Agrochemicals and Shade Complexity Affect Soil Quality in Coffee Home Gardens
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Soil Sample Analysis
2.3. Data Analysis
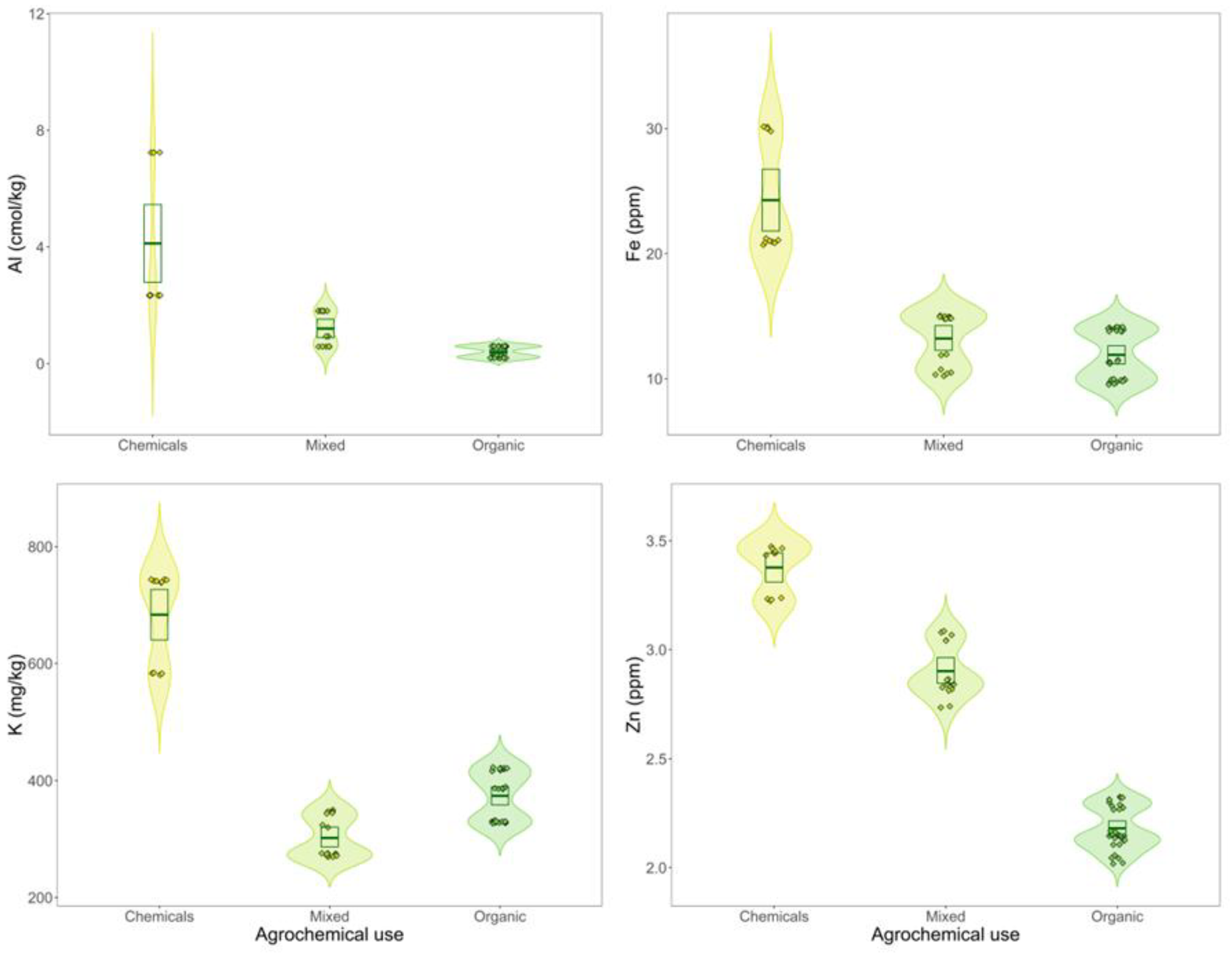
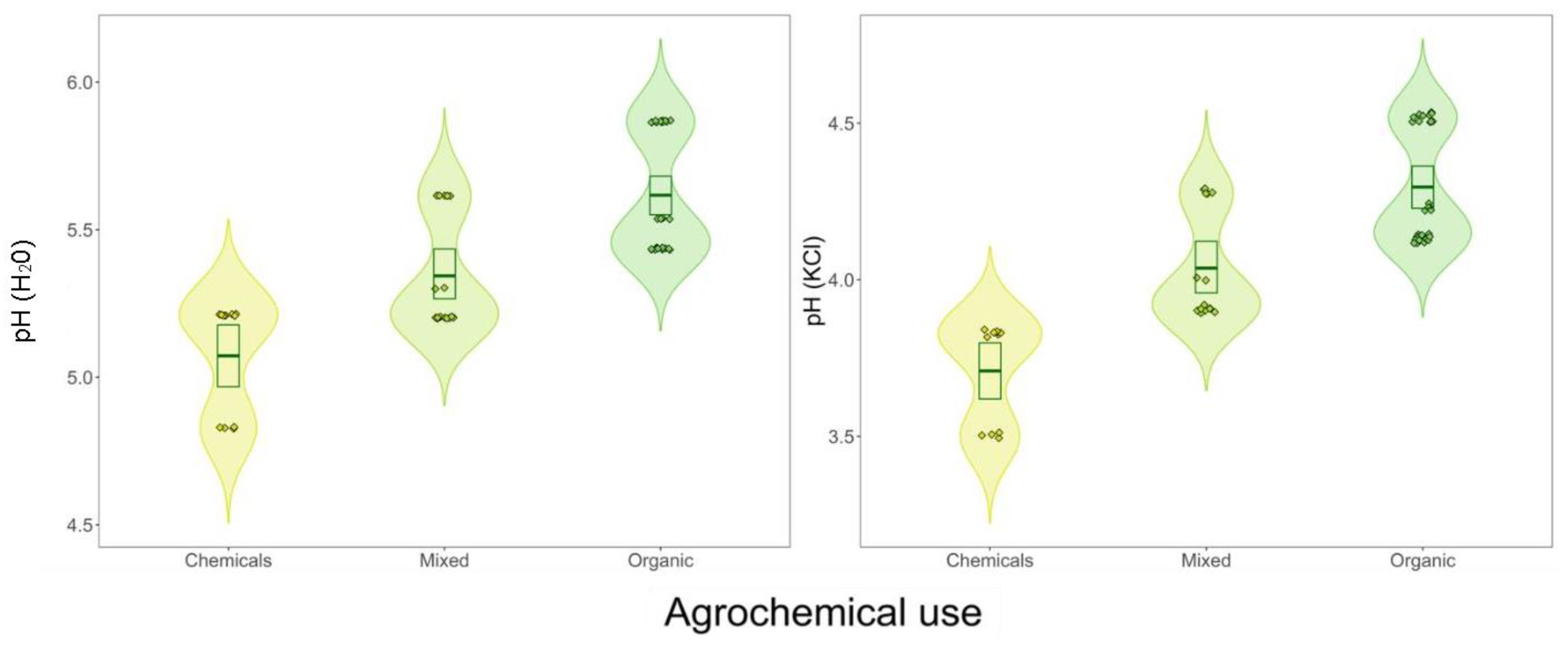
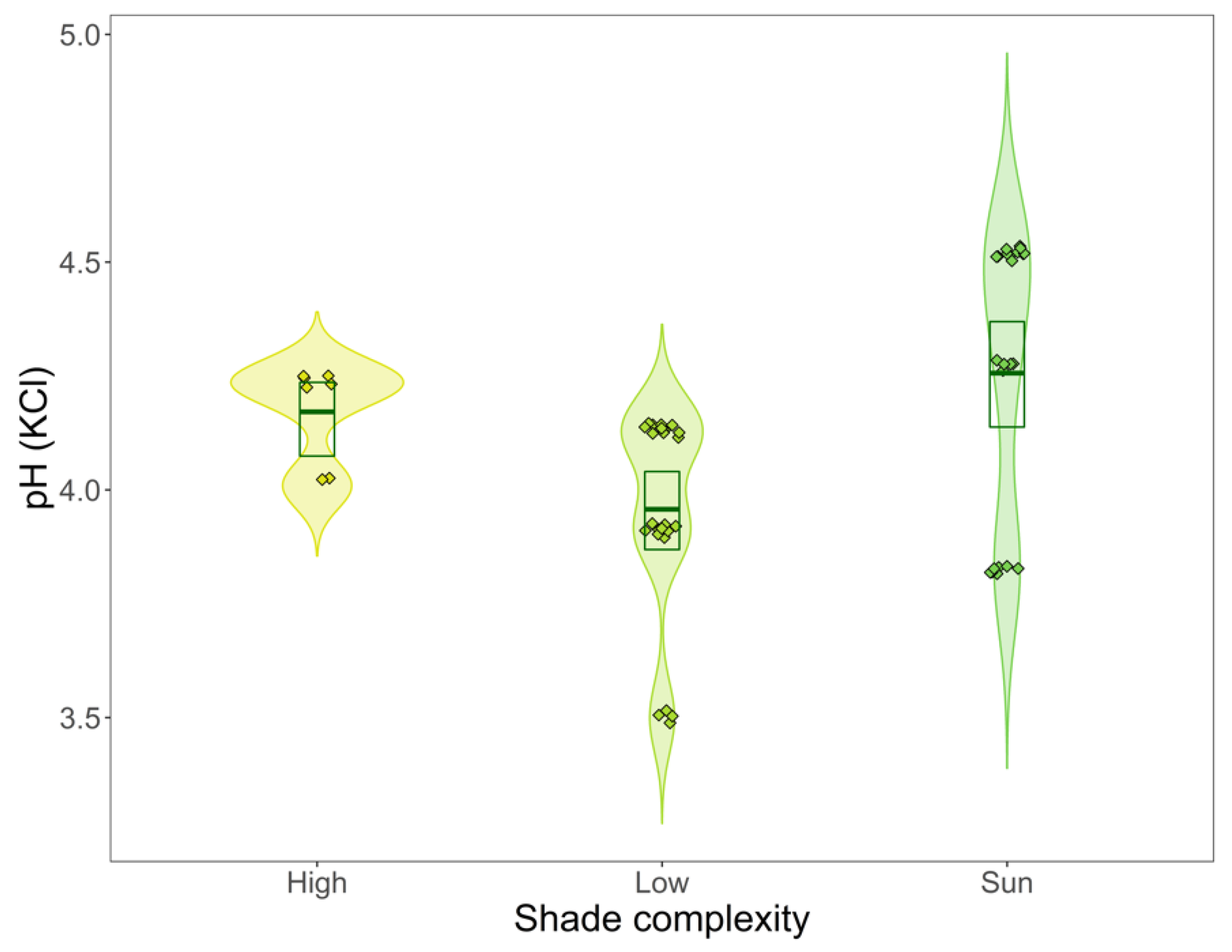
3. Results
3.1. Agrochemical Usage
3.2. Shade Complexity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Vassière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Kemmerling, L.R.; Griffin, S.R.; Haddad, N.M. Optimizing pollination conservation and crop yield among perennial bioenergy crops. Glob. Chang. Biol. Bioenergy 2021, 13, 1030–1042. [Google Scholar] [CrossRef]
- Duffy, C.; Toth, G.G.; Hagan, R.P.O.; McKeown, P.C.; Rahman, S.A.; Widyaningsih, Y.; Sunderland, T.C.H.; Spillane, C. Agroforestry contributions to smallholder farmer food security in Indonesia. Agrofor. Syst. 2021, 95, 1109–1124. [Google Scholar] [CrossRef]
- Campera, M.; Budiadi, B.; Bušina, T.; Fathoni, B.H.; Dermody, J.; Nijman, V.; Imron, M.A.; Nekaris, K.A.I. Abundance and richness of invertebrates in shade-grown versus sun-exposed coffee home gardens in Indonesia. Agrofor. Syst. 2022, 96, 829–841. [Google Scholar] [CrossRef]
- Manson, S.; Nekaris, K.A.I.; Hedger, K.; Balestri, M.; Ahmad, N.; Adinda, E.; Budiadi, B.; Imron, M.A.; Nijman, V.; Campera, M. Flower visitation time and number of visitor species are reduced by the use of agrochemicals in coffee home gardens. Agronomy 2022, 12, 509. [Google Scholar] [CrossRef]
- Ilany, T.; Ashton, M.S.; Montagnini, F.; Martinez, C. Using agroforestry to improve soil fertility: Effects of intercropping on Ilex paraguariensis (yerba mate) plantations with Araucaria angustifolia. Agrofor. Syst. 2010, 80, 399–409. [Google Scholar] [CrossRef]
- Zhao, Q.; Yiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-term coffee monoculture alters soil chemical properties and microbial communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef]
- Saputra, D.D.; Sari, R.R.; Hairiah, K.; Roshetko, J.M.; Suprayogo, D.; van Noordwijk, M. Can cocoa agroforestry restore degraded soil structure following conversion from forest to agricultural use? Agrofor. Syst. 2020, 94, 2261–2276. [Google Scholar] [CrossRef]
