Synergistic Approaches for Navigating and Mitigating Agricultural Pollutants
Abstract
1. Introduction
2. Agricultural Pollutants
2.1. Chemically Synthesized Control Agents
2.2. Fertilizers and Manure
2.3. Post-Harvest Agriculture Waste
3. Ecological Impacts of Agricultural Pollutants
3.1. Environmental Pollution
3.2. Societal Challenges
4. Nano-Remediation: An Emerging Paradigm
4.1. Metal- and Metal Oxide-Based Nanoparticles for Pollutant Remediation
4.1.1. Iron-Based Nanomaterials
4.1.2. Silver Nanoparticles (AgNPs)
4.1.3. Other Metal-Based Nanoparticles
4.2. Carbon-Based Nanomaterials
4.3. Rate-Limiting Variables for Nanomaterial Remediation Approaches
4.3.1. Physical and Chemical Characteristics of Target Pesticide
4.3.2. Physical and Chemical Characteristics of Contaminated Soil
4.4. Mechanism of Nanomaterial-Mediated Pollutant Remediation
Adsorption
5. Protein-Based Biomaterials for Sustainable Agriculture Pollutant Mitigation
6. In Silico-Driven Remediation Model
7. Machine Learning-Based Toxicity Prediction
8. Future Prospects
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duro, J.A.; Lauk, C.; Kastner, T.; Erb, K.-H.; Haberl, H. Global Inequalities in Food Consumption, Cropland Demand and Land-Use Efficiency: A Decomposition Analysis. Glob. Environ. Change 2020, 64, 102124. [Google Scholar] [CrossRef]
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Thouvenot, L.; Phillips, H.R. Pesticide Effects on Soil Fauna Communities—A Meta-analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- New Study: Agricultural Pesticides Cause Widespread Harm to Soil Health, Threaten Biodiversity. Available online: https://biologicaldiversity.org/w/news/press-releases/new-study-agricultural-pesticides-cause-widespread-harm-to-soil-health-threaten-biodiversity-2021-05-04/ (accessed on 27 June 2024).
- Hassaan, M.A.; El Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Sethi, A.; Lin, C.-Y.; Madhavan, I.; Davis, M.; Alexander, P.; Eddleston, M.; Chang, S.-S. Impact of Regional Bans of Highly Hazardous Pesticides on Agricultural Yields: The Case of Kerala. Agric. Food Secur. 2022, 11, 9. [Google Scholar] [CrossRef]
- US EPA Sources and Solutions: Agriculture. Available online: https://www.epa.gov/nutrientpollution/sources-and-solutions-agriculture (accessed on 23 July 2024).
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Dhanapal, A.R.; Thiruvengadam, M.; Vairavanathan, J.; Venkidasamy, B.; Easwaran, M.; Ghorbanpour, M. Nanotechnology Approaches for the Remediation of Agricultural Polluted Soils. ACS Omega 2024, 9, 13522–13533. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.S. Nanotechnology in Agriculture: Prospects and Constraints. Nanotechnol. Sci. Appl. 2014, 7, 63–71. [Google Scholar] [CrossRef]
- Chand Mali, S.; Raj, S.; Trivedi, R. Nanotechnology a Novel Approach to Enhance Crop Productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar] [CrossRef]
- Lalchawimawia, B.; Sil, A.; Banerjee, T.; Singh, N.; Bhatnagar, A.; Mukhopadhyay, R.; Mandal, A. Metal-Organic Framework-Pesticide Interactions in Water: Present and Future Perspectives on Monitoring, Remediation and Molecular Simulation. Coord. Chem. Rev. 2023, 490, 215214. [Google Scholar] [CrossRef]
- Raya, D.; Shreya, A.; Kumar, A.; Giri, S.K.; Salem, D.R.; Gnimpieba, E.Z.; Gadhamshetty, V.; Dhiman, S.S. Molecular Regulation of Conditioning Film Formation and Quorum Quenching in Sulfate Reducing Bacteria. Front. Microbiol. 2022, 13, 1008536. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Q.; Ren, F.; Pang, G.; Zhang, X.; Li, Y. Efficiency and Mechanism of C18-Functionalized Magnetic Nanoparticles for Extracting Weakly Polar Pesticides from Human Serum Determined by UHPLC-QTOF-MS and Molecular Dynamics Simulations. Environ. Pollut. 2022, 293, 118489. [Google Scholar] [CrossRef]
- Manju, K.M.; Srivastava, S.; Singh, B.P.; Raya, D.; Rohit; Fridyanto, R.; Ridwan, R.; Widyastuti, Y.; Loh, T.C.; Foo, H.L.; et al. Functional and Antioxidant Characteristics of the Dietary Fiber Extracted from Pearl Millet. Food Biosci. 2025, 67, 106318. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Y.; Gao, Y.; Zhu, Z. Rational Design Strategies for Nanozymes. ACS Nano 2023, 17, 13062–13080. [Google Scholar] [CrossRef] [PubMed]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, Preparation, and Applications (with Emphasis on Aqueous Media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M. Assess the Influence of Using Treated Wastewater by Nano Hydroxyapatite and Its Modification on Some Soil and Faba Bean Plant Properties. N. Y. Sci. J. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, R.K.; Gupta, S.M.; Gaur, V.S.; Pandey, D. Systems Biology for Smart Crops and Agricultural Innovation: Filling the Gaps between Genotype and Phenotype for Complex Traits Linked with Robust Agricultural Productivity and Sustainability. Omics J. Integr. Biol. 2015, 19, 581–601. [Google Scholar] [CrossRef]
- Anandhi, G.; Iyapparaja, M. Systematic Approaches to Machine Learning Models for Predicting Pesticide Toxicity. Heliyon 2024, 10, e28752. [Google Scholar] [CrossRef]
- Dashti, A.; Navidpour, A.H.; Amirkhani, F.; Zhou, J.L.; Altaee, A. Application of Machine Learning Models to Improve the Prediction of Pesticide Photodegradation in Water by ZnO-Based Photocatalysts. Chemosphere 2024, 362, 142792. [Google Scholar] [CrossRef]
- Allen, C.; Aryal, S.; Do, T.; Gautum, R.; Hasan, M.M.; Jasthi, B.K.; Gnimpieba, E.; Gadhamshetty, V. Deep Learning Strategies for Addressing Issues with Small Datasets in 2D Materials Research: Microbial Corrosion. Front. Microbiol. 2022, 13, 1059123. [Google Scholar] [CrossRef]
- Raya, D.; Peta, V.; Bomgni, A.; Aryal, S.; Do, T.D.; Jawaharraj, K.; Salem, D.R.; Gadhamshetty, V.; Dhiman, S.S.; Gnimpieba, E.Z. Prediction and Validation of Nanowire Proteins in Oleidesulfovibrio Alaskensis G20 Using Machine Learning and Feature Engineering. Comput. Struct. Biotechnol. J. 2025, 27, 1706–1718. [Google Scholar] [CrossRef]
- Khan, A.; Uddin, J.; Ali, F.; Ahmad, A.; Alghushairy, O.; Banjar, A.; Daud, A. Prediction of Antifreeze Proteins Using Machine Learning. Sci. Rep. 2022, 12, 20672. [Google Scholar] [CrossRef]
- Wei, L.; Hu, J.; Li, F.; Song, J.; Su, R.; Zou, Q. Comparative Analysis and Prediction of Quorum-Sensing Peptides Using Feature Representation Learning and Machine Learning Algorithms. Brief. Bioinform. 2020, 21, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, K.; Szymanek, I.; Rogacz, D.; Wrzalik, M.; Łagiewka, J.; Nowik-Zając, A.; Zawierucha, I.; Coseri, S.; Puiu, I.; Falfushynska, H.; et al. Current Trends of Polymer Materials’ Application in Agriculture. Sustainability 2024, 16, 8439. [Google Scholar] [CrossRef]
- Srivastava, S.; Dhiman, S.S. Exploring the Next-Generation Polymers: Synthesis and Emerging Applications of UHMW Protein Polymers. In Microbial Approaches for Sustainable Green Technologies; CRC Press: Boca Raton, FL, USA, 2024; pp. 136–153. [Google Scholar] [CrossRef]
- Bowen, C.H.; Sargent, C.J.; Wang, A.; Zhu, Y.; Chang, X.; Li, J.; Mu, X.; Galazka, J.M.; Jun, Y.-S.; Keten, S.; et al. Microbial Production of Megadalton Titin Yields Fibers with Advantageous Mechanical Properties. Nat. Commun. 2021, 12, 5182. [Google Scholar] [CrossRef] [PubMed]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of Nanomaterials in Agricultural Production and Crop Protection: A Review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Eskandari, H. A General Overview on Intercropping and Its Advantages in Sustainable Agriculture. J. Appl. Environ. Biol. Sci. 2011, 1, 482–486. [Google Scholar]
- Sekhon, B.S. Food Nanotechnology—An Overview. Nanotechnol. Sci. Appl. 2010, 3, 1–15. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural Waste Material as Potential Adsorbent for Sequestering Heavy Metal Ions from Aqueous Solutions—A Review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Shekhar, C.; Khosya, R.; Thakur, K.; Mahajan, D.; Kumar, R.; Kumar, S.; Sharma, A.K. A Systematic Review of Pesticide Exposure, Associated Risks, and Long-Term Human Health Impacts. Toxicol. Rep. 2024, 13, 101840. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a Strategy for the Remediation of Pesticide-Polluted Soil: A Review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Ansari, I.; El-Kady, M.M.; El Din Mahmoud, A.; Arora, C.; Verma, A.; Rajarathinam, R.; Singh, P.; Verma, D.K.; Mittal, J. Persistent Pesticides: Accumulation, Health Risk Assessment, Management and Remediation: An Overview. Desalination Water Treat. 2024, 317, 100274. [Google Scholar] [CrossRef]
- Jones, E.; Barrera, D.; Reeve, J.; Drost, D. Nutrient Management Strategies for Organic Vegetable Production. Utah State University Extension: Logan, UT, USA, 2020; All current publications; Volume 2127, pp. 1–7. Available online: https://digitalcommons.usu.edu/extension_curall/2127 (accessed on 20 July 2025).
- USDA. Chapter 3: Agricultural Wastes And Water, Air, And Animal Resources. In Agricultural Waste Management Field Handbook; United States Department of Agriculture: Washington, DC, USA, 2012. [Google Scholar]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. Chapter Five—A Review of the Use of Organic Amendments and the Risk to Human Health. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 120, pp. 275–379. [Google Scholar]
- Sarkodie, E.K.; Jiang, L.; Li, K.; Yang, J.; Guo, Z.; Shi, J.; Deng, Y.; Liu, H.; Jiang, H.; Liang, Y.; et al. A Review on the Bioleaching of Toxic Metal(Loid)s from Contaminated Soil: Insight into the Mechanism of Action and the Role of Influencing Factors. Front. Microbiol. 2022, 13, 1049277. [Google Scholar] [CrossRef] [PubMed]
- Plant Nutrients in the Soil. Available online: https://www.dpi.nsw.gov.au/agriculture/soils/soil-testing-and-analysis/plant-nutrients (accessed on 22 June 2025).
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive Use of Nitrogenous Fertilizers: An Unawareness Causing Serious Threats to Environment and Human Health. Environ. Sci. Pollut. Res. Int. 2017, 24, 26983–26987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, J.; Li, T.; Shao, P.; Ma, J.; Dong, K. Effects of Nitrogen and Phosphorus Imbalance Input on Rhizosphere and Bulk Soil Bacterial Community of Suaeda Salsa in the Yellow River Delta. Front. Mar. Sci. 2023, 10, 1131713. [Google Scholar] [CrossRef]
- Mulyukin, M.A.; Sutormin, O.S.; Samoylenko, Z.A.; Kravchenko, I.V.; Bulatova, E.V.; Gulakova, N.M.; Baranenko, D.A.; Petrova, Y.Y. Heavy Metal Content in Medicinal Plants Grown in Hydroponics and Forest Soil in the Central Part of Western Siberia. Forests 2024, 15, 1606. [Google Scholar] [CrossRef]
- Sangeetha, T.; Periyathambi, E. Automatic Nutrient Estimator: Distributing Nutrient Solution in Hydroponic Plants Based on Plant Growth. PeerJ Comput. Sci. 2024, 10, e1871. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Kim, D.-W.; Ock, J.; Moon, K.-W.; Park, C.-H. Association between Pb, Cd, and Hg Exposure and Liver Injury among Korean Adults. Int. J. Environ. Res. Public Health 2021, 18, 6783. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Re, M.I.Z.; Tomasek, A.; Hopkins, B.G.; Sullivan, D.M.; Brewer, L. Managing Salt-Affected Soils for Crop Production. Available online: https://extension.oregonstate.edu/catalog/pub/pnw-601-managing-salt-affected-soils-crop-production (accessed on 22 June 2025).
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil Salinization in Agriculture: Mitigation and Adaptation Strategies Combining Nature-Based Solutions and Bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Swetanshu; Singh, P. The Impact of Environmental Toxins on Cardiovascular Diseases. Curr. Probl. Cardiol. 2024, 49, 102120. [Google Scholar] [CrossRef] [PubMed]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil Salinity, a Serious Environmental Issue and Plant Responses: A Metabolomics Perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.R.; Sethunathan, N. Salinity and the Persistence of Parathion in Flooded Soils. Soil Biol. Biochem. 1985, 17, 235–239. [Google Scholar] [CrossRef]
- Rouchaud, J.; Roucourt, P.; Van Himme, M.; Benoit, F.; Ceustermans, N.; Gillet, J.; Plumier, W.; Vulsteke, G. Metabolism of Methabenzthiazuron in the Soil of Pea Crops. J. Agric. Food Chem. 1988, 36, 642–645. [Google Scholar] [CrossRef]
- Chen, C.; Guo, L.; Shen, Y.; Hu, J.; Gu, J.; Ji, G. Oxidative Damage and Cardiotoxicity Induced by 2-Aminobenzothiazole in Zebrafish (Danio Rerio). J. Hazard. Mater. 2024, 476, 135032. [Google Scholar] [CrossRef]
- Huang, G.; Cierpicki, T.; Grembecka, J. 2-Aminobenzothiazoles in Anticancer Drug Design and Discovery. Bioorg. Chem. 2023, 135, 106477. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Doyle, M.P.; Erickson, M.C.; Jiang, X.; Millner, P.; Sharma, M. Composting To Inactivate Foodborne Pathogens for Crop Soil Application: A Review. J. Food Prot. 2018, 81, 1821–1837. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Moriarity, R.J.; Wilton, M.J.; Tsuji, L.J.S.; Sarkar, A.; Liberda, E.N. Evaluating Human Health Risks from Exposure to Agricultural Soil Contaminants Using One- and Two-Dimensional Monte Carlo Simulations. Environ. Res. 2025, 265, 120391. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Moreira, J.V.; Akamine, I.T.; Cardoso, V.S.; Mansoldo, F.R.P. Agricultural Pest Management: The Role of Microorganisms in Biopesticides and Soil Bioremediation. Plants 2024, 13, 2762. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef] [PubMed]
- Ansari, I.; Singh, A.; Kumar, A. Introduction and Sources of Molluscicides. Med. Anal. Chem. Int. J. 2024, 8, 000195. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- US EPA Herbicides. Available online: https://www.epa.gov/caddis/herbicides (accessed on 23 July 2024).
