Greenspace Inversely Associated with the Risk of Alzheimer’s Disease in the Mid-Atlantic United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Alzheimer’s Disease Data
2.3. Land Cover Data
2.4. PM2.5 Data
2.5. Demographic and Socioeconomic Status Data
2.6. Statistical Analysis
3. Results
3.1. Description of AD Data and Explanatory Variables
3.2. Modeled Associations
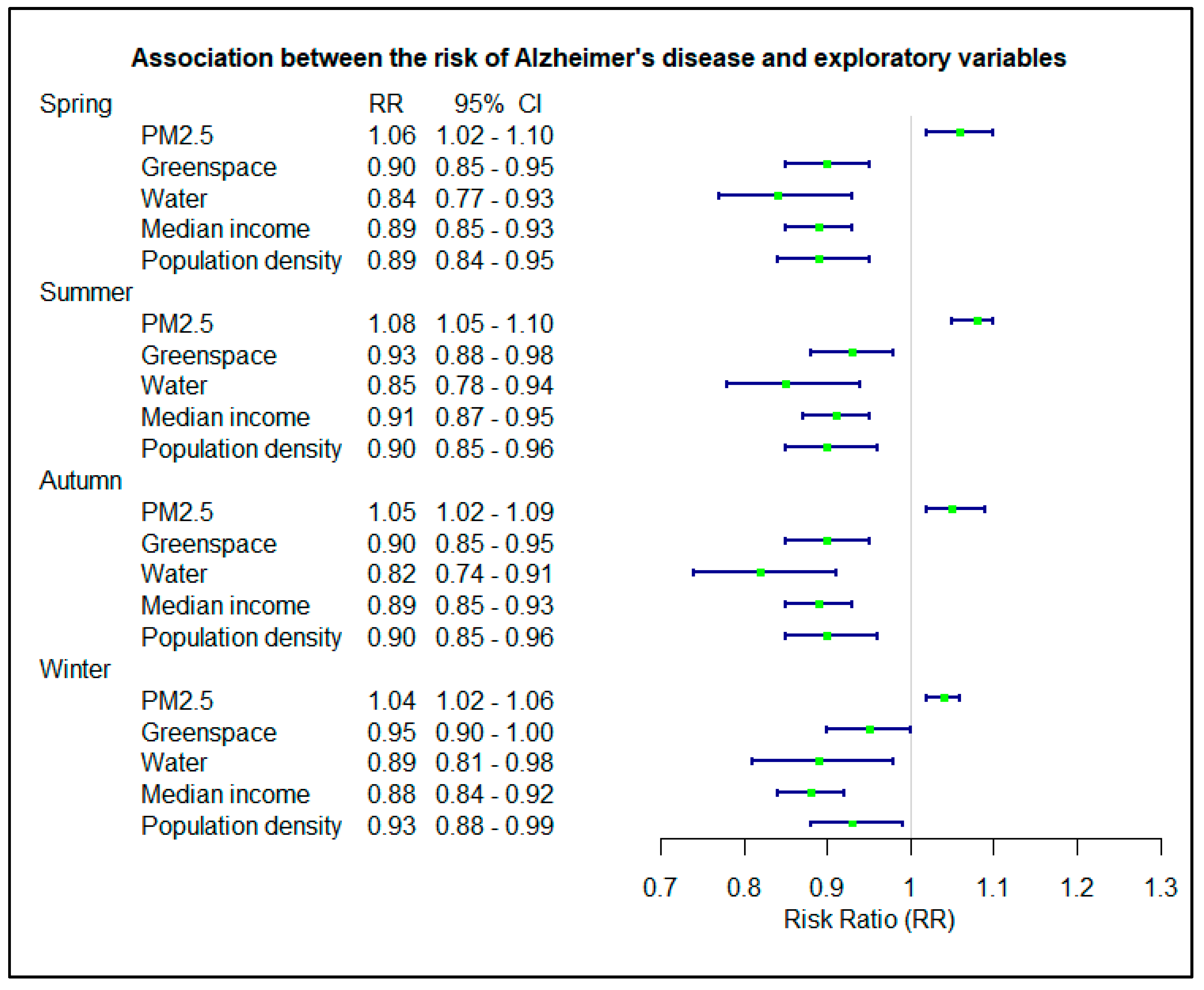
3.3. Seasonal Effects on the Associations
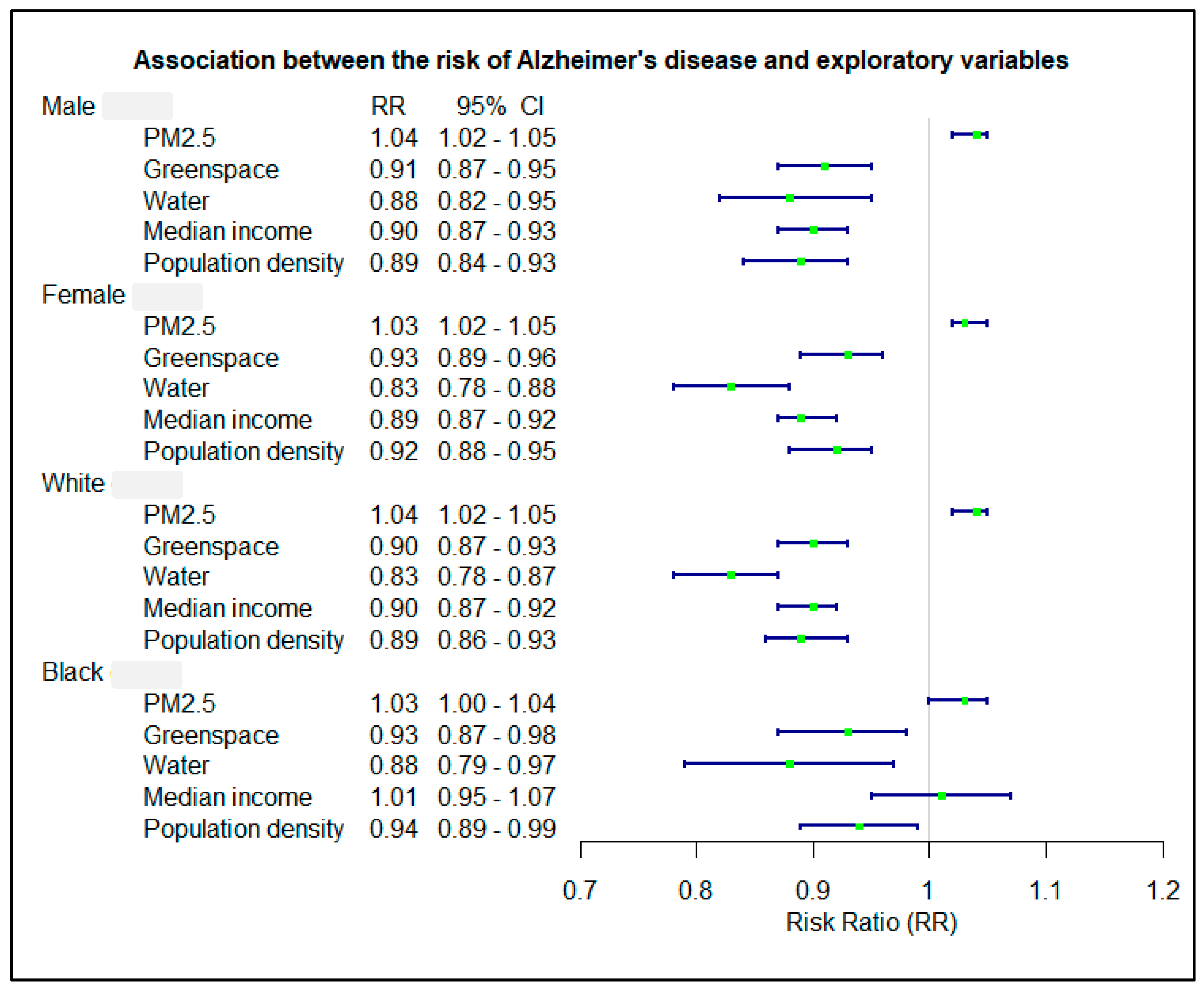
3.4. Association Stratified by Gender and Race
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016, 12, 459–509. [Google Scholar]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006239. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E. The genetics of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006296. [Google Scholar] [CrossRef]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106. [Google Scholar] [CrossRef]
- Yankner, B.A. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 1996, 16, 921–932. [Google Scholar] [CrossRef]
- Moulton, P.V.; Yang, W. Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public Health 2012, 2012. [Google Scholar] [CrossRef]
- Yegambaram, M.; Manivannan, B.; Beach, T.G.; Halden, R.U. Role of environmental contaminants in the etiology of Alzheimer’s disease: A review. Curr. Alzheimer Res. 2015, 12, 116–146. [Google Scholar] [CrossRef]