- da Silva Aragão, O.O.; de Oliveira-Longatti, S.M.; de Castro Caputo, P.S.; Rufini, M.; Carvalho, G.R.; de Carvalho, T.S.; de Souza Moreira, F.M. Microbiological indicators of soil quality are related to greater coffee yield in the Brazilian Cerrado region. Ecol. Indic. 2020, 113, 106205. [Google Scholar] [CrossRef]
- Castro-Tanzi, S.; Dietsch, T.; Urena, N.; Vindas, L.; Chandler, M. Analysis of management and site factors to improve the sustainability of smallholder coffee production in Tarrazú, Costa Rica. Agric. Ecosyst. Environ. 2012, 155, 172–181. [Google Scholar] [CrossRef]
- Meng, W.A.N.G.; Shanshan, L.I.; Xiaoyue, L.I.; Zhongqiu, Z.H.A.O.; Shibao, C.H.E.N. An overview of current status of copper pollution in soil and remediation efforts in China. Front. Earth Sci. 2018, 25, 305. [Google Scholar]
- Sauvadet, M.; den Meersche, K.V.; Allinne, C.; Gay, F.; de Melo Virginio Filho, E.; Chauvat, M.; Becquer, T.; Tixier, P.; Harmand, J.M. Shade trees have higher impact on soil nutrient availability and food web in organic than conventional coffee agroforestry. Sci. Total Environ. 2019, 649, 1065–1074. [Google Scholar] [CrossRef]
- Medina-Sauza, R.M.; Álvarez-Jiménez, M.; Ortíz-Huerta, Y.; Ruiz-Sayago, E.; Blouin, M.; Villain, L.; Guevara, R.; Sangabriel, W.; Reverchon, F.; Barois, I. Bulk and rhizosphere soil properties under two Coffea species influenced by the earthworm Pontoscolex corethrurus. Rhizosphere 2022, 21, 100458. [Google Scholar] [CrossRef]
- Armbrecht, I.; Gallego, M.C. Testing ant predation on the coffee berry borer in shaded and sun coffee plantations in Colombia. Entomol. Exp. Appl. 2007, 124, 261–267. [Google Scholar] [CrossRef]
- Escobar-Ramírez, S.; Grass, I.; Armbrecht, I.; Tscharntke, T. Biological control of the coffee berry borer: Main natural enemies, control success, and landscape influence. Biol. Control 2019, 136, 103992. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarçoni, R.C.; Moreli, A.P.; Pinheiro, P.F.; Pinheiro, C.A.; Moreira, T.R.; Siqueira, E.D.; Caten, C.S. Physicochemical parameters of arabica fermented coffee in different altitudes. Coffee Sci. 2021, 16, e161877. [Google Scholar]