- Mehdizadeh Allaf, M.; Erratt, K.J.; Peerhossaini, H. Comparative Assessment of Algaecide Performance on Freshwater Phytoplankton: Understanding Differential Sensitivities to Frame Cyanobacteria Management. Water Res. 2023, 234, 119811. [Google Scholar] [CrossRef]
- Shah, R. Pesticides and Human Health. In Emerging Contaminants; Nuro, A., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83962-418-6. [Google Scholar]
- Chalam, A.V.; Sasikala, C.; Ramana, C.V.; Raghuveer Rao, P. Effect of Pesticides on Hydrogen Metabolism of Rhodobacter Sphaeroides and Rhodopseudomonas Palustris. FEMS Microbiol. Ecol. 1996, 19, 1–4. [Google Scholar] [CrossRef]
- Chalam, A.V.; Sasikala, C.; Ramana, C.V.; Uma, N.R.; Rao, P.R. Effect of Pesticides on the Diazotrophic Growth and Nitrogenase Activity of Purple Nonsulfur Bacteria. Bull. Environ. Contam. Toxicol. 1997, 58, 463–468. [Google Scholar] [CrossRef]
- Hammouda, O. Response of the Paddy Field Cyanobacterium Anabaena Doliolum to Carbofuran. Ecotoxicol. Environ. Saf. 1999, 44, 215–219. [Google Scholar] [CrossRef]
- Khan, M.; Zaidi, A.; Rizvi, P. Biotoxic Effects of Herbicides on Growth, Nodulation, Nitrogenase Activity, and Seed Production in Chickpeas. Commun. Soil Sci. Plant Anal. 2006, 37, 1783–1793. [Google Scholar] [CrossRef]
- Martínez-Toledo, M.V.; Salmerón, V.; Rodelas, B.; Pozo, C.; González-López, J. Effects of the Fungicide Captan on Some Functional Groups of Soil Microflora. Appl. Soil Ecol. 1998, 7, 245–255. [Google Scholar] [CrossRef]
- Niewiadomska, A. Effect of Carbendazim, Imazetapir and Thiram on Nitrogenase Activity, the Number of Microorganisms in Soil and Yield of Red Clover (Trifolium pratense L.). Pol. J. Environ. Stud. 2004, 13, 403–410. [Google Scholar]
- Niewiadomska, A.; Klama, J. Pesticide Side Effect on the Symbiotic Efficiency and Nitrogenase Activity of Rhizobiaceae Bacteria Family. Pol. J. Microbiol. 2005, 54, 43–48. [Google Scholar] [PubMed]
- Singh, G.; Wright, D. Effects of Herbicides on Nodulation, Symbiotic Nitrogen Fixation, Growth and Yield of Pea (Pisum sativum). J. Agric. Sci. 1999, 133, 21–30. [Google Scholar] [CrossRef]
- Durska, G. Fungicide Effect on Nitrogenase Activity in Methylotrophic Bacteria. Pol. J. Microbiol. 2004, 53, 155–158. [Google Scholar] [PubMed]
- Monkiedje, A.; Spiteller, M. Effects of the Phenylamide Fungicides, Mefenoxam and Metalaxyl, on the Microbiological Properties of a Sandy Loam and a Sandy Clay Soil. Biol. Fertil. Soils 2002, 35, 393–398. [Google Scholar] [CrossRef]
- Tu, C.M. Effects of Some Pesticides on Enzyme Activities in an Organic Soil. Bull. Environ. Contam. Toxicol. 1981, 27, 109–114. [Google Scholar] [CrossRef]
- Madhuri, R.J.; Rangaswamy, V. Influence of Selected Insecticides on Phosphatase Activity in Groundnut (Arachis hypogeae L.) Soils. J. Environ. Biol. 2002, 23, 393–397. [Google Scholar]
- Srinivasulu, M.; Mohiddin, G.J.; Subramanyam, K.; Rangaswamy, V. Effect of Insecticides Alone and in Combination with Fungicides on Nitrification and Phosphatase Activity in Two Groundnut (Arachis hypogeae L.) Soils. Environ. Geochem. Health 2012, 34, 365–374. [Google Scholar] [CrossRef]
- Mayanglambam, T.; Vig, K.; Singh, D.K. Quinalphos Persistence and Leaching Under Field Conditions and Effects of Residues on Dehydrogenase and Alkaline Phosphomonoesterases Activities in Soil. Bull. Environ. Contam. Toxicol. 2005, 75, 1067–1076. [Google Scholar] [CrossRef]
- Singh, J.; Singh, D.K. Dehydrogenase and Phosphomonoesterase Activities in Groundnut (Arachis hypogaea L.) Field after Diazinon, Imidacloprid and Lindane Treatments. Chemosphere 2005, 60, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, B.; Rekhapadmini, A.; Rangaswamy, V. Influence of Tebuconazole and Copper Hydroxide on Phosphatase and Urease Activities in Red Sandy Loam and Black Clay Soils. 3 Biotech 2016, 6, 78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.-K.; Edwards, C.A.; Subler, S. A Microcosm Approach for Evaluating the Effects of the Fungicides Benomyl and Captan on Soil Ecological Processes and Plant Growth. Appl. Soil Ecol. 2001, 18, 69–82. [Google Scholar] [CrossRef]
- Nowak, A.; Nowak, J.; Klodka, D.; Pryzbulewska, K.; Telesiński, A.; Szopa, E. Changes in the Microflora and Biological Activity of the Soil during the Degradation of Isoproturon. J. Plant Prot. 2004, 19, 1003–1016. [Google Scholar]
- Abdel-Mallek, A.Y.; Moharram, A.M.; Abdel-Kader, M.I.; Omar, S.A. Effect of Soil Treatment with the Organophosphorus Insecticide Profenfos on the Fungal Flora and Some Microbial Activities. Microbiol. Res. 1994, 149, 167–171. [Google Scholar] [CrossRef]
- Ingram, C.W.; Coyne, M.S.; Williams, D.W. Effects of Commercial Diazinon and Imidacloprid on Microbial Urease Activity in Soil and Sod. J. Environ. Qual. 2005, 34, 1573–1580. [Google Scholar] [CrossRef]
- Kızılkaya, R.; Akça, İ.; Aşkın, T.; Yılmaz, R.; Olekhov, V.; Samofalova, I.; Mudrykh, N. Effect of Soil Contamination with Azadirachtin on Dehydrogenase and Catalase Activity of Soil. Eurasian J. Soil Sci. 2012, 1, 98–103. [Google Scholar]
- Pertile, M.; Antunes, J.E.L.; Araujo, F.F.; Mendes, L.W.; Van den Brink, P.J.; Araujo, A.S.F. Responses of Soil Microbial Biomass and Enzyme Activity to Herbicides Imazethapyr and Flumioxazin. Sci. Rep. 2020, 10, 7694. [Google Scholar] [CrossRef]
- Nweke, C.O.; Ntinugwa, C.; Obah, I.F.; Ike, S.C.; Eme, G.E.; Opara, E.C.; Okolo, J.C.; Nwanyanwu, C.E. In Vitro Effects of Metals and Pesticides on Dehydrogenase Activity in Microbial Community of Cowpea (Vigna Unguiculata) Rhizoplane. Afr. J. Biotechnol. 2007, 6, 290–295. [Google Scholar]
- Gianfreda, L.; Sannino, F.; Violante, A. Pesticide Effects on the Activity of Free, Immobilized and Soil Invertase. Soil Biol. Biochem. 1995, 27, 1201–1208. [Google Scholar] [CrossRef]
- Sannino, F.; Gianfreda, L. Pesticide Influence on Soil Enzymatic Activities. Chemosphere 2001, 45, 417–425. [Google Scholar] [CrossRef]
- Sukul, P. Enzymatic Activities and Microbial Biomass in Soil as Influenced by Metalaxyl Residues. Soil Biol. Biochem. 2006, 38, 320–326. [Google Scholar] [CrossRef]
- Demanou, J.; Monkiédjé, A.; Njiné, T.; Foto, S.M.; Nola, M.; Zebaze Togouet, S.H.; Kemka, N. Changes in Soil Chemical Properties and Microbial Activities in Response to the Fungicide Ridomil Gold Plus Copper. Int. J. Environ. Res. Public Health 2004, 1, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Arinze, E.; Yubedee, A.G. Effect of Fungicides on Fusarium Grain Rot Endoenzyme Production in Maize. Glob. J. Pure Appl. Sci. 2000, 6, 629–634. [Google Scholar] [CrossRef][Green Version]
- Omar, S.A.; Abdel-Sater, M.A. Microbial Populations and Enzyme Activities in Soil Treated with Pesticides. Water. Air Soil Pollut. 2001, 127, 49–63. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Dumontet, S.; Mazzatura, A.; Pasquale, V. Toxic Effects of Four Sulphonylureas Herbicides on Soil Microbial Biomass. J. Environ. Sci. Health B 2012, 47, 653–659. [Google Scholar] [CrossRef]
- Lan, T.; Yang, G.; Li, J.; Chi, D.; Zhang, K. Residue, Dissipation and Dietary Intake Risk Assessment of Tolfenpyrad in Four Leafy Green Vegetables under Greenhouse Conditions. Food Chem. X 2022, 13, 100241. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Bhargava, P.; Varma, A.; Choudhary, D.K. (Eds.) Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer: Singapore, 2020; ISBN 9789811529849. [Google Scholar]
- Fenlon, K.A.; Jones, K.C.; Semple, K.T. Development of Microbial Degradation of Cypermethrin and Diazinon in Organically and Conventionally Managed Soils. J. Environ. Monit. JEM 2007, 9, 510–515. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Ma, Y.; Fang, S.; Kong, F.; Pang, X. Photocatalytic Degradation of Dinotefuran by Layered Phosphorus-Doped Carbon Nitride and Its Mechanism. J. Photochem. Photobiol. Chem. 2021, 414, 113287. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Kwan, B.H.; Vancov, T. Microbial Degradation of the Organophosphate Pesticide, Ethion. FEMS Microbiol. Lett. 2004, 240, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Mukherjee, I.; Roy, A. Flubendiamide as New Generation Insecticide in Plant Toxicology: A Policy Paper. Adv. Clin. Toxicol. 2017, 2, 000122. [Google Scholar] [CrossRef]
- Pang, S.; Lin, Z.; Zhang, Y.; Zhang, W.; Alansary, N.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the Toxicity and Degradation Mechanisms of Imidacloprid Via Physicochemical and Microbial Approaches. Toxics 2020, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Keighley, N.; Ramwell, C.; Sinclair, C.; Werner, D. Highly Variable Soil Dissipation of Metaldehyde Can Explain Its Environmental Persistence and Mobility. Chemosphere 2021, 283, 131165. [Google Scholar] [CrossRef]
- Da Silva, N.A.; Garcia Birolli, W.; Regali Seleghim, M.H.; Meleiro Porto, A.L. Biodegradation of the Organophosphate Pesticide Profenofos by Marine Fungi; Patil, Y., Ed.; InTech: London, UK, 2013. [Google Scholar]
- Propargite (Pesticide Residues in Food: 1977 Evaluations). Available online: https://www.inchem.org/documents/jmpr/jmpmono/v077pr40.htm (accessed on 7 March 2025).
- Sarkar, S.; Seenivasan, S.; Asir, R.P.S. Biodegradation of Propargite by Pseudomonas Putida, Isolated from Tea Rhizosphere. J. Hazard. Mater. 2010, 174, 295–298. [Google Scholar] [CrossRef]
- Manimozhi, S.; Surendran, A.; Thatheyus, A.J. Biodegradation of the Neonicotinoid Pesticide, Spiromesifen Using the Natural Bacterial Isolate, Serratia Sp. Int. J. Contemp. Microbiol. 2022, 8, 12–20. [Google Scholar] [CrossRef]
- Lebik-Elhadi, H.; Frontistis, Z.; Ait-Amar, H.; Madjene, F.; Mantzavinos, D. Degradation of Pesticide Thiamethoxam by Heat—Activated and Ultrasound—Activated Persulfate: Effect of Key Operating Parameters and the Water Matrix. Process Saf. Environ. Prot. 2020, 134, 197–207. [Google Scholar] [CrossRef]
- Gao, W.; Li, D.; You, H. Functional Characterization and Genomic Analysis of the Chlorantraniliprole-Degrading Strain Pseudomonas Sp. GW13. Bioengineering 2019, 6, 106. [Google Scholar] [CrossRef]
- Chen, S.; Luo, J.; Hu, M.; Geng, P.; Zhang, Y. Microbial Detoxification of Bifenthrin by a Novel Yeast and Its Potential for Contaminated Soils Treatment. PLoS ONE 2012, 7, e30862. [Google Scholar] [CrossRef]
- Shen, G.; Hu, X.; Hu, Y. Kinetic Study of the Degradation of the Insecticide Pymetrozine in a Vegetable-Field Ecosystem. J. Hazard. Mater. 2009, 164, 497–501. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, R.; Hung, Y.; Gao, H.; Zhang, P.; Wang, Y.; Sun, M.; Liu, D.; Wang, S. The Mechanisms and Process of Acephate Degradation by Hydroxyl Radical and Hydrated Electron. Saudi J. Biol. Sci. 2018, 25, 226–233. [Google Scholar] [CrossRef]
- Zheng, L.L.; Mou, H.J.; Li, J. Determination and Microbial Degradation of Lambda-Cyhalothrin. Adv. Mater. Res. 2011, 343–344, 430–437. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.; Lin, Z.; Pang, S.; Mishra, S.; Chen, S. Biodegradation of Fipronil: Current State of Mechanisms of Biodegradation and Future Perspectives. Appl. Microbiol. Biotechnol. 2021, 105, 7695–7708. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, R.; Sherif, B.; Abdel Ghani, S.; Sukar, N. Degradation of Profenofos and λ-Cyhalothrin Using Endogenous Bacterial Isolates and Detection of the Responsible Genes. J. Bioremediat. Biodegrad. 2016, 7, 360. [Google Scholar] [CrossRef]
- Amin, M.; Raza Gurmani, A.; Rafique, M.; Ullah Khan, S.; Mehmood, A.; Muhammad, D.; Hussain Syed, J. Investigating the Degradation Behavior of Cypermethrin (CYP) and Chlorpyrifos (CPP) in Peach Orchard Soils Using Organic/Inorganic Amendments. Saudi J. Biol. Sci. 2021, 28, 5890–5896. [Google Scholar] [CrossRef]
- Tian, K.; Ming, C.; Dai, Y.; Aké, K. Fenton Degradation of Cartap Hydrochloride: Identification of the Main Intermediates and the Degradation Pathway. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2015, 72, 1198–1205. [Google Scholar] [CrossRef]
- Yada, G.M.; Shiraishi, I.S.; Dekker, R.F.H.; Schirmann, J.G.; Barbosa-Dekker, A.M.; de Araujo, I.C.; Abreu, L.M.; Daniel, J.F.S. Soil and Entomopathogenic Fungi with Potential for Biodegradation of Insecticides: Degradation of Flubendiamide in Vivo by Fungi and in Vitro by Laccase. Ann. Microbiol. 2019, 69, 1517–1529. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Liu, Q.; Liu, J. Photodegradation Mechanism of Deltamethrin and Fenvalerate. J. Environ. Sci. 2010, 22, 1123–1128. [Google Scholar] [CrossRef]
- Tang, J.; Lei, D.; Wu, M.; Hu, Q.; Zhang, Q. Biodegradation and Metabolic Pathway of Fenvalerate by Citrobacter Freundii CD-9. AMB Express 2020, 10, 194. [Google Scholar] [CrossRef]
- Burkhard, R.; Binz, H.; Roux, C.A.; Brunner, M.; Ruesch, O.; Wyss, P. Environmental Fate of Emamectin Benzoate after Tree Micro Injection of Horse Chestnut Trees. Environ. Toxicol. Chem. 2015, 34, 297–302. [Google Scholar] [CrossRef]
- Devillers, J. Fate and Ecotoxicological Effects of Pyriproxyfen in Aquatic Ecosystems. Environ. Sci. Pollut. Res. Int. 2020, 27, 16052–16068. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Kim, J.-H.; Kim, Y.-W.; Kim, K.; Li, Q.X. Photodegradation of Diafenthiuron in Water. Pest Manag. Sci. 2002, 58, 496–502. [Google Scholar] [CrossRef]
- Wang, G.; Xu, D.; Xiong, M.; Zhang, H.; Li, F.; Liu, Y. Novel Degradation Pathway and Kinetic Analysis for Buprofezin Removal by Newly Isolated Bacillus Sp. J. Environ. Manag. 2016, 180, 59–67. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Wang, Y.; Chen, Z.; Wang, M.; Shi, H. Photolysis of the Novel Meta-Diamide Insecticide Broflanilide in Solutions: Kinetics, Degradation Pathway, DFT Calculation and Ecotoxicity Assessment. Chemosphere 2023, 320, 138060. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Saghir Khan, M.; Kumar, M. Kitazin-Pea Interaction: Understanding the Fungicide Induced Nodule Alteration, Cytotoxicity, Oxidative Damage and Toxicity Alleviation by Rhizobium Leguminosarum. RSC Adv. 2019, 9, 16929–16947. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Banerjee, D.; Paramasivam, M.; Roy, S.; Banerjee, T.; Banerjee, H. Degradation Dynamics of Propineb (Antracol 70 WP) in Onion and Soil. Pestic. Res. J. 2009, 21, 89–91. [Google Scholar]
- Skanes, B.; Warriner, K.; Prosser, R.S. Hazard Assessment Using an In-Silico Toxicity Assessment of the Transformation Products of Boscalid, Pyraclostrobin, Fenbuconazole and Glyphosate Generated by Exposure to an Advanced Oxidative Process. Toxicol. Vitr. 2021, 70, 105049. [Google Scholar] [CrossRef]
- Skanes, B.; Ho, J.; Warriner, K.; Prosser, R.S. Degradation of Boscalid, Pyraclostrobin, Fenbuconazole, and Glyphosate Residues by an Advanced Oxidative Process Utilizing Ultraviolet Light and Hydrogen Peroxide. J. Photochem. Photobiol. Chem. 2021, 418, 113382. [Google Scholar] [CrossRef]
- Wen, C.; Hasegawa, K.; Kanbara, T.; Kagaya, S.; Yamamoto, T. Visible Light-Induced Catalytic Degradation of Iprobenfos Fungicide by Poly(3-Octylthiophene-2,5-Diyl) Film. J. Photochem. Photobiol. Chem. 2000, 133, 59–66. [Google Scholar] [CrossRef]
- Alvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; Crivellente, F.; De Lentdecker, C.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Sulfur. EFSA J. 2023, 21, e07805. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Xu, Z.; Ren, X.; Chen, L.; Liu, F.; Zhang, H.; Zhao, L.; Chen, Z. Fate Characteristics and Risk Identification of Thifluzamide in Buckwheat across China: Analytical Method Development, Occurrence, and Health Assessment. Ecotoxicol. Environ. Saf. 2024, 270, 115833. [Google Scholar] [CrossRef]
- Lović, J.D.; Mijin, D.Ž.; Jovanović, M.B.; Glavaški, O.S.; Zeremski, T.M.; Petrović, S.D.; Avramov Ivić, M.L. An Investigation of Tebuconazole Degradation Using a Gold Electrode. Comptes Rendus Chim. 2016, 19, 639–645. [Google Scholar] [CrossRef]
- Meenakshi, S.N.; Jeyaramraja, P.R.; Manian, R. Degradation of the Fungicides, Azoxystrobin and Difenoconazole in Soil and their Influence on Soil Microbial Activity. Pestic. Res. J. 2009, 21, 89–91. [Google Scholar]
- Ma, C.; Liu, Z.; Qi, Y.; Wang, S.; Cao, X.; Wang, J.; She, Y.; Shao, Y.; Shen, J.; Zhang, C.; et al. Residue Behavior and Risk Assessment of Thifluzamide in the Maize Field Ecosystem. Environ. Sci. Pollut. Res. Int. 2018, 25, 21195–21204. [Google Scholar] [CrossRef]
- Ayare, S.D.; Gogate, P.R. Degradation of Tricyclazole Fungicide Using Combined Oxidation Strategies Based on Ultrasound, Ultraviolet Irradiation and Microwave. Environ. Technol. Innov. 2022, 26, 102533. [Google Scholar] [CrossRef]
- Gajjar, P.; Kiri, B.; Shah, H. Soil bacterial isolate showing a potential of mancozeb degradation and plant growth enhancement. J. Adv. Zool. 2023, 44, 1525–1533. [Google Scholar] [CrossRef]
- Satapute, P.; Kaliwal, B. Biodegradation of the Fungicide Propiconazole by Pseudomonas Aeruginosa PS-4 Strain Isolated from a Paddy Soil. Ann. Microbiol. 2016, 66, 1355–1365. [Google Scholar] [CrossRef]
- Hong, J.; Boussetta, N.; Enderlin, G.; Merlier, F.; Grimi, N. Degradation of Residual Herbicide Atrazine in Agri-Food and Washing Water. Foods 2022, 11, 2416. [Google Scholar] [CrossRef]
- Mir, N.A.; Khan, A.; Muneer, M.; Vijayalakhsmi, S. Photocatalytic Degradation of Trifluralin, Clodinafop-Propargyl, and 1,2-Dichloro-4-Nitrobenzene As Determined by Gas Chromatography Coupled with Mass Spectrometry. Chromatogr. Res. Int. 2014, 2014, 261683. [Google Scholar] [CrossRef]
- Ahmad, F.; Anwar, S.; Firdous, S.; Da-Chuan, Y.; Iqbal, S. Biodegradation of Bispyribac Sodium by a Novel Bacterial Consortium BDAM: Optimization of Degradation Conditions Using Response Surface Methodology. J. Hazard. Mater. 2018, 349, 272–281. [Google Scholar] [CrossRef]
- Nominee Gold—Bispyribac Sodium 10% SC 500 mL. Available online: https://www.kisanestore.com/index.php?route=product/enquiry&product_id=6757 (accessed on 6 March 2025).
