only at the border and open space.
Rhus glutinosa and
Dodonia angostifolia are dominant species in exclosures in highland areas.
Dodonia angostifolia is a pioneer species that regenerates first in exclosures. This shows that the species are regenerated from soil seed bank or dispersal after the area is enclosed. All species in exclosures at the highland areas have an inverted J-shape structure, showing that most populations are at the sapling stage (
Table 3). This is due to open space, conditions being favorable for light-demanders, and exclosures being young. In grazing land in the highland areas, there are few stressed trees in the DBH range of 14–20 cm. The population structure is a J-shape structure indicating low regeneration (
Table 3). The regeneration may be affected by grazing disturbances.
In mid-altitude areas,
Dodonia angostifolia and
Olea europea were dominant species in the churches with inverted J-shape population structures. Overall, the church forest in mid-altitude areas has an inverse J-shape structure. In churches, most populations are found in DBH range 3–20 cm and there are few big trees (
Table 4).
In exclosures,
Dodonia angostifolia is the only dominant species having a J-shaped structure (
Table 4). In exclosures, most trees are found in the 3–4 cm DBH range, which are newly regenerated after an area is enclosed. However, there are no dominant species in grazing land.
Acacia etbaica and
Euclea divinorum are remnants of shrubs in grazing land, which resist the grazing and other disturbance stresses (
Table 4).
In lowland areas,
Diospyros mesifiliformis and
Oncoba spinosa were the dominant species in churches with a J-shape population structure. Most trees in the church and grazing land have DBH > 10 cm. This shows that there is low regeneration in churches and grazing land (
Table 5), while in exclosures, Acacia asks and Adansonia digitata were the dominant species.
Acacia ask has an inverted J-shaped structure and Adansonia digitata has a J-shape structure.
In lowland areas, there is low regeneration in exclosures. The lowland grazing has an inverted J-shape structure (
Table 5). There are only a few big trees without seedling and sapling populations in grazing lands; this means there is no regeneration in open grazing land.
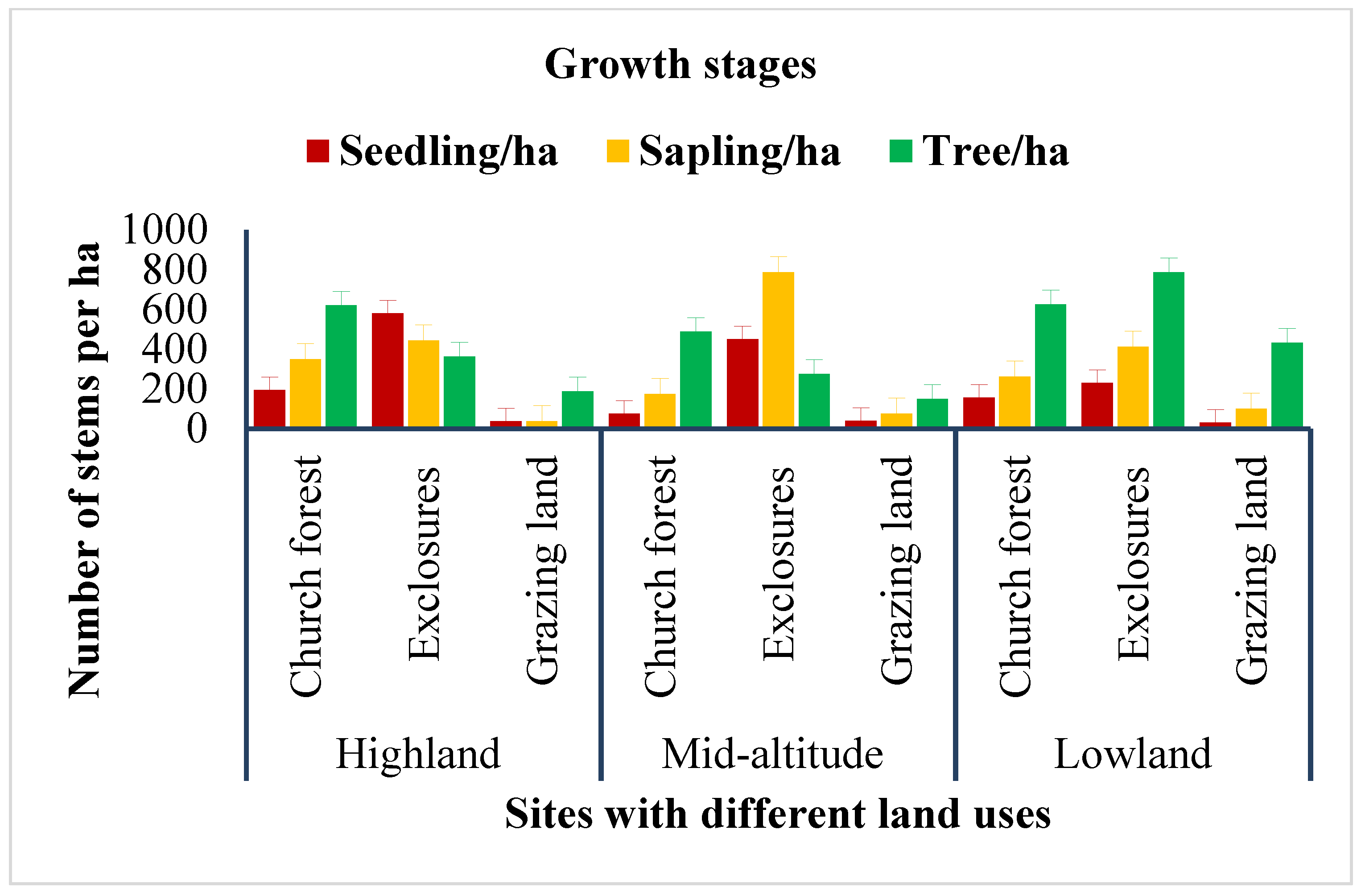
In terms of regeneration, exclosures have high seedling and sapling populations while church forests and grazing lands have low seedling and sapling populations. At highland areas, church forests have J-shaped, exclosure forests have inverted J-shaped, and open grazing lands have J-shaped population structures. At mid-altitude areas, the same trend to highland areas is followed, but at exclosures, there is a high sapling population. In lowland areas, the regeneration status is very low; this means a very low seedling population and, thus, the population structure is J-shaped (