- Barak, P.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 1997, 197, 61–69. [Google Scholar] [CrossRef]
- Ibrahim, H.W.; Zailani, S. A review on the competitiveness of global supply chain in a coffee industry in Indonesia. Int. Bus. Manag. 2010, 4, 105–115. [Google Scholar]
- DaMatta, F.M.; Rahn, E.; Läderach, P.; Ghini, R.; Ramalho, J.C. Why could the coffee crop endure climate change and global warming to a greater extent than previously estimated? Clim. Chang. 2019, 152, 167–178. [Google Scholar] [CrossRef]
- Oka, I.N. Integrated pest management in Indonesia: IPM by Farmers. In Integrated Pest Management in the Global Arena; Maredia, K.M., Dakouo, D., Mota-Sanchez, D., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 223–237. [Google Scholar]
- Imron, M.A.; Campera, M.; Al Bihad, D.; Rachmawati, F.D.; Nugroho, F.E.; Budiadi, B.; Wianti, K.F.; Suprapto, E.; Nijman, V.; Nekaris, K.A.I. Bird assemblages in coffee agroforestry systems and other human modified habitats in Indonesia. Biology 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Campera, M.; Hedger, K.; Birot, H.; Manson, S.; Balestri, M.; Budiadi, B.; Imron, M.A.; Nijman, V.; Nekaris, K.A.I. Does the presence of shade trees and distance to the forest affect detection rates of terrestrial vertebrates in coffee home gardens? Sustainability 2021, 13, 8540. [Google Scholar] [CrossRef]
- Campera, M.; Balestri, M.; Manson, S.; Hedger, K.; Ahmad, N.; Nijman, V.; Budiadi, B.; Imron, M.A.; Nekaris, K.A.I. Shade trees and agrochemical use affect butterfly assemblages in coffee home gardens. Agric. Ecosyst. Environ. 2021, 319, 107547. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Bianchi, F.J.J.A.; Cardoso, I.M.; Tittonell, P.; Peña-Claros, M. Impact of agroecological management on plant diversity and soil-based ecosystem services in pasture and coffee systems in the Atlantic forest of Brazil. Agric. Ecosyst. Environ. 2021, 305, 107171. [Google Scholar] [CrossRef]
- Tassew, A.A.; Yadessa, G.B.; Bote, A.D.; Obso, T.K. Influence of location, elevation gradients, processing methods, and soil quality on the physical and cup quality of coffee in the Kafa Biosphere Reserve of SW Ethiopia. Heliyon 2021, 7, e07790. [Google Scholar] [CrossRef] [PubMed]