- Ma, J.-P.; Wang, Z.; Lu, P.; Wang, H.; Waseem Ali, S.; Li, S.-P.; Huang, X. Biodegradation of the Sulfonylurea Herbicide Chlorimuron-Ethyl by the Strain Pseudomonas Sp. LW3. FEMS Microbiol. Lett. 2009, 296, 203–209. [Google Scholar] [CrossRef]
- Jing, X.; Yao, G.; Liu, D.; Liu, M.; Wang, P.; Zhou, Z. Environmental Fate of Chiral Herbicide Fenoxaprop-Ethyl in Water-Sediment Microcosms. Sci. Rep. 2016, 6, 26797. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wei, Y.; Sun, C.; Yuan, M.; Zeng, W.; Liu, C.; Fu, H. Pinoxaden Degradation Characteristics of Acinetobacter Pittobacter and Prediction of Related Genes. Microbiology 2022, 91, 818–830. [Google Scholar] [CrossRef]
- Ismail, A.; Yousry, A.; Eid, H.; Saleh, R.; Helmy, R.; El-Ganainy, S.; Hajjar, M.J.; Mohamed, H. Exploring the efficacy of indigenous trichoderma asperellum and t. Harzianum for biodegrading thiamethoxam and chlorantraniliprole insecticides. Appl. Ecol. Environ. Res. 2024, 22, 3755–3770. [Google Scholar] [CrossRef]
- Kanat, G. Comparison the Biodegradation of Herbicide Pyroxasulfone by Some Soil Bacteria. Cell. Mol. Biol. 2018, 64, 10–14. [Google Scholar] [CrossRef]
- Mohd Ghazi, R.; Nik Yusoff, N.R.; Abdul Halim, N.S.; Wahab, I.R.A.; Ab Latif, N.; Hasmoni, S.H.; Ahmad Zaini, M.A.; Zakaria, Z.A. Health Effects of Herbicides and Its Current Removal Strategies. Bioengineered 2023, 14, 2259526. [Google Scholar] [CrossRef]
- Lin, Z.; Pang, S.; Zhou, Z.; Wu, X.; Bhatt, P.; Chen, S. Current Insights into the Microbial Degradation for Butachlor: Strains, Metabolic Pathways, and Molecular Mechanisms. Appl. Microbiol. Biotechnol. 2021, 105, 4369–4381. [Google Scholar] [CrossRef]
- El Madani, M.; Harir, M.; Zrineh, A.; El Azzouzi, M. Photodegradation of Imazethapyr Herbicide by Using Slurry and Supported TiO2: Efficiency Comparison. Arab. J. Chem. 2015, 8, 181–185. [Google Scholar] [CrossRef]
- Wahla, A.Q.; Iqbal, S.; Anwar, S.; Firdous, S.; Mueller, J.A. Optimizing the Metribuzin Degrading Potential of a Novel Bacterial Consortium Based on Taguchi Design of Experiment. J. Hazard. Mater. 2019, 366, 1–9. [Google Scholar] [CrossRef]
- Han, Y.; Tang, Z.; Bao, H.; Wu, D.; Deng, X.; Guo, G.; Ye, B.-C.; Dai, B. Degradation of Pendimethalin by the Yeast YC2 and Determination of Its Two Main Metabolites. RSC Adv. 2019, 9, 491–497. [Google Scholar] [CrossRef]
- Duc, H.D.; Oanh, N.T.; Dieu Thuy, N.T.; Kim Xuan, N.T. Degradation of Pretilachlor and Fenclorim and Effects of These Compounds on Bacterial Communities under Anaerobic Condition. Biodegradation 2024, 35, 583–599. [Google Scholar] [CrossRef]
- Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. Paraquat Degradation From Contaminated Environments: Current Achievements and Perspectives. Front. Microbiol. 2019, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Stets, M.; Mazzetto, A.; Andrade, F.; Pileggi, S.; Fávero, P.; Cantú, M.; Carrilho, E.; Carneiro, P.; Pileggi, M. Degradation of 2,4-d Herbicide by Microorganisms Isolated From Brazilian Contaminated Soil. Braz. J. Microbiol. 2007, 38, 522–525. [Google Scholar] [CrossRef]
- Hou, Y.; Tao, J.; Shen, W.; Liu, J.; Li, J.; Li, Y.; Cao, H.; Cui, Z. Isolation of the Fenoxaprop-Ethyl (FE)-Degrading Bacterium Rhodococcus Sp. T1, and Cloning of FE Hydrolase Gene Feh. FEMS Microbiol. Lett. 2011, 323, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Wahla, A.Q.; Ali, T.; Khaliq, S.; Imran, A.; Tawab, A.; Afzal, M.; Iqbal, S. Biodegradation and Subsequent Toxicity Reduction of Co-Contaminants Tribenuron Methyl and Metsulfuron Methyl by a Bacterial Consortium B2R. ACS Omega 2022, 7, 19816–19827. [Google Scholar] [CrossRef]
- Raipuria, N.; Parveen, R. Study of Pretilachlor Degradation by Microbes Isolated From Rice Field of Jabalpur Region. Int. J. Sci. Prog. Res. 2016, 23, 11–19. [Google Scholar]
- Chen, S.-F.; Chen, W.-J.; Song, H.; Liu, M.; Mishra, S.; Ghorab, M.A.; Chen, S.; Chang, C. Microorganism-Driven 2,4-D Biodegradation: Current Status and Emerging Opportunities. Molecules 2024, 29, 3869. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, N.; Banerjee, T.; Singh, S.B. Chemical Degradation of Sulfosulfuron in Aqueous Suspension of Rice and Wheat Straw Ashes. Bull. Environ. Contam. Toxicol. 2019, 103, 484–489. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of Action Classification and Insecticide Resistance Management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Kuck, K.H.; Gisi, U. FRAC Mode of Action Classification and Resistance Risk of Fungicides. In Modern Crop Protection Compounds; Wiley: Hoboken, NJ, USA, 2007; pp. 415–432. ISBN 978-3-527-31496-6. [Google Scholar]
- Barker, A.L.; Pawlak, J.; Duke, S.O.; Beffa, R.; Tranel, P.J.; Wuerffel, J.; Young, B.; Porri, A.; Liebl, R.; Aponte, R.; et al. Discovery, Mode of Action, Resistance Mechanisms, and Plan of Action for Sustainable Use of Group 14 Herbicides. Weed Sci. 2023, 71, 173–188. [Google Scholar] [CrossRef]
- Menne, H.; Köcher, H. HRAC Classification of Herbicides and Resistance Development. In Modern Crop Protection Compounds; Krämer, W., Schirmer, U., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 5–26. ISBN 978-3-527-31496-6. [Google Scholar]
- Wang, L.; Xin, J.; Nai, H.; Zheng, X. Effects of Different Fertilizer Applications on Nitrogen Leaching Losses and the Response in Soil Microbial Community Structure. Environ. Technol. Innov. 2021, 23, 101608. [Google Scholar] [CrossRef]
- Korkmaz, K.; Ibrikci, H.; Ryan, J.; Buyuk, G.; Guzel, N.; Karnez, E.; Oguz, H.; Yagbasanlar, T. Optimizing Nitrogen Fertilizer–Use Recommendations for Winter Wheat in a Mediterranean-Type Environment Using Tissue Nitrate Testing. Commun. Soil Sci. Plant Anal. 2008, 39, 1352–1366. [Google Scholar] [CrossRef]
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. Apcbee Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Fewtrell, L. Drinking-Water Nitrate, Methemoglobinemia, and Global Burden of Disease: A Discussion. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Villa-Galaviz, E.; Smart, S.M.; Ward, S.E.; Fraser, M.D.; Memmott, J. Fertilization Using Manure Minimizes the Trade-Offs between Biodiversity and Forage Production in Agri-Environment Scheme Grasslands. PLoS ONE 2023, 18, e0290843. [Google Scholar] [CrossRef]
- Bolan, N.; Adriano, D.; Mahimairaja, S. Distribution and Bioavailability of Trace Elements in Livestock and Poultry Manure By-Products. Crit. Rev. Environ. Sci. Technol. 2004, 34, 291–338. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compost Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Abdugheni, R.; Li, L.; Yang, Z.-N.; Huang, Y.; Fang, B.-Z.; Shurigin, V.; Mohamad, O.A.A.; Liu, Y.-H.; Li, W.-J. Microbial Risks Caused by Livestock Excrement: Current Research Status and Prospects. Microorganisms 2023, 11, 1897. [Google Scholar] [CrossRef]
- Bentsen, N.S.; Felby, C.; Thorsen, B.J. Agricultural Residue Production and Potentials for Energy and Materials Services. Prog. Energy Combust. Sci. 2014, 40, 59–73. [Google Scholar] [CrossRef]
- Lal, R. World Crop Residues Production and Implications of Its Use as a Biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef]
- Ghimire, N.; Bakke, R.; Bergland, W.H. Liquefaction of Lignocellulosic Biomass for Methane Production: A Review. Bioresour. Technol. 2021, 332, 125068. [Google Scholar] [CrossRef]
- Raya, D.; Ghimire, N.; Flatabø, G.Ø.; Bergland, W.H. Anaerobic Digestion of Aqueous Pyrolysis Liquid in ADM1. In Proceedings of the First SIMS EUROSIM Conference on Modelling and Simulation, Online, 21–23 September 2021. [Google Scholar]
- Smith, K.A.; Searchinger, T.D. Crop-Based Biofuels and Associated Environmental Concerns. GCB Bioenergy 2012, 4, 479–484. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Zou, Y.; Wanger, T.C.; Zhou, W.; Gc, Y.D.; Lu, Y. Agro-Ecology Science Relates to Economic Development but Not Global Pesticide Pollution. J. Environ. Manag. 2022, 307, 114529. [Google Scholar] [CrossRef] [PubMed]
- Luna Juncal, M.J.; Masino, P.; Bertone, E.; Stewart, R.A. Towards Nutrient Neutrality: A Review of Agricultural Runoff Mitigation Strategies and the Development of a Decision-Making Framework. Sci. Total Environ. 2023, 874, 162408. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- FAO. Pesticides Use, Pesticides Trade and Pesticides Indicators; FAO: Rome, Italy, 2022; ISBN 978-92-5-136614-1. [Google Scholar]
- Rasool, S.; Rasool, T.; Gani, K.M. A Review of Interactions of Pesticides within Various Interfaces of Intrinsic and Organic Residue Amended Soil Environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Xu, H.; Fan, Z.; Ahmad, F.; Zhang, D. Exploring the Ecological Protection Impacts of Cultivated Land Transfer: Explanation Based on Fertilizers and Pesticides. Ecol. Indic. 2023, 154, 110681. [Google Scholar] [CrossRef]
- Devlin, M.; Brodie, J. Nutrients and Eutrophication. In Marine Pollution—Monitoring, Management and Mitigation; Reichelt-Brushett, A., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 75–100. ISBN 978-3-031-10127-4. [Google Scholar]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- Stoytcheva, M. (Ed.) Pesticides and Human Health. In Pesticides in the Modern World—Effects of Pesticides Exposure; InTech: London, UK, 2011; ISBN 978-953-307-454-2. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/RFN (accessed on 23 June 2025).