Figure 5).
At churches, the upper canopy affects the regeneration, so the population is only competent trees. It is an indicator of an unbalanced community.
Junipers procera and
Olea european are dominant species in most church forests in the highlands of Ethiopia. However, there is low regeneration because of the low open space and high trampling effect of livestock. This idea is similar to that of [
39], who found that in highland parts of North Wollo,
Junipers procera and
Olea european are common dominant species having J-shaped structures. There are only big trees in dense forest, and there are low seedling and sapling populations. In exclosures, the population structure is an inverted J-shape structure, which means there is a high population of seedlings and saplings. This is an indicator of a healthy community. The exclosure was open grazing land before its establishment. After the exclosure was made, the stressed vegetation started to grow and support natural regeneration as a nurse tree. The space is open, helping to regenerate light-demanders. Thus, the population of exclosure is in the order of seedling > sapling > tree. This idea is supported by [
30] in degraded hillsides of central and northern Ethiopia, [
22] in northern Ethiopia, and [
23] in northeast Ethiopia, showing that the population structures follow an inverted J-shape if there are no livestock interferences, meaning they have been properly protected and managed as area exclosures. Therefore, an area exclosure restores the normal and healthy community after the open common grazing land is excluded from livestock and human interference. Vegetation structure and population status are the key determinant indicators of given forest resources that indicate the health and integrity of the forest ecosystem. Many studies [
25,
38,
40] use this indicator to evaluate the trajectory of area exclosures biophysically. Vegetation structure and population status recover after area exclosure, indicating that the conservation goal of restoration has been achieved. The idea is similar to those of previous studies [
36,
41], where after the degraded forest is enclosed, the vegetation structure become an inverted J-shape with ample natural regeneration.
3.3. Biomass
The highest WBM (613–2594-ton ha
−1) was recorded in lowland, followed by highland (8.7–148.5-ton ha
−1) and mid-altitude (9.9–47.13-ton ha
−1). In terms of land use, the WBM was high in church forests (47.13–2594.5-ton ha
−1), followed by exclosures (12.3–613.4-ton ha
−1) and grazing lands (8.7–821.3-ton ha
−1) (
Table 6).