- Campera, M.; Budiadi, B.; Adinda, E.; Ahmad, N.; Balestri, M.; Hedger, K.; Imron, M.A.; Manson, S.; Nijman, V.; Nekaris, K.A.I. Fostering a wildlife-friendly program for sustainable coffee farming: The case of small-holder farmers in Indonesia. Land 2021, 10, 121. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis, Part 3–Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, J. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. Available online: https://cran.r-project.org/web/packages/DHARMa (accessed on 30 May 2022).
- Lenth, R. Emmean: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.3.4. 2019. Available online: https://cran.r-project.org/package=emmeans (accessed on 30 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Kwiatkowski, C.A.; Harasim, E. Chemical properties of soil in four-field crop rotations under organic and conventional farming systems. Agronomy 2020, 10, 1045. [Google Scholar] [CrossRef]
- Parecido, R.J.; Sorattio, R.P.; Perdona, M.J.; Gitari, H.I.; Dognani, V.; Santos, A.R.; Silveira, L. Liming method and rate effects on soil acidity and Arabica coffee nutrition, growth, and yield. J. Soil Sci. Plant Nutr. 2021, 21, 2613–2625. [Google Scholar] [CrossRef]
- Schaller, M.; Schroth, G.; Beer, J.; Jiménez, F. Species and site characteristics that permit the association of fast-growing trees with crops: The case of Eucalyptus deglupta as coffee shade in Costa Rica. For. Ecol. Manag. 2003, 175, 205–215. [Google Scholar] [CrossRef]
- Latini, A.O.; Silva, D.P.; Souza, F.M.L.; Ferreira, M.C.; de Moura, M.S.; Suarez, N.F. Reconciling coffee productivity and natural vegetation conservation in an agroecosystem landscape in Brazil. J. Nat. Conserv. 2020, 57, 125902. [Google Scholar] [CrossRef]
- del Moral, R.; Muller, C.H. Fog Drip: A Mechanism of toxin transport from Eucalyptus globulus. Bull. Torrey Bot. Club 1969, 96, 467–475. [Google Scholar] [CrossRef]
- Yadessa, A.; Burkhardt, J.; Bekele, E.; Hundera, K.; Goldbach, H. The role of soil nutrient ratios in coffee quality: Their influence on bean size and cup quality in the natural coffee forest ecosystems of Ethiopia. Afr. J. Agric. Res. 2019, 14, 2090–2103. [Google Scholar]