- Ray, S.; Shaju, S.T. Bioaccumulation of Pesticides in Fish Resulting Toxicities in Humans through Food Chain and Forensic Aspects. Environ. Anal. Health Toxicol. 2023, 38, e2023017. [Google Scholar] [CrossRef]
- Lerro, C.C.; Koutros, S.; Andreotti, G.; Friesen, M.C.; Alavanja, M.C.; Blair, A.; Hoppin, J.A.; Sandler, D.P.; Lubin, J.H.; Ma, X.; et al. Organophosphate Insecticide Use and Cancer Incidence among Spouses of Pesticide Applicators in the Agricultural Health Study. Occup. Environ. Med. 2015, 72, 736–744. [Google Scholar] [CrossRef]
- Csitári, G.; Tóth, Z.; Kökény, M. Effects of Organic Amendments on Soil Aggregate Stability and Microbial Biomass in a Long-Term Fertilization Experiment (IOSDV). Sustainability 2021, 13, 9769. [Google Scholar] [CrossRef]
- Cheng, H.; Xing, D.; Twagirayezu, G.; Lin, S.; Gu, S.; Tu, C.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Effects of Field-Aging on the Impact of Biochar on Herbicide Fate and Microbial Community Structure in the Soil Environment. Chemosphere 2024, 348, 140682. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and Aquatic Ecosystems—A Review of Global Sources, Biogeochemical Cycling, and Effects on Fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, N.A. Heavy Metals Exposure and Alzheimer’s Disease: Underlying Mechanisms and Advancing Therapeutic Approaches. Behav. Brain Res. 2025, 476, 115212. [Google Scholar] [CrossRef] [PubMed]
- Alehashem, M.; Alcaraz, A.J.; Hogan, N.; Weber, L.; Siciliano, S.D.; Hecker, M. Linking Pesticide Exposure to Neurodegenerative Diseases: An in Vitro Investigation with Human Neuroblastoma Cells. Sci. Total Environ. 2024, 933, 173041. [Google Scholar] [CrossRef]
- El Nemr, A.; Moneer, A.A.; Khaled, A.; El-Sikaily, A. Contamination and Risk Assessment of Organochlorines in Surface Sediments of Egyptian Mediterranean Coast. Egypt. J. Aquat. Res. 2012, 38, 7–21. [Google Scholar] [CrossRef]
- Salem, D.M.S.A.; Khaled, A.; El Nemr, A.; El-Sikaily, A. Comprehensive Risk Assessment of Heavy Metals in Surface Sediments along the Egyptian Red Sea Coast. Egypt. J. Aquat. Res. 2014, 40, 349–362. [Google Scholar] [CrossRef]
- Kıykım, E. Neurodevelopmental Impact of Pesticides: A Silent Threat. Turk. Arch. Pediatr. 2025, 60, 114–116. [Google Scholar] [CrossRef]
- Jaga, K.; Dharmani, C. Sources of Exposure to and Public Health Implications of Organophosphate Pesticides. Rev. Panam. Salud Pública 2003, 14, 171–185. [Google Scholar] [CrossRef]
- Fukuto, T.R. Mechanism of Action of Organophosphorus and Carbamate Insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Abdollahi, M. Toxic Influence of Organophosphate, Carbamate, and Organochlorine Pesticides on Cellular Metabolism of Lipids, Proteins, and Carbohydrates: A Systematic Review. Hum. Exp. Toxicol. 2011, 30, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Khosrowshahi, A.; Ranjbar, A.; Mousavi, L.; Nili-Ahmadabadi, H.; Ghaffari, F.; Zeinvand-Lorestani, H.; Nili-Ahmadabadi, A. Chronic Exposure to Organophosphate Pesticides as an Important Challenge in Promoting Reproductive Health: A Comparative Study. J. Educ. Health Promot. 2019, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.A.; Akhigbe, T.M.; Adeogun, A.E.; Adesoye, O.B.; Akhigbe, R.E. Impact of Organophosphate Pesticides Exposure on Human Semen Parameters and Testosterone: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1227836. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Kawada, T. Organophosphorus Pesticides Induce Apoptosis in Human NK Cells. Toxicology 2007, 239, 89–95. [Google Scholar] [CrossRef]
- Bernal-González, K.G.; Covantes-Rosales, C.E.; Camacho-Pérez, M.R.; Mercado-Salgado, U.; Barajas-Carrillo, V.W.; Girón-Pérez, D.A.; Montoya-Hidalgo, A.C.; Díaz-Resendiz, K.J.G.; Barcelos-García, R.G.; Toledo-Ibarra, G.A.; et al. Organophosphate-Pesticide-Mediated Immune Response Modulation in Invertebrates and Vertebrates. Int. J. Mol. Sci. 2023, 24, 5360. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lin, C.-L.; Lin, M.-C.; Lai, C.-H.; Lin, H.-H.; Yang, C.-H.; Kao, C.-H. Increased Risk of Dementia in Patients With Acute Organophosphate and Carbamate Poisoning. Medicine 2015, 94, e1187. [Google Scholar] [CrossRef]
- Rothlein, J.; Rohlman, D.; Lasarev, M.; Phillips, J.; Muniz, J.; McCauley, L. Organophosphate Pesticide Exposure and Neurobehavioral Performance in Agricultural and Nonagricultural Hispanic Workers. Environ. Health Perspect. 2006, 114, 691–696. [Google Scholar] [CrossRef]
- Waddell, B.L.; Zahm, S.H.; Baris, D.; Weisenburger, D.D.; Holmes, F.; Burmeister, L.F.; Cantor, K.P.; Blair, A. Agricultural Use of Organophosphate Pesticides and the Risk of Non-Hodgkin’s Lymphoma among Male Farmers (United States). Cancer Causes Control CCC 2001, 12, 509–517. [Google Scholar] [CrossRef]
- Garud, A.; Pawar, S.; Patil, M.S.; Kale, S.R.; Patil, S. A Scientific Review of Pesticides: Classification, Toxicity, Health Effects, Sustainability, and Environmental Impact. Cureus 2024, 16, e67945. [Google Scholar] [CrossRef]
- Dhaneshwar, A.; Hardej, D. Disruption of Mitochondrial Complexes, Cytotoxicity, and Apoptosis Results from Mancozeb Exposure in Transformed Human Colon Cells. Environ. Toxicol. Pharmacol. 2021, 84, 103614. [Google Scholar] [CrossRef]
- Axelstad, M.; Boberg, J.; Nellemann, C.; Kiersgaard, M.; Jacobsen, P.R.; Christiansen, S.; Hougaard, K.S.; Hass, U. Exposure to the Widely Used Fungicide Mancozeb Causes Thyroid Hormone Disruption in Rat Dams but No Behavioral Effects in the Offspring. Toxicol. Sci. 2011, 120, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Zarn, J.A.; Brüschweiler, B.J.; Schlatter, J.R. Azole Fungicides Affect Mammalian Steroidogenesis by Inhibiting Sterol 14 Alpha-Demethylase and Aromatase. Environ. Health Perspect. 2003, 111, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Romeh, A.A.; Ibrahim Saber, R.A. Green Nano-Phytoremediation and Solubility Improving Agents for the Remediation of Chlorfenapyr Contaminated Soil and Water. J. Environ. Manag. 2020, 260, 110104. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, M.; Jonak Dutta, S.; Kuntze, M.; Bading, J.; Rüßbült, J.S.; Fabig, C.; Langfeldt, M.; Schulz, F.; Horcajada, P.; Parak, W.J. Visualization of the High Surface-to-Volume Ratio of Nanomaterials and Its Consequences. J. Chem. Educ. 2024, 101, 3146–3155. [Google Scholar] [CrossRef]
- Sathish, T.; Ahalya, N.; Thirunavukkarasu, M.; Senthil, T.S.; Hussain, Z.; Haque Siddiqui, M.I.; Panchal, H.; Kumar Sadasivuni, K. A Comprehensive Review on the Novel Approaches Using Nanomaterials for the Remediation of Soil and Water Pollution. Alex. Eng. J. 2024, 86, 373–385. [Google Scholar] [CrossRef]
- Kak, V.; Kishor, K.; Singh, K. Nanofertilizers: A New Frontier in Agriculture. In Nanobiostimulants: Emerging Strategies for Agricultural Sustainability; Singh, V., Bhat, R.A., Dar, G.H., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 13–27. ISBN 978-3-031-68138-7. [Google Scholar]
- Mishra, S.; Wang, W.; de Oliveira, I.P.; Atapattu, A.J.; Xia, S.-W.; Grillo, R.; Lescano, C.H.; Yang, X. Interaction Mechanism of Plant-Based Nanoarchitectured Materials with Digestive Enzymes of Termites as Target for Pest Control: Evidence from Molecular Docking Simulation and in Vitro Studies. J. Hazard. Mater. 2021, 403, 123840. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Jabbari, V.; Imam, M.A.; Castro, E.; Kim, H.; Curry, M.L.; Valles-Rosales, D.J.; Noveron, J.C. Nanoscale Nickel Metal Organic Framework Decorated over Graphene Oxide and Carbon Nanotubes for Water Remediation. Sci. Total Environ. 2020, 698, 134214. [Google Scholar] [CrossRef]
- Asghar, N.; Hussain, A.; Nguyen, D.A.; Ali, S.; Hussain, I.; Junejo, A.; Ali, A. Advancement in Nanomaterials for Environmental Pollutants Remediation: A Systematic Review on Bibliometrics Analysis, Material Types, Synthesis Pathways, and Related Mechanisms. J. Nanobiotechnol. 2024, 22, 26. [Google Scholar] [CrossRef]
- Debnath, B.; Majumdar, M.; Bhowmik, M.; Bhowmik, K.L.; Debnath, A.; Roy, D.N. The Effective Adsorption of Tetracycline onto Zirconia Nanoparticles Synthesized by Novel Microbial Green Technology. J. Environ. Manag. 2020, 261, 110235. [Google Scholar] [CrossRef]
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Rangabhashiyam, S.; Khan, M.R.; ALOthman, Z.A. Magnetic Chitosan-Glutaraldehyde/Zinc Oxide/Fe3O4 Nanocomposite: Optimization and Adsorptive Mechanism of Remazol Brilliant Blue R Dye Removal. J. Polym. Environ. 2021, 29, 3932–3947. [Google Scholar] [CrossRef]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Han, Y.; Shi, N.; Wang, H.; Pan, X.; Fang, H.; Yu, Y. Nanoscale Zerovalent Iron-Mediated Degradation of DDT in Soil. Environ. Sci. Pollut. Res. 2016, 23, 6253–6263. [Google Scholar] [CrossRef]
- Zhang, W. Nanoscale Iron Particles for Environmental Remediation: An Overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Kim, H.; Hong, H.-J.; Lee, Y.-J.; Shin, H.-J.; Yang, J.-W. Degradation of Trichloroethylene by Zero-Valent Iron Immobilized in Cationic Exchange Membrane. Desalination 2008, 223, 212–220. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Naseem, T.; Durrani, T. The Role of Some Important Metal Oxide Nanoparticles for Wastewater and Antibacterial Applications: A Review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Fang, G.; Si, Y.; Tian, C.; Zhang, G.; Zhou, D. Degradation of 2,4-D in Soils by Fe3O4 Nanoparticles Combined with Stimulating Indigenous Microbes. Environ. Sci. Pollut. Res. Int. 2012, 19, 784–793. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Z.; Song, Y.; Zheng, X.; Qu, L.; Qian, J.; Wu, Y.; Wu, X.; Wu, Z. Remediation of Phenanthrene Contaminated Soil by G-C3N4/Fe3O4 Composites and Its Phytotoxicity Evaluation. Chemosphere 2019, 221, 554–562. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent Advances on Iron Oxide Magnetic Nanoparticles as Sorbents of Organic Pollutants in Water and Wastewater Treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef]
- Lei, M.; Chao, T.; Lei, Z. Preparation and Characterization of Hollow Magnetic Composite Nanoparticles for Cisplatin Delivery. J. Nanopart. Res. 2014, 16, 2410. [Google Scholar] [CrossRef]
- Anyat, T.; Ali, A.; Alharthi, S.; Santali, E.Y.; Iqbal, M. Facile Synthesis of Amine Functionalized Silica Coated Iron Oxide Nanoparticles for Highly Efficient Removal of Cefixime and Ceftriaxone from Wastewater. Sep. Sci. Technol. 2025, 60, 234–249. [Google Scholar] [CrossRef]
- Lei, T.; Jiang, X.; Zhou, Y.; Chen, H.; Bai, H.; Wang, S.; Yang, X. A Multifunctional Adsorbent Based on 2,3-Dimercaptosuccinic Acid/Dopamine-Modified Magnetic Iron Oxide Nanoparticles for the Removal of Heavy-Metal Ions. J. Colloid Interface Sci. 2023, 636, 153–166. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent Progress on Magnetic Iron Oxide Nanoparticles: Synthesis, Surface Functional Strategies and Biomedical Applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Sun, R. Facile Synthesis of Bifunctional Fe3O4/Au Nanocomposite and Their Application in Catalytic Reduction of 4-Nitrophenol. Mater. Res. Bull. 2014, 57, 293–299. [Google Scholar] [CrossRef]
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Pullabhotla, R.V.S.R. Green Synthesis, Characterization and Application of Silver Nanoparticles Using Bioflocculant: A Review. Bioengineering 2024, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A. Green Synthesis of Silver Nanoparticles Using Pseudoduganella Eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef]
- Manimegalai, G.; Shanthakumar, S.; Sharma, C. Silver Nanoparticles: Synthesis and Application in Mineralization of Pesticides Using Membrane Support. Int. Nano Lett. 2014, 4, 105. [Google Scholar] [CrossRef]
- Eddy, N.O.; Garg, R.; Garg, R.; Ukpe, R.A.; Abugu, H. Adsorption and Photodegradation of Organic Contaminants by Silver Nanoparticles: Isotherms, Kinetics, and Computational Analysis. Environ. Monit. Assess. 2023, 196, 65. [Google Scholar] [CrossRef]