The highest biomass in lowland areas is due to big trees such as
Adansonia digitata L. having a high diameter of up to 178 cm. This tree increases the basal area and biomass in grazing land and exclosures. Additionally,
Acacia asak is dominantly grown in exclosures and open grazing land, where the thorn in the lowland contributes to high biomass in the lowland. The highest litter, grass, and herb biomass (1.35–2.3 t ha
−1) were recorded in church forests, followed by exclosures (1.42–1.96 t ha
−1) and grazing lands (0.57–0.99 t ha
−1). In terms of agro-ecology, the highest LHG was recorded in highland (0.57–2.3 t ha
−1)
, followed by mid-altitude (0.99– 2.1 t ha
−1) and lowland areas (0.75–1.35 t ha
−1) (
Table 6 and
Appendix A Table A2).
In highland and mid-altitudes, the area is much degraded and almost no big trees remain in open grazing land; after exclosures,
Dodonia angostifolia as a pioneer species start to grow to have less diameter and low biomass. Even if in this condition, the woody biomass in exclosures is intermediate between churches and grazing lands. This means excluding open grazing land contributes to the restoration of biomass flow from vegetation to the soil. [
31], in northern Ethiopia, stated the same findings that the aboveground biomass measured inside the exclosures was more than twice that of the adjacent grazed areas and more biomass was produced from the young than the old exclosures. [
42] also stated a similar idea that woody biomass increased with exclosure age, while grass biomass carbon slightly decreased because of canopy cover after a well-developed community. [
37] also stated aboveground biomass and carbon increased following the establishment of exclosures on communal grazing land. [
43] explained that aboveground vegetation biomass across sites follow the order of area exclosures > open grazing land.
In exclosures, the grass and herbaceous species contribute to high LHG biomass, while in church forests, litter fall contributes to the biomass. However, in open grazing lands, the grass, litter, and herbs are browsed by livestock; thus, biomass is lower in highland and mid-altitude areas. [
44] obtained similar findings that litter biomass increases with exclosure age in Northern Ethiopia, Tigray after the open grazing land was excluded from livestock and human interferences.
This confirms that vegetation biomass recovers continuously and close to the nearest reference forest better than the open grazing land after the degraded forest is closed with passive restoration. There are several similar arguments [
37,
41,
42,
45,
46,
47,
48] in different parts of the world supporting that overall biomass and carbon pool increase with the age of the restoration and become comparable with the nearest protected natural forest.
3.4. Soil Attributes of Area Exclosures
There were highly significant differences in sand (
p = 0.008), clay (
p = 0.000), and loam (
p = 0.000) contents between different land uses. However, there was no significant difference in sand, clay, and loam contents of soil in different agro-ecologies (
Table 7). The highest mean clay content was recorded in church forests (6.8%), followed by exclosures (6%) and grazing lands (4%). The highest mean sand content was recorded in grazing lands (88.3%), followed by exclosures (87.4%); church forests had the least sand content (79.56%). The highest mean loam content was recorded in church forests (13.5%), followed by grazing lands (7.2%) and exclosures (6.5%) (
Table 7 and
Appendix A Table A3).
Based on clay, sand, and loam content proportions of the soil, highland areas for all land uses have loam sand textural class. Church forests have loamy sand textural class in all agro-ecologies. In mid-altitude and lowland areas, area exclosures and grazing land have a sandy textural class. Sand, clay, and loam content of the soil increase from grazing land to church forest. However, the sand content of the soil decreases from grazing land to church forest. This tells us the exclosure practices increase the soil clay and loam content from its litterfall and under vegetation decomposition. This is due to the organic matter increment in vegetation-covered church areas and area exclosures. This idea is similar to that of [
49], suggesting that soil organic matter has a habit of increasing the clay and silt content of the soil under vegetation-covered areas. This is due to two mechanisms: first, unions between the surface of clay particles and organic matter delay the decomposition process; then, soils with higher clay content increase the potential for aggregate formation. Under similar climate conditions, the organic matter content in fine-textured (clayey) soils is two to four times that of course-textured (sandy) soils [
49]. Based on [
50] findings, in northern parts of Ethiopia, the sand content reduced after area exclosure but the clay and silt contents of soil increased slightly with the age of exclosure. [
51] in northern Ethiopia concluded a similar idea that sand content of the soil reduced with area exclosure practices while silt and clay content increased after the area was enclosed.
There was a significant difference in pH across land uses and agro-ecologies (
p > 0.05) (
Appendix A Table A2); however, there was no significant difference between soil depths. The highest pH was recorded in lowland areas (6.9–8.4), followed by highland (6.6–7.6) and mid-altitude areas (5.6–7.6) (
Table 8).