- Kopittke, P.M.; Blamey, F.P.C. Theoretical and experimental assessment of nutrient solution composition in short-term studies of aluminium rhizotoxicity. Plant Soil 2016, 406, 311–326. [Google Scholar] [CrossRef]
- Auler, A.C.; Caires, E.F.; Pires, L.F.; Galetto, S.L.; Romaniw, J.; Charnobay, A.C. Lime effects in a no-tillage system on Inceptisols in Southern Brazil. Geoderma Reg. 2019, 16, e00206. [Google Scholar] [CrossRef]
- Takala, B. Ameliorative effects of coffee husk compost and lime amendment on acidic soil of Haru, Western Ethiopia. J. Soil Water Sci. 2020, 4, 141–150. [Google Scholar]
- Nuddin, A. The role of tree crops on nutrient availability, and production of coffee agroforestry. IOP Conf. Ser. Earth Environ. Sci. 2019, 270, 012049. [Google Scholar]
- Thomazini, A.; Mendonça, E.S.; Cardoso, I.M.; Garbin, M.L. SOC dynamics and soil quality index of agroforestry systems in the Atlantic rainforest of Brazil. Geoderma Reg. 2015, 5, 15–24. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Medina-Sauza, R.M.; Alvarez-Jimenez, M.; Delhal, A.; Reverchon, F.; Blouin, M.; Guerrero-Analco, J.A.; Cerdan, C.R.; Guevara, R.; Villain, L.; Barois, I. Earthworms building up soil microbiota, a review. Front. Environ. Sci. 2019, 7, 81. [Google Scholar] [CrossRef]
- Plaas, E.; Meyer-Wolfarth, F.; Banse, M.; Bengtsson, J.; Bergmann, H.; Faber, J.; Potthoff, M.; Runge, T.; Schrader, S.; Taylor, A. Towards valuation of biodiversity in agricultural soils: A case for earthworms. Ecol. Econ. 2019, 159, 291–300. [Google Scholar] [CrossRef]
- Matsuyama, N.; Saigusa, M.; Sakaiya, E.; Tamakawa, K.; Oyamada, Z.; Kudo, K. Acidification and soil productivity of allophanic andosols affected by heavy application of fertilizers. Soil Sci. Plant Nutr. 2006, 51, 117–123. [Google Scholar] [CrossRef][Green Version]
- Herring, M.W.; Garnett, S.T.; Zander, K.K. From boutique to mainstream: Upscaling wildlife-friendly farming through consumer premiums. Conserv. Sci. Pract. 2022, 4, e12730. [Google Scholar] [CrossRef]
- Grüter, R.; Trachsel, T.; Laube, P.; Jaisli, I. Expected global suitability of coffee, cashew and avocado due to climate change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef]