- Bala, R.; Mittal, S.; Sharma, R.K.; Wangoo, N. A Supersensitive Silver Nanoprobe Based Aptasensor for Low Cost Detection of Malathion Residues in Water and Food Samples. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2018, 196, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Rohit, J.V.; Kailasa, S.K. Cyclen Dithiocarbamate-Functionalized Silver Nanoparticles as a Probe for Colorimetric Sensing of Thiram and Paraquat Pesticides via Host–Guest Chemistry. J. Nanopart. Res. 2014, 16, 2585. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Zhou, X. Synthesis of Silver Nanocubes as a SERS Substrate for the Determination of Pesticide Paraoxon and Thiram. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2014, 121, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for Remediation of Environmental Pollutants. Bioinorg. Chem. Appl. 2021, 2021, 1764647. [Google Scholar] [CrossRef]
- Asadishad, B.; Chahal, S.; Cianciarelli, V.; Zhou, K.; Tufenkji, N. Effect of Gold Nanoparticles on Extracellular Nutrient-Cycling Enzyme Activity and Bacterial Community in Soil Slurries: Role of Nanoparticle Size and Surface Coating. Environ. Sci. Nano 2017, 4, 907–918. [Google Scholar] [CrossRef]
- Chauhan, A.; Dhenadhayalan, N.; Yeh, J.-C.; Lin, K.-C. Photocatalytic Degradation-Based Efficient Elimination of Pesticides Using Ruthenium/Gold Metal Nanoparticle-Anchored Zirconium Dioxide. New J. Chem. 2022, 46, 22561–22573. [Google Scholar] [CrossRef]
- Singh, Y.; Saxena, M.K. Insights into the Recent Advances in Nano-Bioremediation of Pesticides from the Contaminated Soil. Front. Microbiol. 2022, 13, 982611. [Google Scholar] [CrossRef]
- Barrios, A.C.; Rico, C.M.; Trujillo-Reyes, J.; Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of Uncoated and Citric Acid Coated Cerium Oxide Nanoparticles, Bulk Cerium Oxide, Cerium Acetate, and Citric Acid on Tomato Plants. Sci. Total Environ. 2016, 563–564, 956–964. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and Other Nanomaterials for Water Remediation from Organic Pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Assadi, A.A.; El Jery, A.; Assadi, A.A.; Amrane, A. Bismuth Sillenite Crystals as Recent Photocatalysts for Water Treatment and Energy Generation: A Critical Review. Catalysts 2022, 12, 500. [Google Scholar] [CrossRef]
- Zarrabi, A.; Ghasemi-Fasaei, R. Preparation of Green Synthesized Copper Oxide Nanoparticles for Efficient Removal of Lead from Wastewaters. Int. J. Phytoremediat. 2021, 24, 855–866. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Green Synthesis Protocols, Toxicity, and Recent Progress in Nanomaterial-Based for Environmental Chemical Sensors Applications. Trends Environ. Anal. Chem. 2023, 39, e00204. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of Pesticides Using TiO2 Based Photocatalytic Strategies: A Review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.E.; Veinot, J.G.C. Removal of Residual Metal Catalysts with Iron/Iron Oxide Nanoparticles from Coordinating Environments. Langmuir ACS J. Surf. Colloids 2008, 24, 7169–7177. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Chen, J.; Zhang, M.; Inyang, M.; Li, Y.; Alva, A.; Yang, L. Engineered Carbon (Biochar) Prepared by Direct Pyrolysis of Mg-Accumulated Tomato Tissues: Characterization and Phosphate Removal Potential. Bioresour. Technol. 2013, 138, 8–13. [Google Scholar] [CrossRef]
- Villafranca, J.C.; Berton, P.; Ferguson, M.; Clausen, R.; Arancibia-Miranda, N.; Martinis, E.M. Aluminosilicates-Based Nanosorbents for Heavy Metal Removal—A Review. J. Hazard. Mater. 2024, 474, 134552. [Google Scholar] [CrossRef]
- Fernanda, C.; Taufikurohmah, T. Green Synthesis Gold Nanoparticles Using Bioreductor of Butterfly Pea (Clitoria ternatea L.) Leaf Extract as an Antioxidant. J. Pijar Mipa 2024, 19, 726–731. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Feng, H.; Song, B.; Huang, C.; Tang, X. Effect of Exogenous Carbonaceous Materials on the Bioavailability of Organic Pollutants and Their Ecological Risks. Soil Biol. Biochem. 2018, 116, 70–81. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; García Kerdan, I.; Escutia-Guadarrama, L.; Reyna-González, J.M.; Magaña, J.J.; Leyva-Gómez, G. Nanoremediation: Nanomaterials and Nanotechnologies for Environmental Cleanup. Front. Environ. Sci. 2021, 9, 793765. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects. J. Xenobiotics 2025, 15, 76. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental Applications of Carbon-Based Nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- John, J.P.; Mary Nancy, T.E.; Bindu Sharmila, T.K. A Comprehensive Review on the Environmental Applications of Graphene–Carbon Nanotube Hybrids: Recent Progress, Challenges and Prospects. Mater. Adv. 2021, 2, 6816–6838. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- Patel, D.K.; Kim, H.-B.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Carbon Nanotubes-Based Nanomaterials and Their Agricultural and Biotechnological Applications. Materials 2020, 13, 1679. [Google Scholar] [CrossRef]
- Safdar, M.; Kim, W.; Park, S.; Gwon, Y.; Kim, Y.-O.; Kim, J. Engineering Plants with Carbon Nanotubes: A Sustainable Agriculture Approach. J. Nanobiotechnol. 2022, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- da Silva Júnior, A.H.; Müller, J.D.O.M.; de Oliveira, C.R.S.; de Noni Junior, A.; Tewo, R.K.; Mhike, W.; da Silva, A.; Mapossa, A.B.; Sundararaj, U. New Insights into Materials for Pesticide and Other Agricultural Pollutant Remediation. Materials 2024, 17, 3478. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, K.; Kaur, H.; Siwal, S.S.; Saini, A.K.; Vo, D.-V.N.; Thakur, V.K. Recent Advances of Carbon-Based Nanomaterials (CBNMs) for Wastewater Treatment: Synthesis and Application. Chemosphere 2022, 299, 134364. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V. Nanotechnology-Based Remediation of Petroleum Impurities from Water. J. Pet. Sci. Eng. 2014, 122, 705–718. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and Water Treatment: Applications and Emerging Opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef]
- Zaib, Q.; Khan, I.A.; Saleh, N.B.; Flora, J.R.V.; Park, Y.-G.; Yoon, Y. Removal of Bisphenol A and 17β-Estradiol by Single-Walled Carbon Nanotubes in Aqueous Solution: Adsorption and Molecular Modeling. Water Air Soil Pollut. 2012, 223, 3281–3293. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, G.; Liu, C.; Luo, S.; Xu, X.; Chen, L.; Wang, B. Magnetic TiO2-Graphene Composite as a High-Performance and Recyclable Platform for Efficient Photocatalytic Removal of Herbicides from Water. J. Hazard. Mater. 2013, 252–253, 115–122. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, W.-H. Graphene Family Nanomaterials (GFN)-TiO2 for the Photocatalytic Removal of Water and Air Pollutants: Synthesis, Characterization, and Applications. Nanomaterials 2021, 11, 3195. [Google Scholar] [CrossRef]
- Huang Kong, E.D.; Lai, C.W.; Juan, J.C.; Pang, Y.L.; Khe, C.S.; Badruddin, I.A.; Gapsari, F.; Anam, K. Recent Advances in Titanium Dioxide Bio-Derived Carbon Photocatalysts for Organic Pollutant Degradation in Wastewater. iScience 2025, 28, 112368. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.; Mao, W.; Jiang, W. Carbon Quantum Dots Modification Reduces TiO2 Nanoparticle Toxicity in an Aquatic Food Chain. J. Hazard. Mater. 2025, 486, 137115. [Google Scholar] [CrossRef] [PubMed]
- Baragaño, D.; Forján, R.; Welte, L.; Gallego, J.L.R. Nanoremediation of As and Metals Polluted Soils by Means of Graphene Oxide Nanoparticles. Sci. Rep. 2020, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal Properties and Stability of Graphene Oxide Nanomaterials in the Aquatic Environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef]
- Yang, W.-D.; Li, Y.-R.; Lee, Y.-C. Synthesis of R-GO/TiO2 Composites via the UV-Assisted Photocatalytic Reduction of Graphene Oxide. Appl. Surf. Sci. 2016, 380, 249–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Sun, Z.; Li, C.; Pan, C. Preparation of Graphene and TiO2 Layer by Layer Composite with Highly Photocatalytic Efficiency. Prog. Nat. Sci. Mater. Int. 2011, 21, 467–471. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, W.; Zou, X.; Xu, F.; Wang, X.; Zhang, B.; Tang, J. One-Pot, Solvothermal Synthesis of TiO2–Graphene Composite Nanosheets. J. Colloid Interface Sci. 2012, 386, 198–204. [Google Scholar] [CrossRef]
- Kong, E.D.H.; Chau, J.H.F.; Lai, C.W.; Khe, C.S.; Sharma, G.; Kumar, A.; Siengchin, S.; Sanjay, M.R. GO/TiO2-Related Nanocomposites as Photocatalysts for Pollutant Removal in Wastewater Treatment. Nanomaterials 2022, 12, 3536. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene Coupled TiO2 Photocatalysts for Environmental Applications: A Review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, K.; Al Ejji, M.; Ahmed, A.K.; Mutahir, H.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. A Carbon Dot-Based Clay Nanocomposite for Efficient Heavy Metal Removal. Nanoscale Adv. 2023, 5, 4224–4232. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.C.; Gomes, C.G.; Pina, J.; Pereira, R.F.P.; Murtinho, D.; Fajardo, A.R.; Valente, A.J.M. Carbon Quantum Dots-Containing Poly(β-Cyclodextrin) for Simultaneous Removal and Detection of Metal Ions from Water. Carbohydr. Polym. 2024, 323, 121464. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Wen, Y.; Bahrami, A.; Karaman, C.; Zare, N.; Karimi-Maleh, H.; Vasseghian, Y. Magnetic-MXene-Based Nanocomposites for Water and Wastewater Treatment: A Review. J. Water Process Eng. 2022, 47, 102696. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon Quantum Dots for Advanced Electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, J. Fluorescent Carbon Quantum Dots for Heavy Metal Sensing. Sci. Total Environ. 2024, 957, 177473. [Google Scholar] [CrossRef]
- Liu, S.; Ge, H.; Wang, C.; Zou, Y.; Liu, J. Agricultural Waste/Graphene Oxide 3D Bio-Adsorbent for Highly Efficient Removal of Methylene Blue from Water Pollution. Sci. Total Environ. 2018, 628–629, 959–968. [Google Scholar] [CrossRef]
- Tsubouchi, L.M.S.; de Almeida, E.A.; Santo, D.E.; Bona, E.; Pereira, G.L.D.; Jegatheesan, V.; Cardozo-Filho, L.; Peron, A.P.; Junior, O.V. Production and Characterization of Graphene Oxide for Adsorption Analysis of the Emerging Pollutant Butylparaben. Water 2024, 16, 3703. [Google Scholar] [CrossRef]
- Coats, J.R. Pesticide Degradation Mechanisms and Environmental Activation. In Pesticide Transformation Products; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1991; Volume 459, pp. 10–30. ISBN 978-0-8412-1994-6. [Google Scholar]
- Beljin, J.; Đukanović, N.; Anojčić, J.; Simetić, T.; Apostolović, T.; Mutić, S.; Maletić, S. Biochar in the Remediation of Organic Pollutants in Water: A Review of Polycyclic Aromatic Hydrocarbon and Pesticide Removal. Nanomaterials 2024, 15, 26. [Google Scholar] [CrossRef]
- Batzill, M. Fundamental Aspects of Surface Engineering of Transition Metal Oxide Photocatalysts. Energy Environ. Sci. 2011, 4, 3275–3286. [Google Scholar] [CrossRef]
- Cork, D.J.; Krueger, J.P. Microbial Transformations of Herbicides and Pesticides. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1991; Volume 36, pp. 1–66. ISBN 978-0-12-002636-4. [Google Scholar]
- Busto, M.D.; Smith, P.P.; Perez-Mateos, M.; Burns, R.G. Degradation of Aliphatic Halogen-Substituted Pesticides by Dehalogenase Isolated from Pseudomonas Alcaligenes. Identification and Properties of the Enzyme. Sci. Total Environ. 1992, 123–124, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Latest Generation of Halogen-Containing Pesticides. Pest Manag. Sci. 2017, 73, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Shi, G.; Yan, Y.; Wang, Q.; Yu, Z.; Yan, L.; Yu, Y. Adsorption of Chlorinated Hydrocarbons by Different Kinds of Soils: Kinetics, Influencing Factors, Mechanism. J. Hazard. Mater. Adv. 2025, 18, 100638. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Removal of Pesticides from Waters by Adsorption: Comparison between Synthetic Zeolites and Mesoporous Silica Materials. A Review. Materials 2021, 14, 3532. [Google Scholar] [CrossRef]
- Topp, G.C.; Reynolds, W.D.; Cook, F.J.; Kirby, J.M.; Carter, M.R. Chapter 2 Physical attributes of soil quality. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 1997; pp. 21–58. [Google Scholar]
- Ge, J.; Fu, W.; Bai, M.; Zhang, L.; Guo, B.; Qiao, Q.; Tao, R.; Kou, J. The Degradation of Residual Pesticides and the Quality of White Clover Silage Are Related to the Types and Initial Concentrations of Pesticides. J. Pestic. Sci. 2021, 46, 342–351. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T. Effect of Concentration, Moisture and Soil Type on the Dissipation of Flufenacet from Soil. Chemosphere 2002, 47, 901–906. [Google Scholar] [CrossRef]