Exclosure forests have higher pH than others in highland areas. This is supported by [
52], where after 7 years of exclosures, soil pH increased from 6 to 7.3. This idea was disproved by [
53], who found that closed areas have lower pH than open grazing land; this is because of vegetation cover. Vegetation cover allows litter decomposition, which leads to high infiltration because of improved soil organic matter and physical characteristics. These leached bases percolate down deep into the soil; the topsoil remains acidic and the pH becomes lower. However, high pH of up to 8.4 was recorded in churches with good vegetation cover in lowland areas due to the presence of buffering compounds such as carbonates. This is based on [
54], who reported that carbonate compounds increase the soil pH to 8.5. This is why in high vegetation cover areas, there is high organic carbon with negative charges. These negative charges attract the positive cations (basic compounds) such as calcium and make carbonate compounds.
Based on [
21] soil critical value, the soil pH of church forest, exclosure, and open grazing land are almost neutral, with pH ranging from 6.8 to 7.3. Therefore, there is no soil pH change with area exclosures in all agro-ecologies. This may be due to the age of exclosures or their need for more time to moderate soil pH. [
55] have proven three soil pH ranges as follows: a pH < 4 indicates the presence of free acids, generally from oxidation of sulfides; a pH < 5.5 suggests the likely occurrence of exchangeable Al; and a pH from 7.8 to 8.2 indicates the presence of CaCO
3. Based on this, our results fall in the third (7.8 to 8.2) range, i.e., the soil of the study areas ranged from neutral to slightly alkaline.
Church forests show a significant difference (
p = 0.02) in soil organic carbon and soil organic matter across depths in all agro-ecologies. There was a significant difference in SOC between land uses (
p = 0.003) in all agro-ecologies (
Appendix A Table A2). The highest SOC was recorded in church forests (0.58–2.9%), followed by exclosures (0.13–2.27%) and open grazing lands (0.5–1.1%) (
Figure 6).
Based on [
20,
21] soil critical values, our SOC and SOM at church forest have medium organic carbon (2.1–4.2%) at highland and mid-altitude areas but high at lowland areas (4.3 to 5). Exclosures have low SOC and SOM in all agro-ecologies. Open grazing land has very low SOC and SOM in all agro-ecologies. This shows that exclosures have SOM and SOC contents, which are transitional between church forest and open grazing land. Therefore, exclosure contributes to the development of soil organic matter, which is important for soil fertility and soil biology. This idea is alike to [
53] in the West Hararghe Zone of Oromia that there is high soil organic carbon in exclosures. Thus, exclosure practice substitutes the loss of soil by erosion, overexploitation, and aboveground biomass deduction by consequent grazing.
In [
56], soil organic matter increased with the age of exclosures after exclosures were developed in northern Ethiopia. This means vegetation restoration leads to biomass production increases and, subsequently, soil productivity increases. This idea is shown by [
55], where many soils—specifically those under the forest—have good organic soil materials at the surface (defined as containing >20% organic carbon), also called forest floor or litterfall. This is why the most recently deposited, relatively undecomposed foliage, twigs, etc. are present on the surface. In general, SOM is a large and active component of the global carbon cycle, containing three times the amount of carbon contained in the earth and twice the carbon contained in the atmosphere.