- Jung, C.-R.; Lin, Y.-T.; Hwang, B.-F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J. Alzheimer’s Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Kioumourtzoglou, M.-A.; Schwartz, J.D.; Weisskopf, M.G.; Melly, S.J.; Wang, Y.; Dominici, F.; Zanobetti, A. Long-term PM2. 5 exposure and neurological hospital admissions in the northeastern United States. Environ. Health Perspect. 2015, 124, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Oudin, A.; Forsberg, B.; Adolfsson, A.N.; Lind, N.; Modig, L.; Nordin, M.; Nordin, S.; Adolfsson, R.; Nilsson, L.-G. Traffic-related air pollution and dementia incidence in Northern Sweden: A longitudinal study. Environ. Health Perspect. 2015, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Maheswaran, R. The health benefits of urban green spaces: A review of the evidence. J. Public Health 2011, 33, 212–222. [Google Scholar] [CrossRef]
- Markevych, I.; Schoierer, J.; Hartig, T.; Chudnovsky, A.; Hystad, P.; Dzhambov, A.M.; De Vries, S.; Triguero-Mas, M.; Brauer, M.; Nieuwenhuijsen, M.J. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ. Res. 2017, 158, 301–317. [Google Scholar] [CrossRef]
- Van den Berg, M.; van Poppel, M.; van Kamp, I.; Andrusaityte, S.; Balseviciene, B.; Cirach, M.; Danileviciute, A.; Ellis, N.; Hurst, G.; Masterson, D. Visiting green space is associated with mental health and vitality: A cross-sectional study in four european cities. Health Place 2016, 38, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jackson, L. Inverse relationship between urban green space and childhood autism in California elementary school districts. Environ. Int. 2017, 107, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Völker, S.; Kistemann, T. The impact of blue space on human health and well-being–Salutogenetic health effects of inland surface waters: A review. Int. J. Hyg. Environ. Health 2011, 214, 449–460. [Google Scholar] [CrossRef]