- Yu, Y.L.; Chen, Y.X.; Luo, Y.M.; Pan, X.D.; He, Y.F.; Wong, M.H. Rapid Degradation of Butachlor in Wheat Rhizosphere Soil. Chemosphere 2003, 50, 771–774. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F. Bioremediation of Agricultural Soils Polluted with Pesticides: A Review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef]
- Gold, R.E.; Howell, H.N.; Pawson, B.M.; Wright, M.S.; Lutz, J.C. Evaluation of Termiticides Residues and Bioavailability from Five Soil Types and Locations in Texas. In Proceedings of the 2nd International Conference on Insects Pests in the Environment, Edinburgh, Scotland, 7–10 July 1996. [Google Scholar]
- Schneiders, G.E.; Koeppe, M.K.; Naidu, M.V.; Horne, P.; Brown, A.M.; Mucha, C.F. Fate of Rimsulfuron in the Environment. J. Agric. Food Chem. 1993, 41, 2404–2410. [Google Scholar] [CrossRef]
- Jones, W.J.; Ananyeva, N.D. Correlations between Pesticide Transformation Rate and Microbial Respiration Activity in Soil of Different Ecosystems. Biol. Fertil. Soils 2001, 33, 477–483. [Google Scholar] [CrossRef]
- Hafez, H.F.H.; Thiemann, W.H.P. Persistence and Biodegradation of Diazinon and Imidacloprid in Soil; La Goliardica Pavese s.r.l.: Piacenza, Italy, 2003; Volume 2003, pp. 35–42. [Google Scholar]
- Pal, R.; Chakrabarti, K.; Chakraborty, A.; Chowdhury, A. Pencycuron Application to Soils: Degradation and Effect on Microbiological Parameters. Chemosphere 2005, 60, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Walter-Echols, G.; Lichtenstein, E.P. Movement and Metabolism of [14C]Phorate in a Flooded Soil System. J. Agric. Food Chem. 1978, 26, 599–604. [Google Scholar] [CrossRef]
- Chowdhury, A.; Pradhan, S.; Saha, M.; Sanyal, N. Impact of Pesticides on Soil Microbiological Parameters and Possible Bioremediation Strategies. Indian J. Microbiol. 2008, 48, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Racke, K.D.; Skidmore, M.W.; Hamilton, D.J.; Unsworth, J.B.; Miyamoto, J.; Cohen, S.Z.; Holland, P.T. Pesticide Fate in Tropical Soils. Pure Appl. Chem. 1997, 69, 1349–1371. Available online: https://old.iupac.org/publications/pac/1997/pdf/6906x1349.pdf (accessed on 10 July 2025). [CrossRef]
- Perucci, P.; Vischetti, C.; Battistoni, F. Rimsulfuron in a Silty Clay Loam Soil: Effects upon Microbiological and Biochemical Properties under Varying Microcosm Conditions. Soil Biol. Biochem. 1999, 31, 195–204. [Google Scholar] [CrossRef]
- Vischetti, C.; Perucci, P.; Scarponi, L. Relationship between Rimsulfuron Degradation and Microbial Biomass Content in a Clay Loam Soil. Biol. Fertil. Soils 2000, 31, 310–314. [Google Scholar] [CrossRef]
- Ghimire, N.; Bakke, R.; Bergland, W.H. Mesophilic Anaerobic Digestion of Hydrothermally Pretreated Lignocellulosic Biomass (Norway Spruce (Picea Abies)). Processes 2021, 9, 190. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of Engineered Nanoparticles as Fertilizers for Increasing Agronomic Productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Alkhaza’leh, H.; Rahbeh, M.; Hamadneh, I.; AL-Mashakbeh, H.; Albalawna, Z. Nanoparticles in Soil Reclamation: A Review of Their Role in Reducing Soil Compaction. Air Soil Water Res. 2025, 18, 11786221241311725. [Google Scholar] [CrossRef]
- Umair, M.; Huma Zafar, S.; Cheema, M.; Usman, M. New Insights into the Environmental Application of Hybrid Nanoparticles in Metal Contaminated Agroecosystem: A Review. J. Environ. Manag. 2024, 349, 119553. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Kakkar, R. Hierarchically Structured Magnesium Based Oxides: Synthesis Strategies and Applications in Organic Pollutant Remediation. CrystEngComm 2017, 19, 6913–6926. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.; Morin-Crini, N.; Wilson, L.D. Adsorption-Oriented Processes Using Conventional and Non-Conventional Adsorbents for Wastewater Treatment Adsorption-Oriented Processes Using Conventional and Non-Conventional Adsorbents for Wastewater Treatment. In Green Adsorbents for Pollutant Removal Adsorption-Oriented Processes Using Conventional and Non-conventional Adsorbents for Wastewater Treatment; Environmental Chemistry for a Sustainable World Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 18, pp. 23–71. [Google Scholar] [CrossRef]
- Adesanmi, B.O.; Mantripragada, S.; Ayivi, R.D.; Tukur, P.; Obare, S.O.; Wei, J. Adsorptive Removal of Organophosphate Pesticides from Aqueous Solution Using Electrospun Carbon Nanofibers. Front. Chem. 2024, 12, 1454367. [Google Scholar] [CrossRef]
- Huber, F.; Berwanger, J.; Polesya, S.; Mankovsky, S.; Ebert, H.; Giessibl, F.J. Chemical Bond Formation Showing a Transition from Physisorption to Chemisorption. Science 2019, 366, 235–238. [Google Scholar] [CrossRef]
- Kecili, R.; Hussain, C.M. Chapter 4—Mechanism of Adsorption on Nanomaterials. In Nanomaterials in Chromatography; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–115. ISBN 978-0-12-812792-6. [Google Scholar]
- Králik, M. Adsorption, Chemisorption, and Catalysis. Chem. Pap. 2014, 68, 1625–1638. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of Pesticides from Water and Wastewater: Chemical, Physical and Biological Treatment Approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Momić, T.; Pašti, T.L.; Bogdanović, U.; Vodnik, V.; Mraković, A.; Rakočević, Z.; Pavlović, V.B.; Vasić, V. Adsorption of Organophosphate Pesticide Dimethoate on Gold Nanospheres and Nanorods. J. Nanomater. 2016, 2016, 8910271. [Google Scholar] [CrossRef]
- Liu, G.; Dai, Z.; Liu, X.; Dahlgren, R.A.; Xu, J. Modification of Agricultural Wastes to Improve Sorption Capacities for Pollutant Removal from Water—A Review. Carbon Res. 2022, 1, 24. [Google Scholar] [CrossRef]
- Guo, F.; Li, D.; Fein, J.B.; Xu, J.; Wang, Y.; Huang, Q.; Rong, X. Roles of Hydrogen Bond and Ion Bridge in Adsorption of Two Bisphenols onto Montmorillonite: An Experimental and DFT Study. Appl. Clay Sci. 2022, 217, 106406. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Connor, A.J.; Koffas, M.; Zha, R.H. Chemical Synthesis of Silk-Mimetic Polymers. Materials 2019, 12, 4086. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening Doors for a Sustainable Future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin—Based Materials for Biomedical Applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Luhach, V.; Yadav, J.; Barala, P.; Sangwan, P.; Suhag, S.; Hooda, V. The Future of Herbicide Application: Controlled-Release Formulations for Enhanced Agricultural Sustainability. Des. Monomers Polym. 2025, 28, 24–43. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as Agricultural Polymers for Packaging Production. Cereal Chem. J. 1998, 75, 1–9. [Google Scholar] [CrossRef]
- Cortés-Martínez, C.I.; Ruiz-Vega, J.; Martínez-Gutiérrez, G.A. Moisture Evaporation from Granular Biopesticides Containing Quiescent Entomopathogenic Nematodes. In Current Perspective to Predict Actual Evapotranspiration; Bucur, D., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3173-1. [Google Scholar]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern Seed Technology: Seed Coating Delivery Systems for Enhancing Seed and Crop Performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-Based Smart Pesticide Formulations: Emerging Opportunities for Agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Saberi-Riseh, R.; Moradi-Pour, M.; Mohammadinejad, R.; Thakur, V.K. Biopolymers for Biological Control of Plant Pathogens: Advances in Microencapsulation of Beneficial Microorganisms. Polymers 2021, 13, 1938. [Google Scholar] [CrossRef]
- De Frates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef]
- Gong, H.; Fu, H.; Zhang, J.; Zhang, Q.; Wang, Y.; Wang, D.; Cai, L.; Chen, J.; Yu, H.; Lyu, B. Preparation of Soybean Protein-Based Nanoparticles and Its Application as Encapsulation Carriers of Bioactive Substances. LWT 2024, 191, 115680. [Google Scholar] [CrossRef]
- Mgadi, K.; Ndaba, B.; Roopnarain, A.; Rama, H.; Adeleke, R. Nanoparticle Applications in Agriculture: Overview and Response of Plant-Associated Microorganisms. Front. Microbiol. 2024, 15, 1354440. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Marmiroli, M. Editorial: Polymeric Nanoparticles for Sustainable Plant Agriculture and Food Industry. Front. Plant Sci. 2023, 14, 1183938. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, T.; Wang, Y.; Li, T.; Chi, Q. Multifaceted Impacts of Nanoparticles on Plant Nutrient Absorption and Soil Microbial Communities. Front. Plant Sci. 2024, 15, 1497006. [Google Scholar] [CrossRef]
- Dutta, S.; Pal, S.; Panwar, P.; Sharma, R.K.; Bhutia, P.L. Biopolymeric Nanocarriers for Nutrient Delivery and Crop Biofortification. ACS Omega 2022, 7, 25909–25920. [Google Scholar] [CrossRef]
- Dissanayake, T.; Bandara, N. Protein-Based Encapsulation Systems for Codelivery of Bioactive Compounds: Recent Studies and Potential Applications. Curr. Opin. Food Sci. 2024, 57, 101181. [Google Scholar] [CrossRef]
- Chen, F.-P.; Ou, S.-Y.; Tang, C.-H. Core–Shell Soy Protein–Soy Polysaccharide Complex (Nano)Particles as Carriers for Improved Stability and Sustained Release of Curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. [Google Scholar] [CrossRef] [PubMed]
- Vinzant, K.; Rashid, M.; Khodakovskaya, M.V. Advanced Applications of Sustainable and Biological Nano-Polymers in Agricultural Production. Front. Plant Sci. 2023, 13, 1081165. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebova, L.; Trinick, J. Titin: Properties and Family Relationships. Nat. Rev. Mol. Cell Biol. 2003, 4, 679–689. [Google Scholar] [CrossRef]
- Granzier, H.L.; Labeit, S. Discovery of Titin and Its Role in Heart Function and Disease. Circ. Res. 2025, 136, 135–157. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Y.; Li, J.; Liu, K.; Wu, F. A State-of-the-Art Review of Organic Polymer Modifiers for Slope Eco-Engineering. Polymers 2023, 15, 2878. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Mahmoud Abdelshafy, A.; Luo, Z.; Belwal, T.; Lin, X.; Xu, Y.; Wang, L.; Yang, M.; Qi, M.; Dong, Y.; et al. Occurrence, Detection, and Dissipation of Pesticide Residue in Plant-Derived Foodstuff: A State-of-the-Art Review. Food Chem. 2022, 384, 132494. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Lin, X.; Khan, F.; Bennett, A.E.; Winter, J.O. Translating Controlled Release Systems from Biomedicine to Agriculture. Front. Biomater. Sci. 2022, 1, 1011877. [Google Scholar] [CrossRef]
- Petersen, R.S.; Nielsen, L.H.; Rindzevicius, T.; Boisen, A.; Keller, S.S. Controlled Drug Release from Biodegradable Polymer Matrix Loaded in Microcontainers Using Hot Punching. Pharmaceutics 2020, 12, 1050. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Hassanisaadi, M.; Thakur, V.K.; Kennedy, J.F. Use of Whey Protein as a Natural Polymer for the Encapsulation of Plant Biocontrol Bacteria: A Review. Int. J. Biol. Macromol. 2023, 234, 123708. [Google Scholar] [CrossRef]
- Sasson, E.; Pinhasi, R.V.O.; Margel, S.; Klipcan, L. Engineering and Use of Proteinoid Polymers and Nanocapsules Containing Agrochemicals. Sci. Rep. 2020, 10, 9171. [Google Scholar] [CrossRef]
- Geitner, N.K.; Zhao, W.; Ding, F.; Chen, W.; Wiesner, M.R. Mechanistic Insights from Discrete Molecular Dynamics Simulations of Pesticide–Nanoparticle Interactions. Environ. Sci. Technol. 2017, 51, 8396–8404. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Chen, W.-J.; Huang, Y.; Xiao, Y.; Wu, S.; Lei, Q.; Zhong, J.; Zhu, X.; Chen, S. Bioremediation Potential of Laccase for Catalysis of Glyphosate, Isoproturon, Lignin, and Parathion: Molecular Docking, Dynamics, and Simulation. J. Hazard. Mater. 2023, 443, 130319. [Google Scholar] [CrossRef]
- Park, H.; May, A.; Portilla, L.; Dietrich, H.; Münch, F.; Rejek, T.; Sarcletti, M.; Banspach, L.; Zahn, D.; Halik, M. Magnetite Nanoparticles as Efficient Materials for Removal of Glyphosate from Water. Nat. Sustain. 2020, 3, 129–135. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate: Environmental Fate and Impact. Weed Sci. 2020, 68, 201–207. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, T.; Bhatt, K.; Zhang, W.; Huang, Y.; Chen, S. Binding Interaction of Glyphosate with Glyphosate Oxidoreductase and C–P Lyase: Molecular Docking and Molecular Dynamics Simulation Studies. J. Hazard. Mater. 2021, 409, 124927. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, C.; Yang, L.; Liu, C.-Z. Magnetic Mesoporous Silica Nanoparticles: Fabrication and Their Laccase Immobilization Performance. Bioresour. Technol. 2010, 101, 8931–8935. [Google Scholar] [CrossRef] [PubMed]