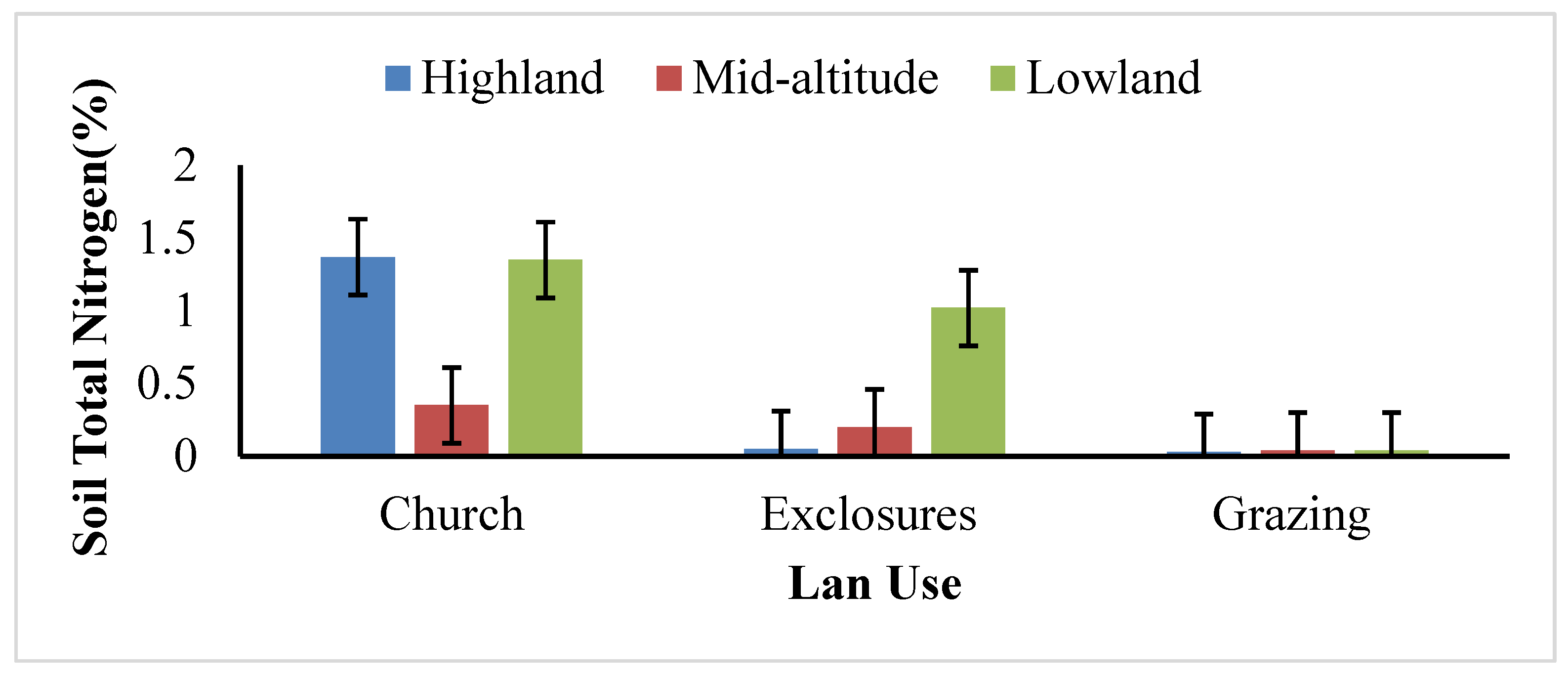
There was a highly significant difference (
p = 0.000) in Total Nitrogen (TN) between land uses and agro-ecology (
Appendix A Table A2). Nevertheless, there was no significant difference in TN between soil depths in all agro-ecologies. The highest TN was recorded in highland, followed by lowland and mid-altitude. In terms of land uses, church forests have the highest TN, followed by exclosures and open grazing lands (
Figure 7).
Based on [
21] soil critical value, church forests have very high TN in highland and mid-altitude areas but very low TN in lowland areas. On the other hand, exclosures have low TN in all agro-ecologies. Compared with church forests and exclosures, open grazing lands have very low TN in all agro-ecologies. This shows that, after exclosures, there was nitrogen fixation in the soil. This idea is similar to [
53], who reported that TN is increased slightly after exclosures. Thus, the enclosed forest has TN in the intermediate of open grazing lands and church forests.
Ref. [
52] discussed the fact that TN and SOC in the exclosures forest have no difference with open grazing land in 7-year exclosures in northern Ethiopia. This is why regaining this type of element in the soil needs more time after area exclosure.
There was no significant difference in available phosphorus (AvaP) across land uses and soil depth in all agro-ecologies. Nevertheless, there was a significant difference in AvaP across different agro-ecologies (
p < 0.05) (
Table 2). The highest values were recorded in church forests (6.3–38.81 ppm), followed by exclosures (2.46–14.9 ppm) and open grazing lands (3.1–14.6 ppm). In terms of ago-ecology, highland areas had the highest values (4.36–38.81 ppm), followed by mid-altitude (3.22–24.6 ppm) and lowland areas (2.46–21.8 ppm) (
Table 8).