- Völker, S.; Kistemann, T. Reprint of:“I’m always entirely happy when I’m here!” Urban blue enhancing human health and well-being in Cologne and Düsseldorf, Germany. Soc. Sci. Med. 2013, 91, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Triguero-Mas, M.; Martínez, D.; Dadvand, P.; Forns, J.; Plasència, A.; Nieuwenhuijsen, M.J. Mental health benefits of long-term exposure to residential green and blue spaces: A systematic review. Int. J. Environ. Res. Public Health 2015, 12, 4354–4379. [Google Scholar] [CrossRef]
- Amoly, E.; Dadvand, P.; Forns, J.; López-Vicente, M.; Basagaña, X.; Julvez, J.; Alvarez-Pedrerol, M.; Nieuwenhuijsen, M.J.; Sunyer, J. Green and blue spaces and behavioral development in Barcelona schoolchildren: The BREATHE project. Environ. Health Perspect. 2014, 122, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J. Air Pollution Removal by Chicago’s Urban Forest; Gen. Tech. Rep. NE-186; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1994; pp. 83–94.
- Cheapeake Bay Watershed Land Cover 2013/2014. Available online: https://chesapeakeconservancy.org/conservation-innovation-center/high-resolution-data (accessed on 27 February 2021).
- US ZIP Code Boundary GIS Layer. Available online: https://www.census.gov/programs-surveys/geography/guidance/geo-areas/zctas.html (accessed on 27 February 2021).
- US EPA. Air Quality Data. Available online: https://www.epa.gov/hesc/rsig-related-downloadable-data-files (accessed on 27 February 2021).