- Borron, S. Building Resilience for an Unpredictable Future: How Organic Agriculture Can Help Farmers Adapt to Climate Change; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006.
- Scialabba, N.E.; Müller-Lindenlauf, M. Organic agriculture and climate change. Renew. Agric. Food Syst. 2010, 25, 158–169. [Google Scholar] [CrossRef]
- Rahn, E.; Läderach, P.; Baca, M.; Cressy, C.; Schroth, G.; Malin, D.; van Rikxoort, H.; Shriver, J. Climate change adaptation, mitigation and livelihood benefits in coffee production: Where are the synergies? Mitig. Adapt. Strateg. Glob. Chang. 2013, 19, 1119–1137. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankagote, P.; Baert, G.; Van Ranst, E. Coffee waste as an alternative fertilizer with soil improving properties for sandy soils in humid tropical environments. Soil Use Manag. 2010, 27, 94–102. [Google Scholar] [CrossRef]
- Dzung, N.A.; Dzung, T.T.; Khanh, V.T.P. Evaluation of coffee husk compost for improving soil fertility and sustainable coffee production in rural central highland of Vietnam. Resour. Environ. 2013, 3, 77–82. [Google Scholar]
- Guimarães, N.D.; Gallo, A.D.; Fontanetti, A.; Meneghin, S.P.; de Souza, M.D.B.; Morinigo, K.P.G.; da Silva, R.F. Biomass and soil microbial activity in different systems of coffee cultivation. Rev. Fac. Cienc. Agrar. 2017, 40, 34–44. [Google Scholar]
- Henrique, N.S.; Maltoni, K.L.; Faria, G.A. Soil quality in two coffee crop systems in the Amazon biome. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 379–384. [Google Scholar] [CrossRef]


| Soil Component | Treatment | Ratio | Standard Error | Lower CL | Upper CL | t-Ratio | p-Value |
|---|---|---|---|---|---|---|---|
| pH (H2O) | C:M | 0.928 | 0.042 | 0.830 | 1.038 | −1.653 | 0.157 |
| C:O | 0.888 | 0.036 | 0.803 | 0.983 | −2.980 | 0.016 * | |
| M:O | 0.957 | 0.033 | 0.879 | 1.042 | −1.282 | 0.206 | |
| H:L | 1.019 | 0.048 | 0.907 | 1.15 | 0.401 | 0.690 | |
| H:S | 0.944 | 0.046 | 0.838 | 1.06 | −1.192 | 0.359 | |
| L:S | 0.926 | 0.030 | 0.856 | 1.00 | −2.396 | 0.061 | |
| pH (KCl) | C:M | 0.895 | 0.046 | 0.788 | 1.017 | −2.151 | 0.055 |
| C:O | 0.847 | 0.040 | 0.755 | 0.951 | −3.561 | 0.003 * | |