Based on [
21] soil critical value, AvaP (ppm) in church forest is low at highland, optimum at mid-altitude, and very low in lowland areas. In exclosures, AvaP (ppm) is low in highland areas and very low in mid-altitude and lowland areas. Exclosures exhibited a trajectory of nutrient build from open grazing to church (reference) in soil restoration. This is similar to the findings of [
44] in the northern highlands of Tigray, where the AvaP in the enclosed forest (2.95 ppm) increased from open grazing lands (1.28 ppm) to church forests (10 ppm). However, according to [
52], when the area exclosures age increases, there is high nutrient cycling; then, AvaP decreases with soil and accumulates in the wood growing system.
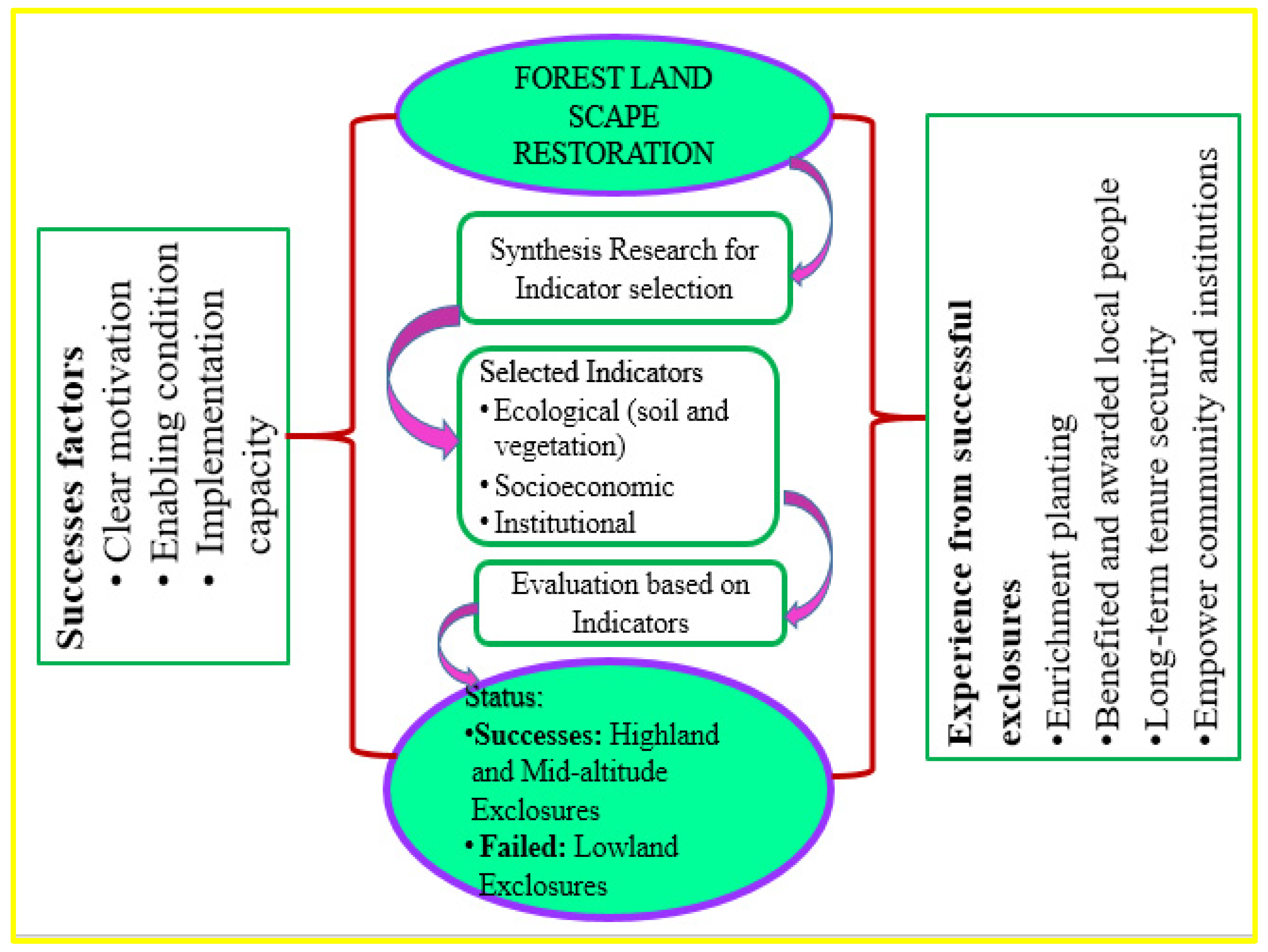
Finally, the conceptual framework for the evaluation of forest landscape restoration was developed (
Figure 8).