- Daumann, L.J.; Larrabee, J.A.; Ollis, D.; Schenk, G.; Gahan, L.R. Immobilization of the Enzyme GpdQ on Magnetite Nanoparticles for Organophosphate Pesticide Bioremediation. J. Inorg. Biochem. 2014, 131, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Limon, A.; García Suárez, P.C.; Arellano-García, E.; Contreras, O.E.; Aguila, S.A. Enhanced Degradation of Pesticide Dichlorophen by Laccase Immobilized on Nanoporous Materials: A Cytotoxic and Molecular Simulation Investigation. Bioconjug. Chem. 2018, 29, 1073–1080. [Google Scholar] [CrossRef]
- Peixoto, R.S.; Vermelho, A.B.; Rosado, A.S. Petroleum-Degrading Enzymes: Bioremediation and New Prospects. Enzyme Res. 2011, 2011, 475193. [Google Scholar] [CrossRef]
- Sharma, K.; Tewatia, P.; Kaur, M.; Pathania, D.; Banat, F.; Rattan, G.; Singhal, S.; Kaushik, A. Bioremediation of Multifarious Pollutants Using Laccase Immobilized on Magnetized and Carbonyldiimidazole-Functionalized Cellulose Nanofibers. Sci. Total Environ. 2023, 864, 161137. [Google Scholar] [CrossRef]
- Paul, C.; Pal, N.; Maitra, M.; Das, N. Laccase-Assisted Bioremediation of Pesticides: Scope and Challenges. Mini-Rev. Org. Chem. 2024, 21, 633–654. [Google Scholar] [CrossRef]
- Medina, F.; Aguila, S.; Baratto, M.C.; Martorana, A.; Basosi, R.; Alderete, J.B.; Vazquez-Duhalt, R. Prediction Model Based on Decision Tree Analysis for Laccase Mediators. Enzyme Microb. Technol. 2013, 52, 68–76. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Fan, H.; Wang, Z.; Zheng, J.-J.; Fan, K.; Yan, X.; Gao, X. Mechanism and Kinetics-Guided Discovery of Nanometal Scissors to Cut Phosphoester Bonds. ACS Catal. 2023, 13, 504–514. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Gao, X.; Zhao, Y. Simultaneous Enzyme Mimicking and Chemical Reduction Mechanisms for Nanoceria as a Bio-Antioxidant: A Catalytic Model Bridging Computations and Experiments for Nanozymes. Nanoscale 2019, 11, 13289–13299. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, L.; Song, K.; Cheng, M.; Fang, L.; Kong, L.; Yu, L.; Wang, R.; Fu, Z.; Sun, M.; et al. Stable Peptide-Assembled Nanozyme Mimicking Dual Antifungal Actions. Nat. Commun. 2024, 15, 5636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, X.; Gao, X. Density Functional Theory Mechanistic Insight into the Peroxidase- and Oxidase-like Activities of Nanoceria. J. Phys. Chem. C 2021, 125, 23098–23104. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J. Artificial Intelligence-Based Toxicity Prediction of Environmental Chemicals: Future Directions for Chemical Management Applications. Environ. Sci. Technol. 2022, 56, 7532–7543. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Q.; Mu, L.; Hu, X. Machine Learning in the Identification, Prediction and Exploration of Environmental Toxicology: Challenges and Perspectives. J. Hazard. Mater. 2022, 438, 129487. [Google Scholar] [CrossRef]
- Huang, R.; Xia, M.; Nguyen, D.-T.; Zhao, T.; Sakamuru, S.; Zhao, J.; Shahane, S.A.; Rossoshek, A.; Simeonov, A. Tox21Challenge to Build Predictive Models of Nuclear Receptor and Stress Response Pathways as Mediated by Exposure to Environmental Chemicals and Drugs. Front. Environ. Sci. 2016, 3, 85. [Google Scholar] [CrossRef]
- Golbraikh, A.; Wang, X.S.; Zhu, H.; Tropsha, A. Predictive QSAR Modeling: Methods and Applications in Drug Discovery and Chemical Risk Assessment. In Handbook of Computational Chemistry; Springer: Cham, Switzerland, 2017; pp. 2303–2340. ISBN 978-3-319-27282-5. [Google Scholar]
- Guha, R.; Willighagen, E. A Survey of Quantitative Descriptions of Molecular Structure. Curr. Top. Med. Chem. 2012, 12, 1946–1956. [Google Scholar] [CrossRef]
- Danishuddin; Khan, A.U. Descriptors and Their Selection Methods in QSAR Analysis: Paradigm for Drug Design. Drug Discov. Today 2016, 21, 1291–1302. [Google Scholar] [CrossRef]
- Richard, A.M.; Huang, R.; Waidyanatha, S.; Shinn, P.; Collins, B.J.; Thillainadarajah, I.; Grulke, C.M.; Williams, A.J.; Lougee, R.R.; Judson, R.S.; et al. The Tox21 10K Compound Library: Collaborative Chemistry Advancing Toxicology. Chem. Res. Toxicol. 2021, 34, 189–216. [Google Scholar] [CrossRef]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards Direct Deposition of Bioassay Data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef]
- Wetmore, B.A.; Wambaugh, J.F.; Ferguson, S.S.; Sochaski, M.A.; Rotroff, D.M.; Freeman, K.; Clewell, H.J., III; Dix, D.J.; Andersen, M.E.; Houck, K.A.; et al. Integration of Dosimetry, Exposure, and High-Throughput Screening Data in Chemical Toxicity Assessment. Toxicol. Sci. 2012, 125, 157–174. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, C.; Zhang, Y. A Comparative Analysis of Linear Regression, Neural Networks and Random Forest Regression for Predicting Air Ozone Employing Soft Sensor Models. Sci. Rep. 2023, 13, 22420. [Google Scholar] [CrossRef] [PubMed]
- Cristianini, N.; Shawe-Taylor, J. An Introduction to Support Vector Machines and Other Kernel-Based Learning Methods; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-78019-3. [Google Scholar]
- Zhong, M.; Nie, X.; Yan, A.; Yuan, Q. Carcinogenicity Prediction of Noncongeneric Chemicals by a Support Vector Machine. Chem. Res. Toxicol. 2013, 26, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Xu, S.; Zheng, H.; Zhang, L.; Chen, J.; Hong, H.; Kusko, R.; Li, R. Quantitative Structure–Activity Relationship Models for Predicting Inflammatory Potential of Metal Oxide Nanoparticles. Environ. Health Perspect. 2020, 128, 067010. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Basu, S.; Heng, J.J.H.; Loo, L.-H.; Zink, D. Predicting Direct Hepatocyte Toxicity in Humans by Combining High-Throughput Imaging of HepaRG Cells and Machine Learning-Based Phenotypic Profiling. Arch. Toxicol. 2020, 94, 2749–2767. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Jain, S.; Siramshetty, V.B.; Alves, V.M.; Muratov, E.N.; Kleinstreuer, N.; Tropsha, A.; Nicklaus, M.C.; Simeonov, A.; Zakharov, A.V. Large-Scale Modeling of Multispecies Acute Toxicity End Points Using Consensus of Multitask Deep Learning Methods. J. Chem. Inf. Model. 2021, 61, 653–663. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Cai, Y.; Cao, Q.; Sun, L.; Wang, Z.; Li, W.; Liu, G.; Lee, P.W.; Tang, Y. In Silico Prediction of Chemical Aquatic Toxicity for Marine Crustaceans via Machine Learning. Toxicol. Res. 2019, 8, 341–352. [Google Scholar] [CrossRef]
- Shen, C.; Zhu, K.; Ruan, J.; Li, J.; Wang, Y.; Zhao, M.; He, C.; Zuo, Z. Screening of Potential Oestrogen Receptor α Agonists in Pesticides via in Silico, in Vitro and in Vivo Methods. Environ. Pollut. 2021, 270, 116015. [Google Scholar] [CrossRef]
- Ke, M.; Xu, N.; Zhang, Z.; Qiu, D.; Kang, J.; Lu, T.; Wang, T.; Peijnenburg, W.J.G.M.; Sun, L.; Hu, B.; et al. Development of a Machine-Learning Model to Identify the Impacts of Pesticides Characteristics on Soil Microbial Communities from High-Throughput Sequencing Data. Environ. Microbiol. 2022, 24, 5561–5573. [Google Scholar] [CrossRef]
- Jia, X.; Wang, T.; Zhu, H. Advancing Computational Toxicology by Interpretable Machine Learning. Environ. Sci. Technol. 2023, 57, 17690–17706. [Google Scholar] [CrossRef] [PubMed]
- Elmarakeby, H.A.; Hwang, J.; Arafeh, R.; Crowdis, J.; Gang, S.; Liu, D.; AlDubayan, S.H.; Salari, K.; Kregel, S.; Richter, C. Biologically Informed Deep Neural Network for Prostate Cancer Discovery. Nature 2021, 598, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.; Scott, A.M.; Karlsson, C.; Mohanty, T.; Vaara, S.T.; Linder, A.; Malmström, L.; Malmström, J. Interpreting Biologically Informed Neural Networks for Enhanced Proteomic Biomarker Discovery and Pathway Analysis. Nat. Commun. 2023, 14, 5359. [Google Scholar] [CrossRef] [PubMed]
- Kuenzi, B.M.; Park, J.; Fong, S.H.; Sanchez, K.S.; Lee, J.; Kreisberg, J.F.; Ma, J.; Ideker, T. Predicting Drug Response and Synergy Using a Deep Learning Model of Human Cancer Cells. Cancer Cell 2020, 38, 672–684.e6. [Google Scholar] [CrossRef]
- Ma, J.; Yu, M.K.; Fong, S.; Ono, K.; Sage, E.; Demchak, B.; Sharan, R.; Ideker, T. Using Deep Learning to Model the Hierarchical Structure and Function of a Cell. Nat. Methods 2018, 15, 290–298. [Google Scholar] [CrossRef]
- Adamczyk, J.; Poziemski, J.; Siedlecki, P. Evaluating Machine Learning Models for Predicting Pesticides Toxicity to Honey Bees. arXiv 2025, arXiv:2503.24305. [Google Scholar] [CrossRef]
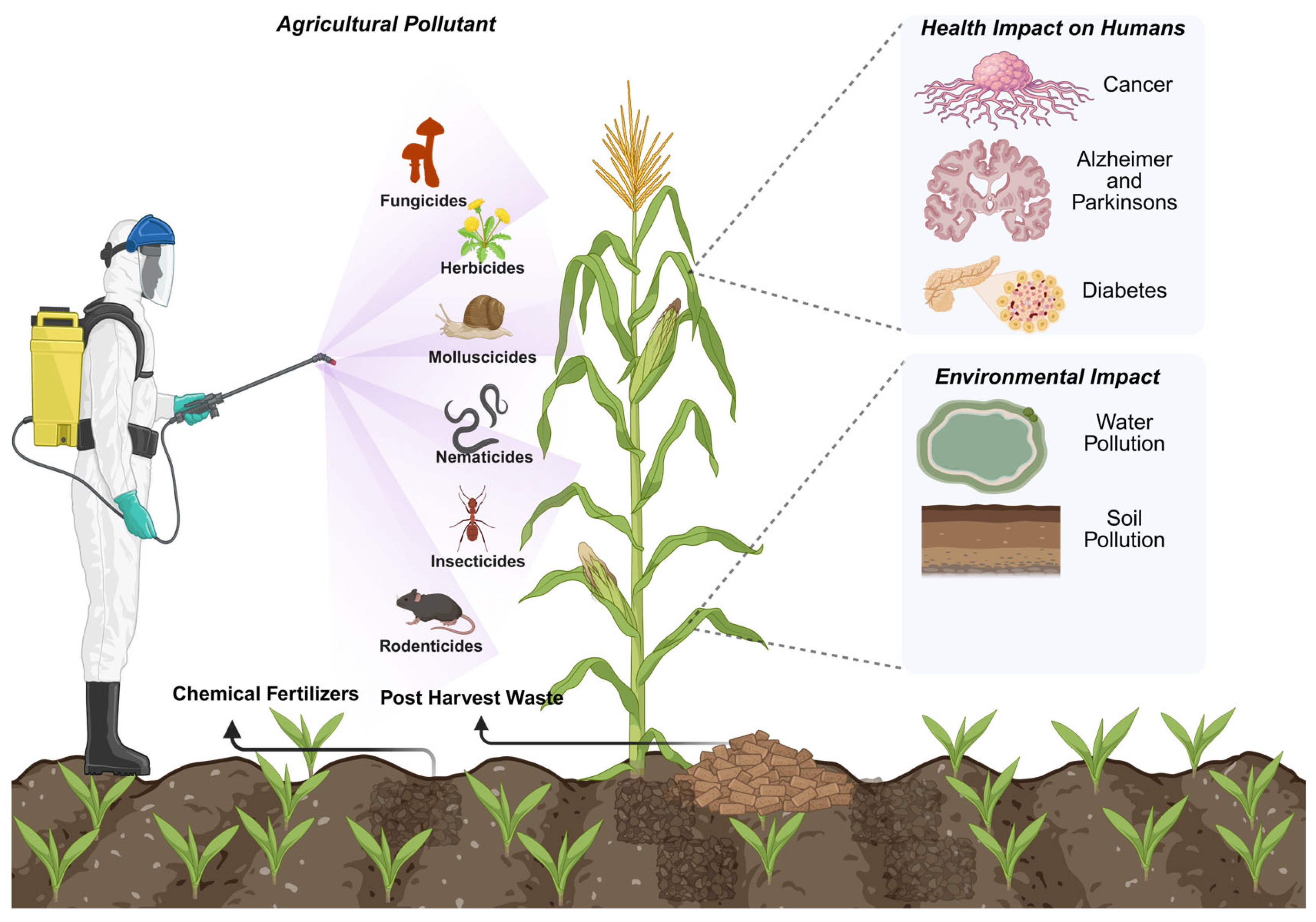
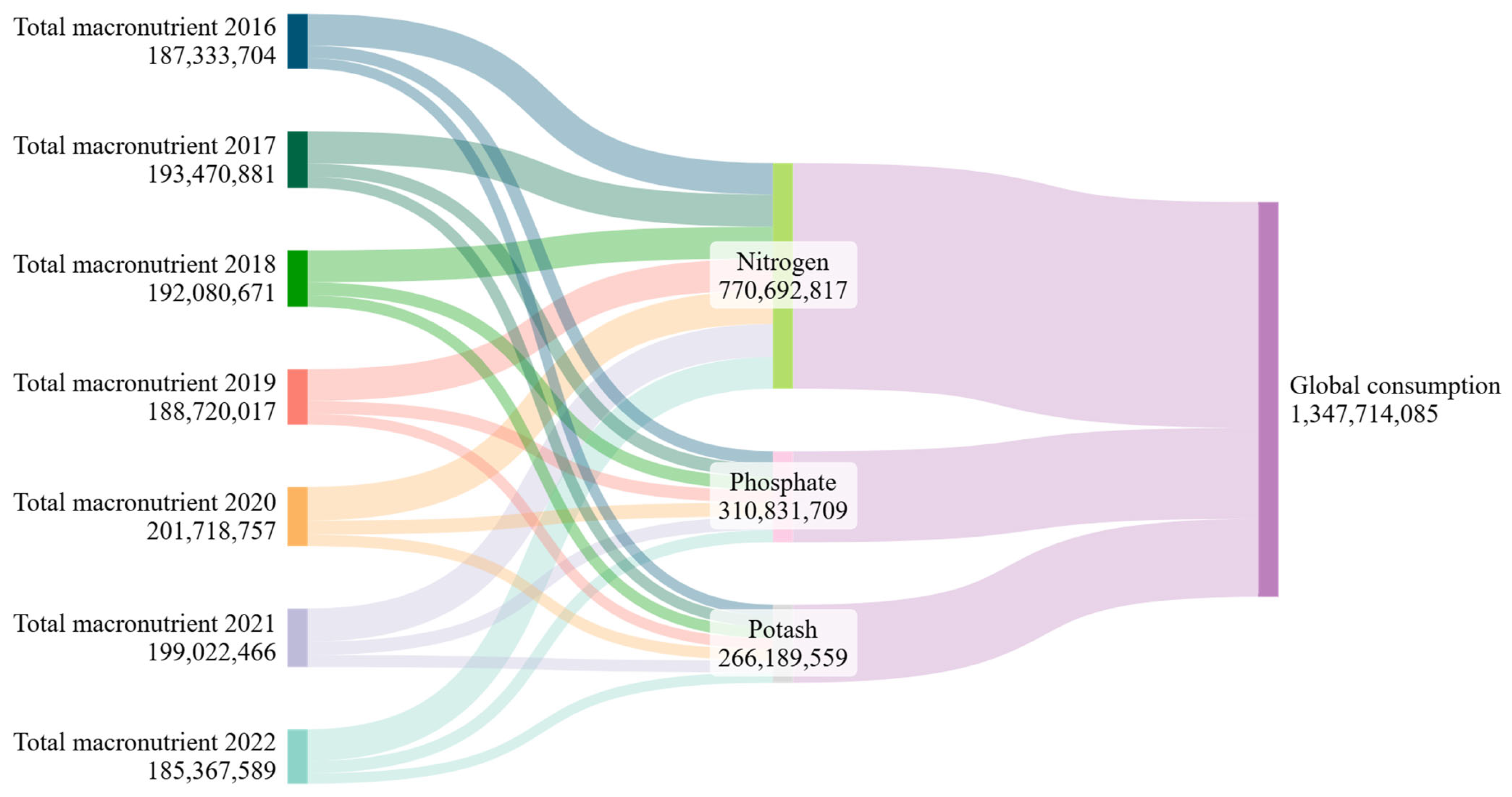
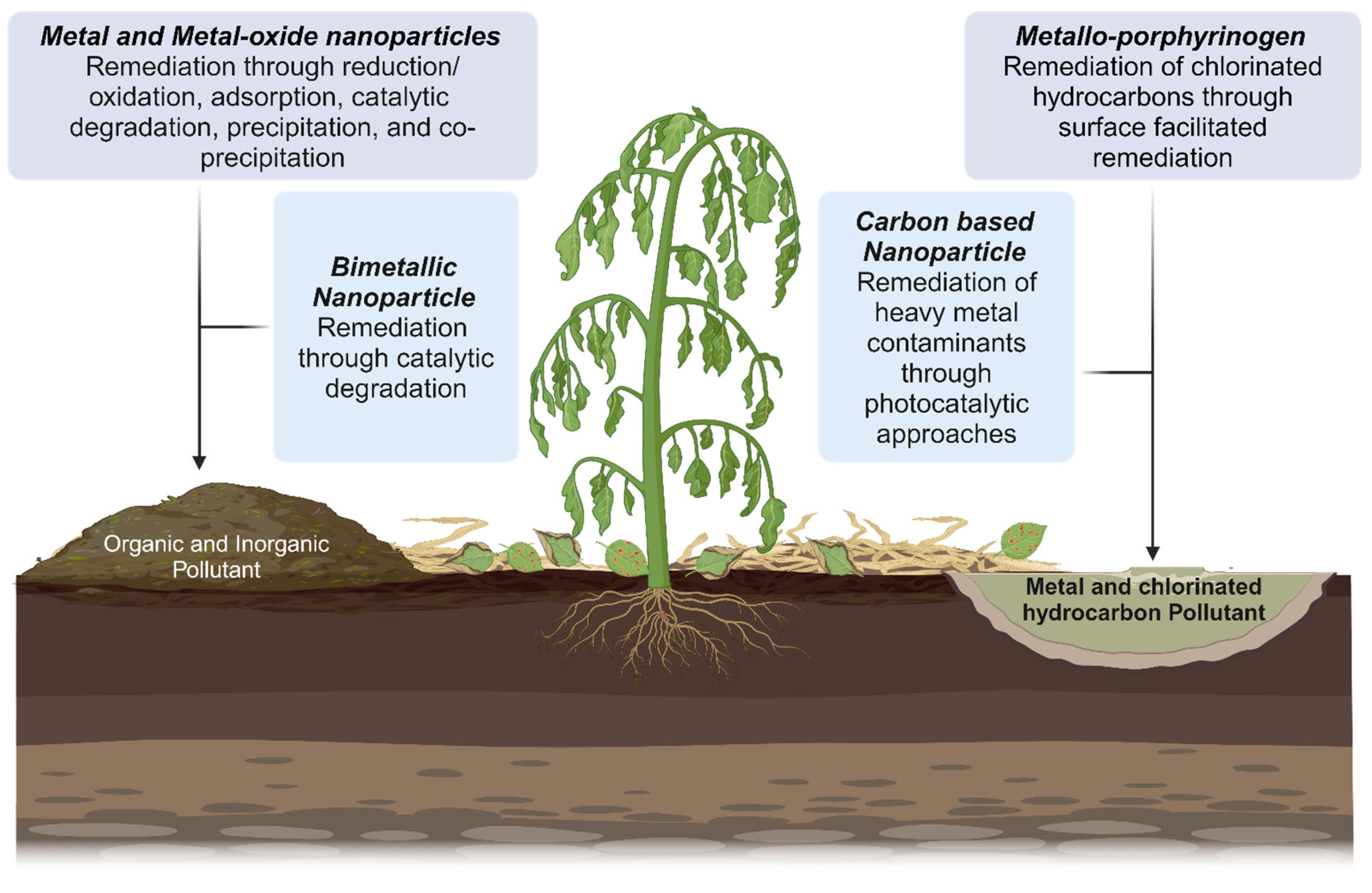
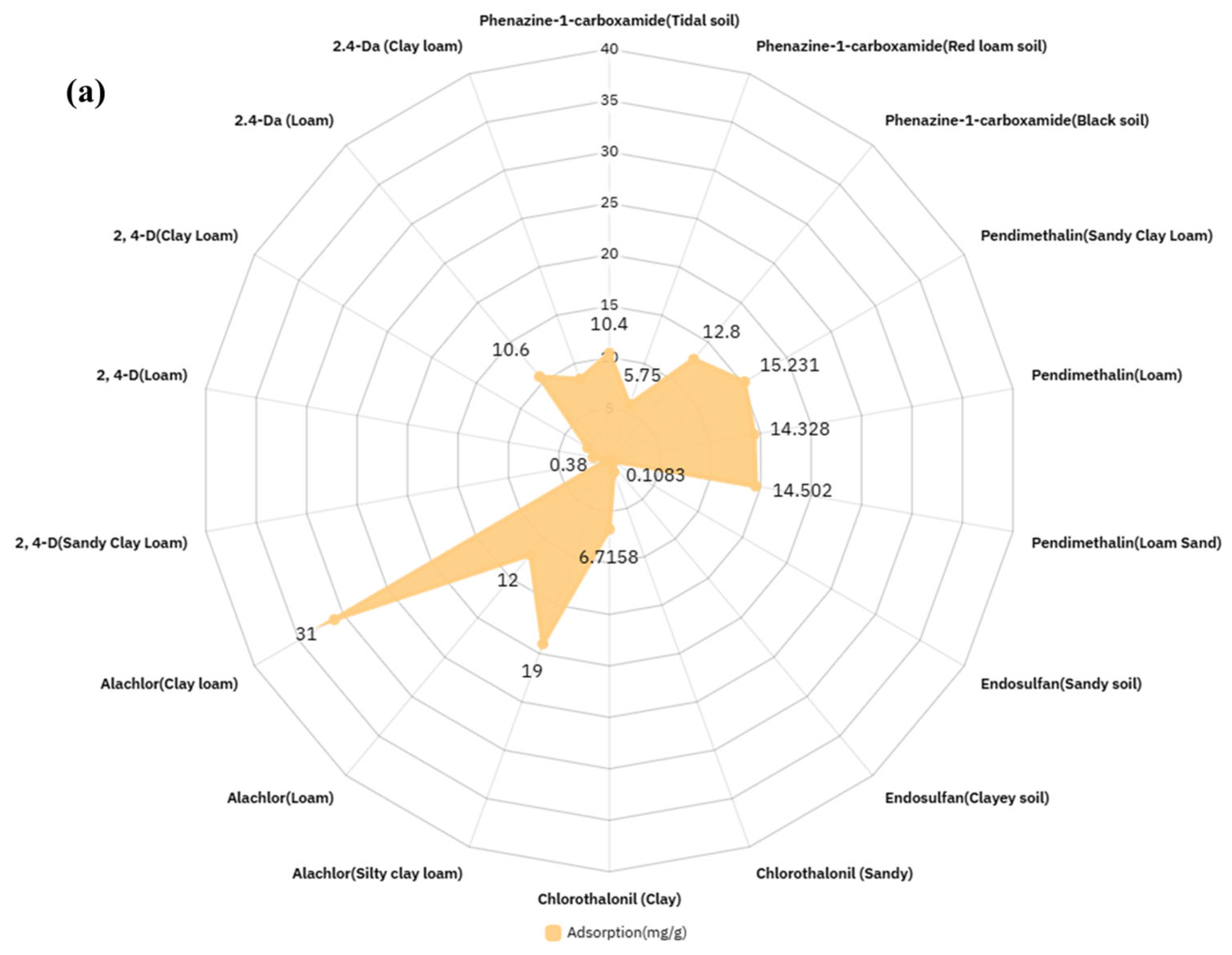
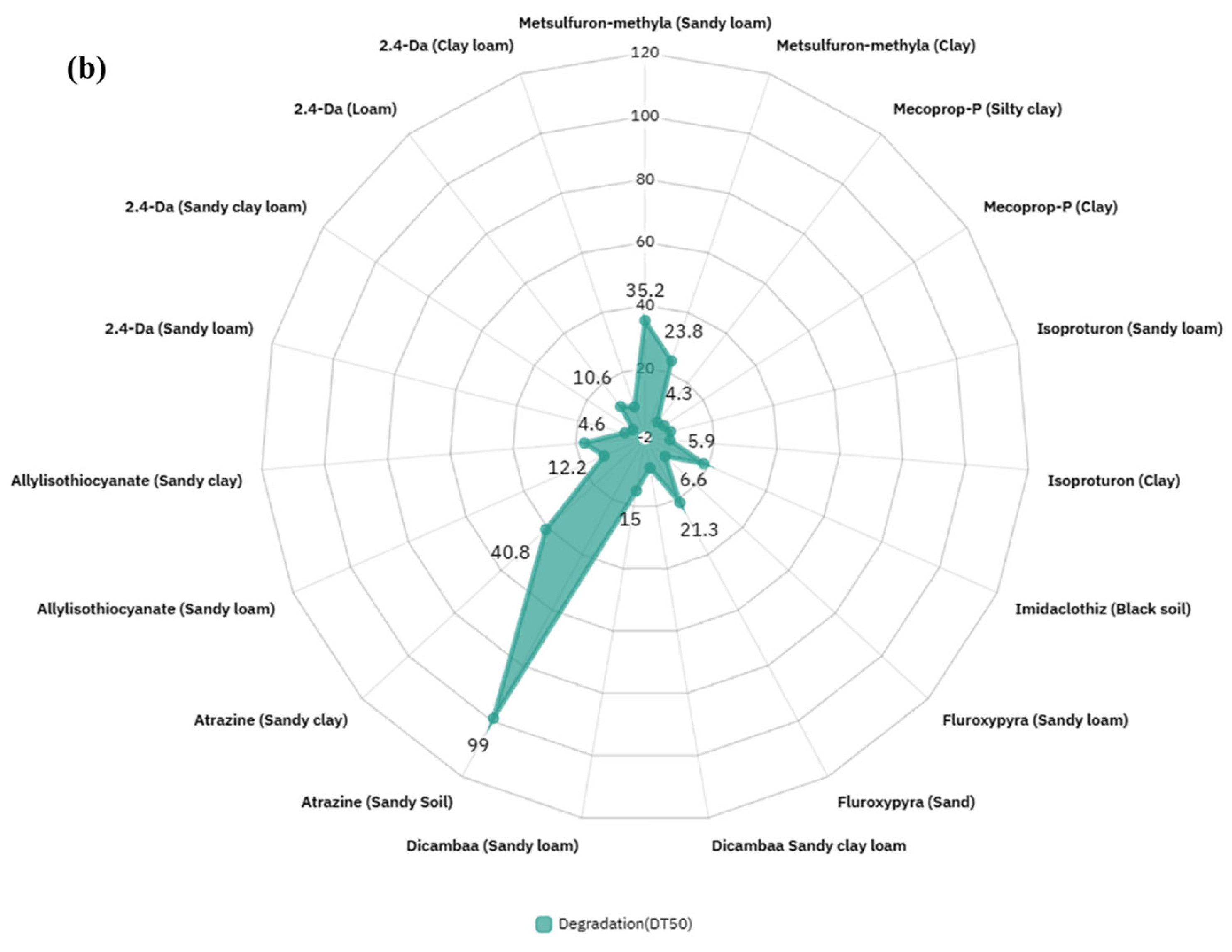
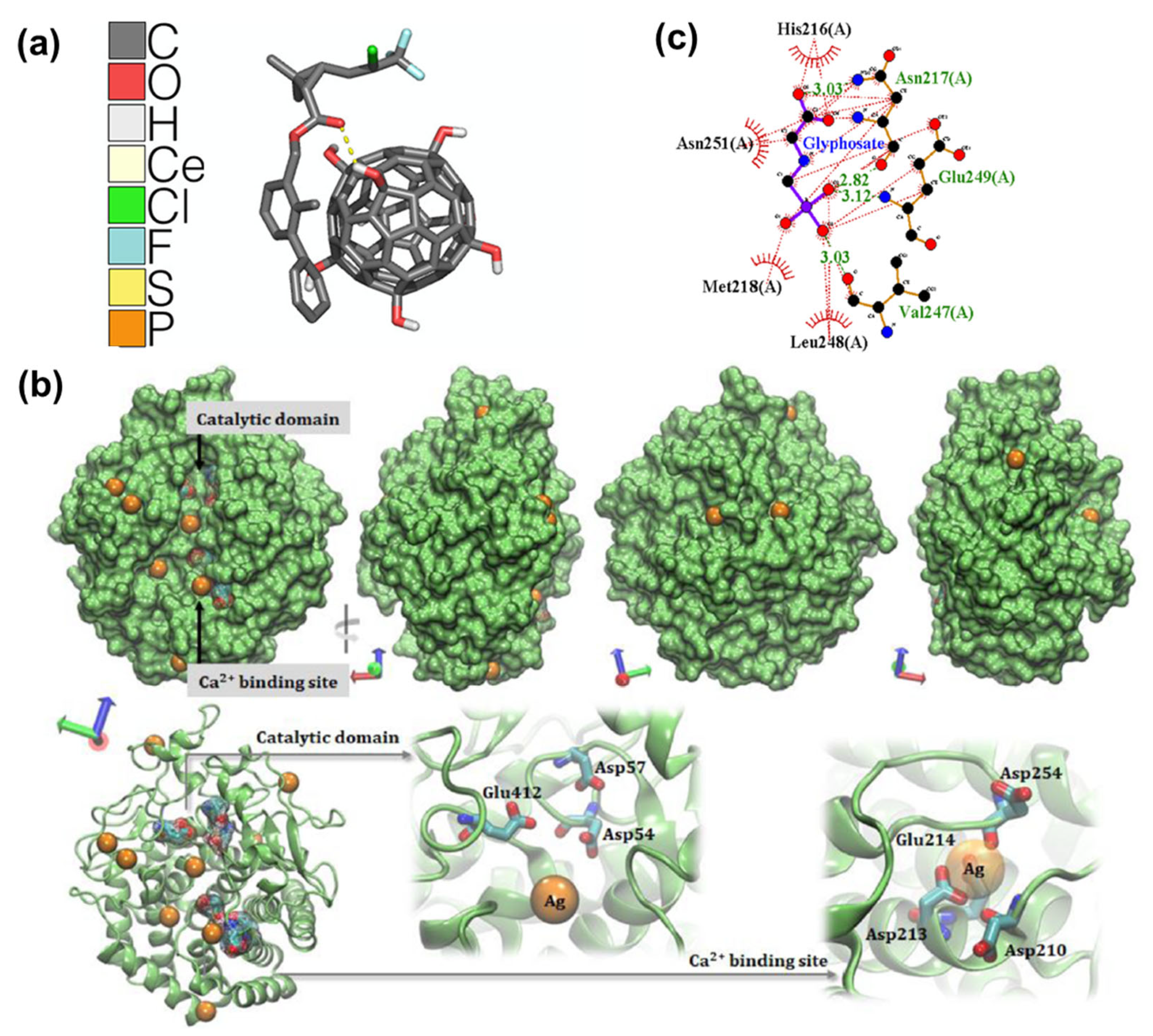
| Pollutant | Example | Source | Ecotoxicity | Health Impact | Life Cycle Remarks |
|---|---|---|---|---|---|
| Pesticides | Organophosphate, carbamates, pyrethroids [34] | Herbicides, insecticides, fungicides, bactericides [34] | High [34] | Carcinogenic [34] | Bioaugmentation [35]; persist up to ~50 years [36] |
| Organic matter | Organic material, e.g., plant, livestock wastes [37,38] | Chemical or biochemical oxygen-demanding materials [37,38] | Moderate [38] | Diarrhea, trachoma, and helminth infection [39] | Bioleaching-based release and ecotoxicity [40] |
| Nutrients | Supplements of nitrogen, phosphorus [6] | Chemical fertilizers, organic material [6] | Low [6,41] | Alzheimer, diabetes mellitus [42] | Imbalance of nitrogen and phosphorus contents of soil [43] |
| Metals | Se, Pb, Cu, Hg, As, Mg [44] | Nutrient supplements, specifically for small-scale hydroponics and greenhouses [44,45] | High [46] | Kidney, liver, lung failure [46,47,48] | Compromised nutrient status of food materials [49] |
| Salts | N, P, K, Na, Cl, bicarbonates of Mg, Ca [50] | Nutrient supplements, fertilizers, growth stimulants [51] | High [52] | Cardiac arrhythmias, hypercalcemia [52] | Compromised nutrient status of food materials [53,54] |
| Emerging pollutants | 2-aminobenzothiazole (ABA) [55] | Drug residues, synthetic hormones, and feed additives [55] | High [56] | Cardiovascular toxicity [56] | ABA acts as cytokines in human [57] |
| Pathogens | Fecal coliforms, Salmonella, Enterococci [58] | Organic manures, bio-manufactured supplements [58,59] | Moderate [58] | Carcinogenic, viral, and parasitic infection [60] | Repeating cycle of pathogen leads to multiple infection cycles and disease spread [60] |
| Enzyme (Function in Soil) | Pesticides | Remarks |
|---|---|---|
| Nitrogenase (fixes atmospheric N2) | Carbendazim, imazetapir, thiram, captan, carbofuran, 2,4-D, quinalphos, monocrotophos, endosulfan, γ-HCH, butachlors Terbutryn, simazine, prometryn Brominal, fenvalerate, Cuprosan Oxafun, Funaben, Baytan, pretilachlor, benthiocarb, cinmethylin, anilofos, Methabenzthiazuron, terbutryn, linuron | Reduced or no nitrogenase activity [68,69,70,71,72,73,74,75] Field doses of the fungicides did not affect nitrogenase activity of methylotrophic bacteria Higher doses suppressed activity [76] |
| Phosphatase (mineralizes organic phosphorus to inorganic) | 2,4-D, nitrapyrin, mefenoxam, metalaxyl Monocrotophos, chlorpyrifos, mancozeb, and carbendazim Quinalphos Diazinon, imidacloprid, lindane Glufosinate ammonium | Inhibited [77,78] Higher concentration or increasing incubation period had inhibitory effects [79,80] Initially inhibited but later activity was restored [81] Diazinon did not affect; imidacloprid increased while lindane decreased the enzyme when applied as seed treatment in groundnut field [82] Initial inhibition of phosphatase in sandy loam and clay loam soils [83] |
| Urease (hydrolyses urea into CO2 and NH3; key component of N2 cycle in soil) | Isoproturon, benomyl, captan, diazinon, profenofos | Increased activity [84,85] Reduced/inhibited activity [86,87] |
| DHA (removal of H2) | Azadirachtin, Acetamiprid, Quinalphos, Glyphosate Atrazine, and Northrin | Positive/stimulatory influence on DHA [88] Initially inhibited followed by activity restoration [89] Herbicides stimulated DHA of the microbial community at lower and inhibited it at higher concentrations [90] |