- Lambert, D. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics 1992, 34, 1–14. [Google Scholar] [CrossRef]
- Wu, J.; Tschakert, P.; Klutse, E.; Ferring, D.; Ricciardi, V.; Hausermann, H.; Oppong, J.; Smithwick, E.A. Buruli Ulcer Disease and Its Association with Land Cover in Southwestern Ghana. PLoS Negl. Trop. Dis. 2015, 9, e0003840. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in Tree Species Ability to Capture and Retain Airborne Fine Particulate Matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef]
- Nguyen, T.; Yu, X.; Zhang, Z.; Liu, M.; Liu, X. Relationship between types of urban forest and PM2.5 capture at three growth stages of leaves. J. Environ. Sci. 2015, 27, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Greenfield, E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 2014, 193, 119–129. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Hirabayashi, S.; Ziv, G.; Bakshi, B.R. Air quality and human health impacts of grasslands and shrublands in the United States. Atmos. Environ. 2018, 182, 193–199. [Google Scholar] [CrossRef]
- Stephen, R.; Hongisto, K.; Solomon, A.; Lönnroos, E. Physical activity and Alzheimer’s disease: A systematic review. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 733–739. [Google Scholar] [CrossRef]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Coutts, C. Greenway accessibility and physical-activity behavior. Environ. Plan. B Plan. Des. 2008, 35, 552–563. [Google Scholar] [CrossRef]
- Shafer, C.S.; Scott, D.; Mixon, J. A Greenway Classification System: Defining the Function and Character of Greenways in Urban Areas. J. Park Recreat. Adm. 2000, 18, 88–106. [Google Scholar]
- Wu, J.; Rappazzo, K.M.; Simpson Jr, R.J.; Joodi, G.; Pursell, I.W.; Mounsey, J.P.; Cascio, W.E.; Jackson, L.E. Exploring links between greenspace and sudden unexpected death: A spatial analysis. Environ. Int. 2018, 113, 114–121. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Rovner, B.W.; Broadhead, J.; Spencer, M.; Carson, K.; Folstein, M.F. The American Journal of Psychiatry. Am. J. Psychiatry 1989, 146, 350–353. [Google Scholar]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 2013, 202, 329–335. [Google Scholar] [CrossRef]
- Mazumdar, S.; Dunshea, A.; Chong, S.; Jalaludin, B. Tree Canopy Cover Is Best Associated with Perceptions of Greenspace: A Short Communication. Int. J. Environ. Res. Public Health 2020, 17, 6501. [Google Scholar] [CrossRef]
- Astell-Burt, T.; Navakatikyan, M.A.; Feng, X. Urban green space, tree canopy and 11-year risk of dementia in a cohort of 109,688 Australians. Environ. Int. 2020, 145, 106102. [Google Scholar] [CrossRef]
- Miles, R.; Coutts, C.; Mohamadi, A. Neighborhood urban form, social environment, and depression. J. Urban Health 2012, 89, 1–18. [Google Scholar] [CrossRef]
- Roe, J.; Thompson, C.; Aspinall, P.; Brewer, M.; Duff, E.; Miller, D.; Mitchell, R.; Clow, A. Green space and stress: Evidence from cortisol measures in deprived urban communities. Int. J. Environ. Res. Public Health 2013, 10, 4086–4103. [Google Scholar] [CrossRef]
- Thompson, C.W.; Roe, J.; Aspinall, P.; Mitchell, R.; Clow, A.; Miller, D. More green space is linked to less stress in deprived communities: Evidence from salivary cortisol patterns. Landsc. Urban Plan. 2012, 105, 221–229. [Google Scholar] [CrossRef]
- Holtan, M.T.; Dieterlen, S.L.; Sullivan, W.C. Social life under cover: Tree canopy and social capital in Baltimore, Maryland. Environ. Behav. 2015, 47, 502–525. [Google Scholar] [CrossRef]
- Kweon, B.-S.; Sullivan, W.C.; Wiley, A.R. Green common spaces and the social integration of inner-city older adults. Environ. Behav. 1998, 30, 832–858. [Google Scholar] [CrossRef]
- Carrus, G.; Scopelliti, M.; Lafortezza, R.; Colangelo, G.; Ferrini, F.; Salbitano, F.; Agrimi, M.; Portoghesi, L.; Semenzato, P.; Sanesi, G. Go greener, feel better? The positive effects of biodiversity on the well-being of individuals visiting urban and peri-urban green areas. Landsc. Urban Plan. 2015, 134, 221–228. [Google Scholar] [CrossRef]
