Preliminary Study Using Sensor Measurements in Selected Homes in Cornwall, England, over a One-Year Period Confirms Increased Indoor Exposure from Second-Hand Smoking but Not from Second-Hand Vaping
Abstract
1. Introduction
2. Materials and Methods
2.1. Context
2.2. Participant Questionnaire and Indoor Sensors
- They had withdrawn from the Smartline project, or sensors were not installed at the property.
- The Smartline baseline survey questionnaire was missing information on indoor smoking and vaping.
- PM2.5 sensor data for the property did not span the complete date range.
2.3. Data Analysis
3. Results
3.1. Descriptive Statistics
3.2. Comparison of Mean PM2.5 Concentrations Between Households
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
4.3. Strengths and Limitations in Relation to Existing Literature
4.4. Implications for Clinicians, Policy and Practice
4.5. Unanswered Research Questions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, K.; Ho, H.C.; Su, H.; Huang, C.; Zheng, H.; Zhang, W.; Tao, J.; Hossain, M.Z.; Zhang, Y.; Hu, K.; et al. A systematic review and meta-analysis of intraday effects of ambient air pollution and temperature on cardiorespiratory morbidities: First few hours of exposure matters to life. eBioMedicine 2022, 86, 104327. [Google Scholar] [CrossRef]
- Sharpe, R.; Taylor, T.; Fleming, L.; Morrissey, K.; Morris, G.; Wigglesworth, R. Making the Case for “Whole System” Approaches: Integrating Public Health and Housing. Int. J. Environ. Res. Public Health 2018, 15, 2345. [Google Scholar] [CrossRef]
- Brown, V.M.; Crump, D.R.; Harrison, P.T.C. Assessing and controlling risks from the emission of organic chemicals from construction products into indoor environments. Environ. Sci. Process. Impacts 2013, 15, 2164. [Google Scholar] [CrossRef]
- Hodas, N.; Loh, M.; Shin, H.-M.; Li, D.; Bennett, D.; McKone, T.E.; Jolliet, O.; Weschler, C.J.; Jantunen, M.; Lioy, P.; et al. Indoor inhalation intake fractions of fine particulate matter: Review of influencing factors. Indoor Air 2015, 26, 836–856. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. “Every breath we take: The lifelong impact of air pollution”—A call for action. Clin. Med. 2017, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.; Garden, C.; Coggins, M.; Galea, K.S.; Whelan, P.; Cowie, H.; Sánchez-Jiménez, A.; Thorne, P.S.; Hurley, J.F.; Ayres, J.G. Contribution of solid fuel, gas combustion, or tobacco smoke to indoor air pollutant concentrations in Irish and Scottish homes. Indoor Air 2011, 22, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Amalia, B.; Fu, M.; Tigova, O.; Ballbè, M.; Paniello-Castillo, B.; Castellano, Y.; Vyzikidou, V.K.; O’donnell, R.; Dobson, R.; Lugo, A.; et al. Exposure to secondhand aerosol from electronic cigarettes at homes: A real-life study in four European countries. Sci. Total Environ. 2023, 854, 158668. [Google Scholar] [CrossRef]
- Global Burden of Disease 2021 Findings from the GBD 2021 Study. Available online: https://www.healthdata.org/sites/default/files/2024-05/GBD_2021_Booklet_FINAL_2024.05.16.pdf (accessed on 28 February 2025).
- Copas, J.B. Reanalysis of epidemiological evidence on lung cancer and passive smoking. BMJ 2000, 320, 417–418. [Google Scholar] [CrossRef]
- Thirlway, F. Explaining the social gradient in smoking and cessation: The peril and promise of social mobility. Sociol. Health Illn. 2020, 42, 565–578. [Google Scholar] [CrossRef]
- Deprivation and the Impact on Smoking Prevalence, England and Wales—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/bulletins/deprivationandtheimpactonsmokingprevalenceenglandandwales/2017to2021#cite-this-statistical-bulletin (accessed on 28 February 2025).
- Service.gov.uk. 2025. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/817002/FA3101_demographic_and_economic_characteristics_of_social_and_privately_renting_households.xlsx (accessed on 28 February 2025).
- Social Housing and Employment Helping Social Housing Be the Springboard to a Better Life. 2018. Available online: https://www.centreforsocialjustice.org.uk/core/wp-content/uploads/2018/07/CSJ6364-Social-Housing-and-Employment-Report-180706-WEB.pdf (accessed on 28 February 2025).