| Invertase (hydrolyze sucrose to fructose and glucose) | Atrazine, Carbaryl, Paraquat | Activity inhibition [91,92] |
| β-Glucosidase (hydrolyzes disaccharides in soil to form β-glucose) | Metalaxyl, Ridomil Gold Plus Copper | Enzyme activity increased and then decreased [93]; inhibited [94] |
| Cellulase (hydrolyzes cellulose to D-glucose) | Benlate, Captan, Brominal | Inhibited enzyme activity [95,96] |
| Arylsulphatase (hydrolyzes aryl sulfates) | Cinosulfuron, Prosulfuron, Thifensulfuron methyl, Triasulfuron | Activity reduction [97] |
| Brand Name | Causative Ingredient | Target Organism or Disease | Degradation Technique | Reference |
|---|---|---|---|---|
| Keefun | Tolfenpyard | Diamond black moth | Microbial | [98] |
| Colt | Cypermethrin | Lepidopteron pest | Photo and microbial | [99,100] |
| Osheen | Dinotefuran | Brown plant hoppers | Photocatalytic | [101] |
| Colfos | Ethion and Cypermethrin | Bollworms on cotton | Photo and microbial | [99,100,102] |
| Fosmite | Ethion | Mites, scales, thrips, beetles | Microbial | [102] |
| Fluton | Flubendiamide | Tobacco caterpillar, American bollworm | Direct aqueous photolysis | [103] |
| Jumbo | Imidacloprid | Rice hoppers, thrips, turf insects | Photolysis and microbial | [104] |
| Snailkill | Metaldehyde | Snails and slugs | Biodegradation | [105] |
| Roket | Profenofos and Cypermethrin | Insect pests (both chewing and sucking) | Biodegradation | [106] |
| Carina | Profenofos | Insect pests (both chewing and sucking) | Biodegradation | [106] |
| Simbaa | Propargite | Red spider mites | Biodegradation and phytodegradation | [107,108] |
| Voltage | Spiromesifen | Whiteflies and mites | Biodegradation | [109] |
| Maxima | Thiamethoxam | Thrips and tea mosquito bug | Activated persulfate | [110] |
| Vibrant | Thiocyclam Hydrogen Oxalate | Stem borer | ||
| Cosko GR | Chlorantraniliprole (0.4%) | Lepidoptera | Photolysis, hydrolysis and biodegradation | [111] |
| Cosko SC | Chlorantraniliprole (18.5%) | Lepidoptera | Photolysis, hydrolysis and biodegradation | [111] |
| Rodeo | Bifenthrin | Whitefly, mites and jassids | Microbial | [112] |
| Distruptor | Dinotifuran and Pymetrozine | Brown planthopper, white-backed plant hopper | Photocatalytic and biodegradation | [101,113] |
| Aceveer | Acephate 75% SP | Pink bollworm, armyworm | Acid hydrolysis, ozone, and electrolyzed treatment | [114] |
| Imexo | Thiamethoxam 25% WDGs | Stem borer, gall midge, leaf folder, WBPH, BPH, GLH, Thrips, whorl maggot, jassid, aphids, whiteflies | Activated presulfated process | [110] |
| Virtako 0.6 GR | 4 gm/kg Thiamethoxam + 2 gm/kg chlorantraniliprole | Stem borer, borers of sugarcane, and borer of corn | Acid hydrolysis, ozone, and electrolyzed treatment | [110,111] |
| Lambda Double | Lambda Cyhalothrin 5% EC | Bollworms, jassids, thrips, stem borer, shoot and fruit borer, mite, pod borer, pod fly, hopper, leaf hopper | Microbial | [115] |
| Lambda Strong | Lambda Cyhalothrin 4.9% CS | Bollworms, jassids, thrips, stem borer, shoot and fruit borer, mite, pod borer, pod fly, hopper, leaf hopper | Microbial | [115] |
| Onvix | Chlorantraniliprole 0.4% Grs | Chilo infuscatellus, Scirphophaga excerptalis, Scirphophaga insurtulas, | Photolysis, hydrolysis, and biodegradation | [111] |
| Pevota | Chlorantraniliprole 18.5% SC | Scirphophaga insurtulas, Chiloinfuscatellus medinalist | Photolysis, hydrolysis, and biodegradation | [111] |
| Stembo | Fipronil 0.3% Grs | rice gall midge, whorl maggot, white backed plant hopper | Photolysis, hydrolysis, and biodegradation | [116] |
| Veercombi 44 | Profenofos 40% + Cyper 4% EC | Thrips, bollworm, aphid, jassid, mealybug | Biodegradation | [106,117] |
| Veercombi 505 | Chlorpyrifos 50% + Cyper 5% EC | Spotted bollworm, American bollworm | Biodegradation | [118] |
| Veertap Power | Cartap Hydrochloride 4% Grs | Stem borer, leaf folder, whorl maggot | Fenton degradation | [119] |
| Uttam Flue 3935 | Flubendiamide 39.35% w/w | Spotted boll worm, stem borer, leaf folder, fruit borer | Soil photolysis | [120] |
| Fenveer DP | Fenvalerate 0.4% dust | General insects | Photo and microbial | [121,122] |
| Imidaveer | Imidacloprid 17.8% SL | Thrips, hopper, aphid, jassid, termite, whitefly, leaf miner | Photolysis and microbial | [104] |
| Uttam Metroz | Pymetrozine 50% WDGs | Brown plant hoppers | Biodegradation | [113] |
| Toro-10 | Bifenthrin 10% EC | Whitefly, aphids, stem borer, leaf folder, green leaf hopper | Microbial | [112] |
| Chloroveer | Chlorpyriphos 20% EC | Control of sucking, chewing, and boring insects | Biodegradation | [118] |
| Chlorveer Strong | Chlorpyriphos 50% EC | Control of sucking, chewing, and boring insects | Biodegradation | [118] |
| Uttam EMA | Emamectin Benzoate 5% SG | Pod borer, bollworms, tea looper, fruit and shoot borer | Foliar spray and ultraviolent radiation | [123] |
| Uttam Reon | Pyriproxyfen 5% and Diafenthiuron 25% SE | Whitefly, aphid, jassids, thrips | Photodegradation | [124,125] |
| Bruno | Buprofezin 25% SC | Whitefly, aphid, jassids | Biodegradation | [126] |
| Brofreya | Broflanilide | Leps and sucking pests | Photolysis | [127] |
| Brand Name | Causative Ingredient | Target Organism or Disease | Degradation Technique | Reference |
|---|---|---|---|---|
| KItazin | Kitazin | Blast and sheath blight of rice, fruit rot of chili, and purple blotch of onion | Microbial | [128] |
| Sanipeb | Propineb | Broad spectrum of fungi | Biodegradation | [129] |
| Clutch | Pyraclostrobin and Metiram | Early blight disease, late blight disease and downy mildew disease | Irradiation with UV | [130] |
| Header | Pyraclostrobin | Rice blast | Irradiation with UV | [130] |
| Visma | Pyraclostrobin and Boscalid | Downy mildew, powdery mildew, anthracnose, botrytis | Irradiation with UV | [130,131] |
| Shield | Iprobenfos | Wide range of fungal species | Light-induced catalyst | [132] |
| Veersulp DP | Sulphur 85% DP | Mildew, rust, tikka leaf spot | Microbial degradation | [133,134] |
| Uttam Fulcot | Thifluzamide 24% SC | Sheath blight | Microbial degradation | [135] |
| Uttam Lexon | Azoxystrobin 11% + Tebuconazole 18.3% SC | Sheath blight, early blight, late blight, yellow rust, purple blotch | Bacterial degradation | [136,137] |
| Uttam Azole | Azoxystrobin 18.2% and Difenoconazole 11.4% SC | Anthracnose, powdery mildew, early blight and late blight, downy mildew | Bacterial degradation | [137] |
| Wagon | Thifluzamide | Sheath blight in rice | Bacterial degradation | [138] |
| Figo | Tricyclazole 75% WP | Blast disease | Use of hydrogen peroxide | [139] |
| Manzim | Manco 63 and Carben 12% | Blast disease, leaf spot, rust diseases | Microbial degradation | [140] |
| Sulfino | Sulphur 80% WDGs | Powdery mildew, scab | Microbial degradation | [133,134] |
| Veercon | Propiconazole 25% EC | Karnal bunt, leaf rust, sheath blight, rust, leaf spot | Biodegradation | [141] |
| Brand Name | Causative Ingredient | Target Organism or Disease | Degradation Technique | Reference |
|---|---|---|---|---|
| Solaro | Atrazine | Trianthama monogyna, Digera arvensis, Echinochloa spp., Eleusine spp., Xantheium strumarium, Brachiara spp., Digitaria spp. | Ozonation | [142] |
| Wicket | Clodinafop-Propargyl | Phalaris minor | Photocatalytic | [143] |
| Nominee Gold | Bispyribac Sodium | Fimbristylis miliacea, Eclipta alba, Ludwigia parviflora, Monochoria vaginalis, Alternanthera philoxeroides, Sphenoclcea zeylenica | Microbial | [144,145] |
| Pimix | Metsulfuron methyl and Chlorimuron ethyl | Cyperus iria, Cyperus difformis, Fimbristylis miliacea, Eclipta alba, Ludwigia parviflora, Cynotis axillaris, Monochoria vaginalis | Biodegradation | [146] |
| Legacee | Fenoxaprop-p-ethyl | Echinochloa spp. (barnyard grass) | Hydrolysis | [147] |
| Melsa | Pinoxaden | Canary grass, Phalaris minor (wild oat), Avena ludoviciana | Microbial | [148] |
| Elite | Topramezone | Elusine indica, Digitaria sanguinalis, Dactyloctenium aegyptium, Echinocloa spp., Chloris barbata, Parthenium hysterophorus | Biodegradation | [149] |
| Awkira | Pyroxasulfone | Echinochloa colonum, Celosia argentia, Trianthema portulacastrum, Amaranthus viridis, Digeria arvensis | Biodegradation | [150] |
| Londax Powder | Bensulfuron Methyl and Pretilachlor | Echinochloa crusgalli, Echinochloa colonum, Cynodon dactylon, Sedges, Cyperus iria, Cyperus difformis, Cyperus rotundus | Microbial | [151] |
| Attract | Atrazine 50% WP | Trianthama monogyna, Digera arvensis, Echinochloa spp., Eleusine spp., Xantheium strumarium, Brachiara spp., Digitaria spp. | Ozonation | [142] |
| Butaveer | Butachlor 50% EC | Cyperusdifformis, Cyperusiria, Echinochloacrusgalli, Echinochloacolonum, Elusine Indica, Eclipta alba, Fimbristy lismiliacea | Microbial | [152] |
| Dhoomketu | Imazethapyr 30% SL | Echinocloa crusgalli, Digera arvensis, Commelina benghalensis, Amaranthus viridis | Photodegradation | [153] |
| Lido | Butachlor 50% EW | Cyperusdifformis, Cyperusiria, Monochoria vaginalis, Eclipta alba | Microbial | [152] |
| Metaveer | Metribuzin 75% WP | Capeweed, doublegee, wild radish | Biodegradation | [154] |
| Penveer | Pendimethalin 30% EC | Echinochloa, Euphorbia, wild amaranthus, Phyllanthus, Paspalum, Phalaris, Carnoplus | Biodegradation and phytodegradation | [155] |
| Penveer Plus | Pendimethalin 38.7% CS | Annual grasses and broad-leaved weeds like E. colonum, Digitaria sanguinalis, Dactyloctenium aegyptium, Amaranthus viridis | Biodegradation and phytodegradation | [155] |
| Pretilaveer | Pretilachlor 50% EC | Echinochloa crusgalli, Echinochloa colonum, Cyperusdifformis, Cyperusiria Fimbristylismilliacea, Eclipta alba | Microbial | [151,156] |
| Totto | Paraquat Dichloride 24% SL | Imperata cylindrica, Setaria spp., Commelina benghalensis, Boerhavia hispida, Paspalum conjugatum, Chenopodium spo., Anagallis arvensis | Microbial | [157] |
| Veerkill 80 | 2,4-D Sodium Salt 80% WP | Leucasaspera, Chenopodium album, Argemonemexlcana, Fimbrlstyllismiliacea, Anagallisarvensis, Vicia saliva | Microbial | [158] |
| Zeto | Fenoxaprop Ethyl 9.3% EC | Echinochloa colonum, Echinochloa crusgalli, Digitaria spp., Eleusine indica, Setaria spp., Brachiaria spp., Digitaria spp. | Microbial | [159] |
| Moto | Metsulfuron Methyl 20% WP | Chenopodium album, Melilotus indica, Lathyrus aphaca, Anagalis arvensis, Vicia sativa, Ludwigia parviflora, Sphenoclea zeylanica | Microbial | [160] |
| Prido | Pretilachlor 37% EW | Echinochloa colonum, E. crusgall, Cyperus difformis, C. iria, Digitaria sanguinalis, Frimbristylis miliacea, Eclipta alba | Microbial | [161] |
| Veerkill | 2,4-D Ethyl Ester 38% EC | Cyperus Iria, Digera Arvensis, Convolvulus Arvensis, Trianthema Sp., Tridax Procumbens, Euphorbia Hirta, Phyllanthus Niruri, Trianthema | Microbial | [162] |
| Weedkil | 2,4-D Amine Salt 58% SL | Cyperusiria, Striga sp. Trianthema sp., Tridaxprocumbens, Digeriaarvensis, Convolvulus arvensis, Euphorbia hirta, Phyllanthusniruri | Microbial | [158] |
| Weeza | Clodinofop Propargyl 15% WP | Phalaris minor | Photocatalytic | [143] |
| Wheto | Sulfosulfuron 75% WDGs | Phalaris minor, Chenopodium album, Melilotus alba | Hydrolytic | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.; Raya, D.; Sharma, R.; Giri, S.K.; Priya, K.; Kumar, A.; Singh, G.; Dhiman, S.S. Synergistic Approaches for Navigating and Mitigating Agricultural Pollutants. Pollutants 2025, 5, 37. https://doi.org/10.3390/pollutants5040037
Srivastava S, Raya D, Sharma R, Giri SK, Priya K, Kumar A, Singh G, Dhiman SS. Synergistic Approaches for Navigating and Mitigating Agricultural Pollutants. Pollutants. 2025; 5(4):37. https://doi.org/10.3390/pollutants5040037
Chicago/Turabian StyleSrivastava, Swati, Dheeraj Raya, Rajni Sharma, Shiv Kumar Giri, Kanu Priya, Anil Kumar, Gulab Singh, and Saurabh Sudha Dhiman. 2025. "Synergistic Approaches for Navigating and Mitigating Agricultural Pollutants" Pollutants 5, no. 4: 37. https://doi.org/10.3390/pollutants5040037
APA StyleSrivastava, S., Raya, D., Sharma, R., Giri, S. K., Priya, K., Kumar, A., Singh, G., & Dhiman, S. S. (2025). Synergistic Approaches for Navigating and Mitigating Agricultural Pollutants. Pollutants, 5(4), 37. https://doi.org/10.3390/pollutants5040037