| M:O | 0.947 | 0.037 | 0.860 | 1.042 | −1.420 | 0.162 | |
| H:L | 1.025 | 0.055 | 0.899 | 1.17 | 0.471 | 0.640 | |
| H:S | 0.937 | 0.051 | 0.819 | 1.07 | −1.190 | 0.360 | |
| L:S | 0.914 | 0.033 | 0.836 | 1.00 | −2.485 | 0.049 * | |
| Al | C:M | 4.010 | 2.350 | 0.934 | 17.2 | 2.373 | 0.033 * |
| C:O | 12.130 | 7.080 | 2.837 | 51.8 | 4.276 | <0.001 ** | |
| M:O | 3.020 | 1.640 | 0.785 | 11.6 | 2.041 | 0.047 * | |
| H:L | 0.520 | 0.385 | 0.082 | 3.29 | −0.882 | 0.574 | |
| H:S | 1.610 | 1.369 | 0.193 | 13.4 | 0.556 | 0.581 | |
| L:S | 3.090 | 1.610 | 0.845 | 11.3 | 2.166 | 0.107 | |
| Fe | C:M | 2.000 | 0.517 | 1.057 | 3.80 | 2.693 | 0.014 * |
| C:O | 2.150 | 0.502 | 1.204 | 3.83 | 3.270 | 0.006 * | |
| M:O | 1.070 | 0.214 | 0.654 | 1.76 | 0.350 | 0.728 | |
| H:L | 0.802 | 0.219 | 0.408 | 1.58 | −0.809 | 0.626 | |
| H:S | 1.149 | 0.325 | 0.570 | 2.31 | 0.491 | 0.626 | |
| L:S | 1.433 | 0.269 | 0.899 | 2.28 | 1.912 | 0.185 | |
| K | C:M | 2.136 | 0.605 | 1.059 | 4.31 | 2.679 | 0.030 * |
| C:O | 1.766 | 0.456 | 0.932 | 3.35 | 2.204 | 0.048 * | |
| M:O | 0.827 | 0.186 | 0.473 | 1.44 | −0.845 | 0.402 | |
| H:L | 1.178 | 0.365 | 0.548 | 2.54 | 0.531 | 0.808 | |
| H:S | 0.926 | 0.291 | 0.425 | 2.02 | −0.244 | 0.808 | |
| L:S | 0.786 | 0.162 | 0.471 | 1.31 | −1.166 | 0.748 | |
| C:N | C:M | 1.066 | 0.041 | 0.969 | 1.17 | 1.664 | 0.307 |
| C:O | 1.031 | 0.036 | 0.946 | 1.12 | 0.885 | 0.380 | |
| M:O | 0.967 | 0.029 | 0.898 | 1.04 | −1.106 | 0.380 | |
| H:L | 1.070 | 0.044 | 0.964 | 1.18 | 1.594 | 0.117 | |
| H:S | 1.150 | 0.049 | 1.035 | 1.28 | 3.278 | 0.006 * | |
| L:S | 1.080 | 0.030 | 1.005 | 1.15 | 2.639 | 0.017 * | |
| Mn | C:M | 1.469 | 0.272 | 0.928 | 2.32 | 2.076 | 0.130 |
| C:O | 1.298 | 0.219 | 0.856 | 1.97 | 1.550 | 0.191 | |
| M:O | 0.884 | 0.132 | 0.610 | 1.28 | −0.826 | 0.413 | |
| H:L | 1.390 | 0.285 | 0.840 | 2.31 | 1.623 | 0.145 | |
| H:S | 1.700 | 0.350 | 1.025 | 2.83 | 2.598 | 0.037 * | |
| L:S | 1.220 | 0.166 | 0.873 | 1.71 | 1.480 | 0.145 | |
| Zn | C:M | 1.130 | 0.228 | 0.686 | 1.86 | 0.610 | 0.545 |
| C:O | 1.510 | 0.276 | 0.959 | 2.37 | 2.250 | 0.087 | |
| M:O | 1.330 | 0.216 | 0.894 | 1.99 | 1.786 | 0.120 | |
| H:L | 0.959 | 0.212 | 0.554 | 1.66 | −0.190 | 0.850 | |