- ASH. Smoking and Social Housing Supporting Residents, Addressing Inequalities 2030. 2022. Available online: https://ash.org.uk/uploads/ASH-Housing-LIN-Smoking-and-Social-Housing-May-2022.pdf?v=1652284469 (accessed on 28 February 2025).
- Marcham, C.L.; Springston, J.P. Electronic cigarettes in the indoor environment. Rev. Environ. Health 2019, 34, 105–124. [Google Scholar] [CrossRef]
- Nguyen, C.; Li, L.; Sen, C.A.; Ronquillo, E.; Zhu, Y. Fine and ultrafine particles concentrations in vape shops. Atmos. Environ. 2019, 211, 159–169. [Google Scholar] [CrossRef]
- McNeill, A.; Simonavičius, E.; Brose, L.; Taylor, E.; East, K.; Zuikova, E.; Taylor, E.; Zuikova, E. Nicotine Vaping in England: An Evidence Update Including Health Risks and Perceptions, 2022; A report commissioned by the Office for Health Improvement and Disparities; King’s College London: London, UK, 2022. [Google Scholar]
- Yingst, J.; Veldheer, S.; Hammett, E.; Hrabovsky, S.; Foulds, J. Should electronic cigarette use be covered by clean indoor air laws? Tob. Control 2016, 26, e16–e18. [Google Scholar] [CrossRef]
- Freeman, B.; Peters, M.J.; Bittoun, R.; Brightwell, R.; English, D.R.; Thomas, D.P.; Otlowski, M.F.; Zwar, N.A.; Chamberlain, C. National Health and Medical Research Council statement on electronic cigarettes: 2022 update. Med. J. Aust. 2023, 220, 100–106. [Google Scholar] [CrossRef]
- Banks, E.; Yazidjoglou, A.; Brown, S.; Nguyen, M.; Martin, M.; Beckwith, K.; Daluwatta, A.; Campbell, S.; Joshy, G. Electronic cigarettes and health outcomes: Umbrella and systematic review of the global evidence. Med. J. Aust. 2023, 218, 267–275. [Google Scholar] [CrossRef]
- DEFRA. Emissions of Air Pollutants in the UK—Particulate Matter (PM10 and PM2.5). 2025. Available online: https://www.gov.uk/government/statistics/emissions-of-air-pollutants/emissions-of-air-pollutants-in-the-uk-particulate-matter-pm10-and-pm25 (accessed on 28 February 2025).
- Tzortzi, A.; Teloniatis, S.; Matiampa, G.; Bakelas, G.; Tzavara, C.; Vyzikidou, V.K.; Vardavas, C.; Behrakis, P.; Fernandez, E.; Fernández, E.; et al. Passive exposure of non-smokers to E-Cigarette aerosols: Sensory irritation, timing and association with volatile organic compounds. Environ. Res. 2020, 182, 108963. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L. Smoking and Cerebral Oxidative Stress and Air Pollution: A Dreadful Equation with Particulate Matter Involved and One More Powerful Reason Not to Smoke Anything! J. Alzheimer’s Dis. 2016, 54, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Pimpin, L.; Retat, L.; Fecht, D.; de Preux, L.; Sassi, F.; Gulliver, J.; Belloni, A.; Ferguson, B.; Corbould, E.; Jaccard, A.; et al. Estimating the costs of air pollution to the National Health Service and social care: An assessment and forecast up to 2035. PLoS Med. 2018, 15, e1002602. [Google Scholar] [CrossRef]
- Oh, A.Y.; Kacker, A. Do electronic cigarettes impart a lower potential disease burden than conventional tobacco cigarettes?: Review on e-cigarette vapor versus tobacco smoke. Laryngoscope 2014, 124, 2702–2706. [Google Scholar] [CrossRef]
- Protano, C.; Manigrasso, M.; Avino, P.; Vitali, M. Second-hand smoke generated by combustion and electronic smoking devices used in real scenarios: Ultrafine particle pollution and age-related dose assessment. Environ. Int. 2017, 107, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Koger, F.; Klingelhöfer, D.; Müller, R.; Groneberg, D.A. Particulate Matter Emissions of Four Different Cigarette Types of One Popular Brand: Influence of Tobacco Strength and Additives. Int. J. Environ. Res. Public Health 2019, 16, 263. [Google Scholar] [CrossRef]
- Li, L.; Lin, Y.; Xia, T.; Zhu, Y. Effects of Electronic Cigarettes on Indoor Air Quality and Health. Annu. Rev. Public Health 2020, 41, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Zainol Abidin, N.; Zainal Abidin, E.; Zulkifli, A.; Karuppiah, K.; Syed Ismail, S.N.; Amer Nordin, A.S. Electronic cigarettes and indoor air quality: A review of studies using human volunteers. Rev. Environ. Health 2017, 32, 235–244. [Google Scholar] [CrossRef]