- Maas, J.; Van Dillen, S.M.; Verheij, R.A.; Groenewegen, P.P. Social contacts as a possible mechanism behind the relation between green space and health. Health Place 2009, 15, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.; Franke, T.; McKay, H.; Sims-Gould, J. Therapeutic landscapes and wellbeing in later life: Impacts of blue and green spaces for older adults. Health Place 2015, 34, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zeng, Y. Effects of urban lake wetlands on the spatial and temporal distribution of air PM10 and PM2. 5 in the spring in Wuhan. Urban For. Urban Green. 2018, 31, 142–156. [Google Scholar] [CrossRef]
- Kupryś-Lipińska, I.; Kuna, P.; Wagner, I. Water in the urban space and the health of residents. Water City Sendzimir Found. Kraków 2014, 88, 47–55. [Google Scholar]

| Variables | No. Observations | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Medicare claims data | |||||
| Initial AD claims/month | 106,763 | 0.067 | 0.290 | 0 | 5 |
| Monthly claim rate | 106,763 | 2.6 × 10−6 | 1.3 × 10−6 | 0 | 3.98 × 103 |
| Environmental data | |||||
| PM2.5 (µg/m3) | 106,763 | 9.073 | 2.252 | 4.485 | 0.053 |
| Greenspace (%) | 106,763 | 85.013 | 17.508 | 7.08 | 99.77 |
| Water (%) | 106,763 | 3.735 | 9.248 | 0 | 85.73 |
| Covariates | |||||
| Median income ($) | 105,073 | 29315 | 11805 | 2542 | 135865 |
| Male population (%) | 106,763 | 50.028 | 7.753 | 0 | 100 |
| Female population (%) | 106,763 | 49.972 | 7.753 | 0 | 100 |
| White population (%) | 106,763 | 85.623 | 19.828 | 0 | 100 |
| Black population (%) | 106,763 | 9.338 | 16.976 | 0 | 100 |
| ZIP code area (km2) | 106,763 | 80.564 | 100.716 | 0.007 | 872.515 |
| Population density (1000/km2) | 106,763 | 5.300 | 18.961 | 0.001 | 712.326 |
| Road density (km/km2) | 109,405 | 0.789 | 1.711 | 0 | 58.555 |
| Variables (Unit) | Monthly Initial Records | Monthly Rate | ||
|---|---|---|---|---|
| r | p | r | p | |
| PM2.5 (µg/m3) | 0.069 | <0.001 | 0.050 | <0.001 |
| Greenspace (%) | −0.099 | <0.001 | −0.057 | <0.001 |
| Water (%) | −0.002 | <0.001 | −0.021 | <0.001 |
| Median income ($) | 0.030 | <0.001 | 0.027 | <0.001 |
| Population density | 0.059 | <0.001 | 0.060 | <0.001 |
| Male population (%) | −0.047 | <0.001 | −0.026 | <0.001 |
| Female population (%) | 0.0465 | <0.001 | 0.026 | <0.001 |
| White population (%) | −0.125 | <0.001 | −0.076 | <0.001 |
| Black population (%) | 0.104 | <0.001 | 0.061 | <0.001 |
| Road density (km/km2) | 0.031 | <0.001 | 0.025 | <0.001 |
| ZIP code area (km2) | 0.062 | <0.001 | 0.062 | <0.001 |
| Exploratory Variables (Unit) | RR | 95% CI | p |
|---|---|---|---|
| PM2.5 (µg/m3) | 1.03 | 1.02–1.05 | <0.001 |
| Greenspace (10%) | 0.91 | 0.89–0.94 | <0.001 |
| Water (10%) | 0.85 | 0.81–0.89 | <0.001 |
| Median income ($10,000) | 0.90 | 0.88–0.92 | <0.001 |
| Population density (1000/km2) | 0.91 | 0.88–0.93 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Jackson, L. Greenspace Inversely Associated with the Risk of Alzheimer’s Disease in the Mid-Atlantic United States. Earth 2021, 2, 140-150. https://doi.org/10.3390/earth2010009
Wu J, Jackson L. Greenspace Inversely Associated with the Risk of Alzheimer’s Disease in the Mid-Atlantic United States. Earth. 2021; 2(1):140-150. https://doi.org/10.3390/earth2010009
Chicago/Turabian StyleWu, Jianyong, and Laura Jackson. 2021. "Greenspace Inversely Associated with the Risk of Alzheimer’s Disease in the Mid-Atlantic United States" Earth 2, no. 1: 140-150. https://doi.org/10.3390/earth2010009
APA StyleWu, J., & Jackson, L. (2021). Greenspace Inversely Associated with the Risk of Alzheimer’s Disease in the Mid-Atlantic United States. Earth, 2(1), 140-150. https://doi.org/10.3390/earth2010009