| H:S | 0.891 | 0.199 | 0.512 | 1.55 | −0.519 | 0.850 | |
| L:S | 0.929 | 0.137 | 0.644 | 1.34 | −0.498 | 0.850 | |
| Ca | C:M | 0.906 | 0.143 | 0.612 | 1.34 | −0.625 | 0.667 |
| C:O | 0.860 | 0.123 | 0.604 | 1.23 | −1.055 | 0.667 | |
| M:O | 0.950 | 0.113 | 0.706 | 1.28 | −0.432 | 0.667 | |
| H:L | 0.990 | 0.163 | 0.658 | 1.49 | −0.061 | 0.952 | |
| H:S | 0.937 | 0.159 | 0.616 | 1.43 | −0.383 | 0.952 | |
| L:S | 0.947 | 0.105 | 0.718 | 1.25 | −0.492 | 0.952 | |
| Mg | C:M | 1.015 | 0.187 | 0.643 | 1.60 | 0.081 | 0.936 |
| C:O | 0.957 | 0.160 | 0.632 | 1.45 | −0.266 | 0.936 | |
| M:O | 0.942 | 0.136 | 0.659 | 1.35 | −0.411 | 0.936 | |
| H:L | 0.897 | 0.176 | 0.551 | 1.46 | −0.554 | 0.718 | |
| H:S | 1.077 | 0.219 | 0.651 | 1.78 | 0.363 | 0.718 | |
| L:S | 1.200 | 0.160 | 0.863 | 1.67 | 1.371 | 0.530 | |
| Cu | C:M | 0.040 | 0.203 | −0.462 | 0.542 | 0.196 | 0.845 |
| C:O | 0.220 | 0.184 | −0.235 | 0.674 | 1.195 | 0.389 | |
| M:O | 0.180 | 0.157 | −0.210 | 0.570 | 1.142 | 0.389 | |
| H:L | −0.202 | 0.217 | −0.738 | 0.335 | −0.930 | 0.535 | |
| H:S | −6.35 × 10−3 | 0.224 | −0.560 | 0.548 | −0.028 | 0.978 | |
| L:S | 0.195 | 0.147 | −0.169 | 0.559 | 1.329 | 0.535 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manson, S.; Nekaris, K.A.I.; Rendell, A.; Budiadi, B.; Imron, M.A.; Campera, M. Agrochemicals and Shade Complexity Affect Soil Quality in Coffee Home Gardens. Earth 2022, 3, 853-865. https://doi.org/10.3390/earth3030049
Manson S, Nekaris KAI, Rendell A, Budiadi B, Imron MA, Campera M. Agrochemicals and Shade Complexity Affect Soil Quality in Coffee Home Gardens. Earth. 2022; 3(3):853-865. https://doi.org/10.3390/earth3030049
Chicago/Turabian StyleManson, Sophie, K. A. I. Nekaris, Andrew Rendell, Budiadi Budiadi, Muhammad Ali Imron, and Marco Campera. 2022. "Agrochemicals and Shade Complexity Affect Soil Quality in Coffee Home Gardens" Earth 3, no. 3: 853-865. https://doi.org/10.3390/earth3030049
APA StyleManson, S., Nekaris, K. A. I., Rendell, A., Budiadi, B., Imron, M. A., & Campera, M. (2022). Agrochemicals and Shade Complexity Affect Soil Quality in Coffee Home Gardens. Earth, 3(3), 853-865. https://doi.org/10.3390/earth3030049