- Shearston, J.; Lee, L.; Eazor, J.; Meherally, S.; Park, S.H.; Vilcassim, M.R.; Weitzman, M.; Gordon, T. Effects of exposure to direct and secondhand hookah and e-cigarette aerosols on ambient air quality and cardiopulmonary health in adults and children: Protocol for a panel study. BMJ Open 2019, 9, e029490. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Ballbè, M.; Sureda, X.; Fu, M.; Saltó, E.; Martínez-Sánchez, J.M. Particulate Matter from Electronic Cigarettes and Conventional Cigarettes: A Systematic Review and Observational Study. Curr. Environ. Health Rep. 2015, 2, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, F.C.; Buonanno, G.; Stabile, L.; Vigo, P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut. 2014, 184, 523–529. [Google Scholar] [CrossRef]
- Ingebrethsen, B.J.; Cole, S.K.; Alderman, S.L. Electronic cigarette aerosol particle size distribution measurements. Inhal. Toxicol. 2012, 24, 976–984. [Google Scholar] [CrossRef]
- Schripp, T.; Markewitz, D.; Uhde, E.; Salthammer, T. Does e-cigarette consumption cause passive vaping? Indoor Air 2012, 23, 25–31. [Google Scholar] [CrossRef]
- Flouris, A.D.; Chorti, M.S.; Poulianiti, K.P.; Jamurtas, A.Z.; Kostikas, K.; Tzatzarakis, M.N.; Wallace Hayes, A.; Tsatsakis, A.M.; Koutedakis, Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal. Toxicol. 2013, 25, 91–101. [Google Scholar] [CrossRef]
- Ballbè, M.; Martínez-Sánchez, J.M.; Sureda, X.; Fu, M.; Pérez-Ortuño, R.; Pascual, J.A.; Saltó, E.; Fernández, E. Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environ. Res. 2014, 135, 76–80. [Google Scholar] [CrossRef]
- Chen, R.; Aherrera, A.; Isichei, C.; Olmedo, P.; Jarmul, S.; Cohen, J.E.; Navas-Acien, A.; Rule, A.M. Assessment of indoor air quality at an electronic cigarette (Vaping) convention. J. Expo. Sci. Environ. Epidemiol. 2017, 28, 522–529. [Google Scholar] [CrossRef]
- Protano, C.; Avino, P.; Manigrasso, M.; Vivaldi, V.; Perna, F.; Valeriani, F.; Vitali, M. Environmental Electronic Vape Exposure from Four Different Generations of Electronic Cigarettes: Airborne Particulate Matter Levels. Int. J. Environ. Res. Public Health 2018, 15, 2172. [Google Scholar] [CrossRef]
- Lampos, S.; Kostenidou, E.; Farsalinos, K.; Zagoriti, Z.; Ntoukas, A.; Dalamarinis, K.; Savranakis, P.; Lagoumintzis, G.; Poulas, K. Real-Time Assessment of E-Cigarettes and Conventional Cigarettes Emissions: Aerosol Size Distributions, Mass and Number Concentrations. Toxics 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Pisinger, C.; Døssing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 2014, 69, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Hess, I.; Lachireddy, K.; Capon, A. A systematic review of the health risks from passive exposure to electronic cigarette vapour. Public Health Res. Pract. 2016, 26, e2621617. [Google Scholar] [CrossRef]
- Moses, L.; Morrissey, K.; Sharpe, R.A.; Taylor, T. Exposure to Indoor Mouldy Odour Increases the Risk of Asthma in Older Adults Living in Social Housing. Int. J. Environ. Res. Public Health 2019, 16, 2600. [Google Scholar] [CrossRef]
- Morrissey, K.; Taylor, T.; Tu, G. Estimating the Impact of Relative Financial Circumstances in Childhood on Adult Mental Wellbeing: A Mediation Analysis. Appl. Res. Qual. Life 2022, 18, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Maguire, K.; Morrissey, K.; Taylor, T.; Wyatt, K. Social Cohesion, Mental Wellbeing and Health-related Quality of Life among a Cohort of Social Housing Residents in Cornwall: A Cross Sectional Study. BMC Public Health 2020, 20, 985. [Google Scholar] [CrossRef]
- Williams, A.J.; Menneer, T.; Sidana, M.; Walker, T.; Maguire, K.; Mueller, M.; Paterson, C.; Leyshon, M.; Leyshon, C.; Seymour, E.; et al. Fostering Engagement With Health and Housing Innovation: Development of Participant Personas in a Social Housing Cohort. JMIR Public Health Surveill. 2021, 7, e25037. [Google Scholar] [CrossRef]
- Menneer, T.; Mueller, M.; Sharpe, R.A.; Townley, S. Modelling mould growth in domestic environments using relative humidity and temperature. Build. Environ. 2022, 208, 108583. [Google Scholar] [CrossRef]
- Kuholski, K.; Tohn, E.; Morley, R. Healthy Energy-Efficient Housing. J. Public Health Manag. Pract. 2010, 16, S68–S74. [Google Scholar] [CrossRef]
- Cornwall Council. Index of Multiple Deprivation. 2019. Available online: https://www.cornwall.gov.uk/media/cftltjsd/2019-imd-cornwall-headline-data.pdf (accessed on 28 February 2025).
- Sharpe, R.A.; Thornton, C.R.; Nikolaou, V.; Osborne, N.J. Higher energy efficient homes are associated with increased risk of doctor diagnosed asthma in a UK subpopulation. Environ. Int. 2015, 75, 234–244. [Google Scholar] [CrossRef]
- Smartline. 2020. Available online: https://www.smartline.org.uk/ (accessed on 28 February 2025).
- Sharpe, R.A.; Thornton, C.R.; Nikolaou, V.; Osborne, N.J. Fuel poverty increases risk of mould contamination, regardless of adult risk perception & ventilation in social housing properties. Environ. Int. 2015, 79, 115–129. [Google Scholar] [CrossRef]
- Melstrom, P.; Koszowski, B.; Thanner, M.H.; Hoh, E.; King, B.; Bunnell, R.; McAfee, T. Measuring PM2.5, Ultrafine Particles, Nicotine Air and Wipe Samples Following the Use of Electronic Cigarettes. Nicotine Tob. Res. 2017, 19, 1055–1061. [Google Scholar] [CrossRef]
- Protano, C.; Manigrasso, M.; Cammalleri, V.; Biondi Zoccai, G.; Frati, G.; Avino, P.; Vitali, M. Impact of Electronic Alternatives to Tobacco Cigarettes on Indoor Air Particular Matter Levels. Int. J. Environ. Res. Public Health 2020, 17, 2947. [Google Scholar] [CrossRef]
- Yuan, S.; Xu, W.; Liu, Z. A Study on the Model for Heating Influence on PM2.5 Emission in Beijing China. Procedia Eng. 2015, 121, 612–620. [Google Scholar] [CrossRef]
- Zhao, B.; Zheng, H.; Wang, S.; Smith, K.R.; Lu, X.; Aunan, K.; Gu, Y.; Wang, Y.; Ding, D.; Xing, J.; et al. Change in household fuels dominates the decrease in PM2.5 exposure and premature mortality in China in 2005–2015. Proc. Natl. Acad. Sci. USA 2018, 115, 12401–12406. [Google Scholar] [CrossRef]
- Rostami, A.; Pithawalla, Y.; Liu, J.; Oldham, M.; Wagner, K.; Frost-Pineda, K.; Sarkar, M.A. A Well-Mixed Computational Model for Estimating Room Air Levels of Selected Constituents from E-Vapor Product Use. Int. J. Environ. Res. Public Health 2016, 13, 828. [Google Scholar] [CrossRef]
- Barn, P.; Gombojav, E.; Ochir, C.; Laagan, B.; Beejin, B.; Naidan, G.; Boldbaatar, B.; Galsuren, J.; Byambaa, T.; Janes, C.; et al. The effect of portable HEPA filter air cleaners on indoor PM2.5 concentrations and second hand tobacco smoke exposure among pregnant women in Ulaanbaatar, Mongolia: The UGAAR randomized controlled trial. Sci. Total Environ. 2018, 615, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Isiugo, K.; Ryan, P.; Grinshpun, S.A.; Yermakov, M.; Desmond, C.; Jandarov, R.; Vesper, S.; Ross, J.; Chillrud, S.; et al. Effectiveness of a Portable Air Cleaner in Removing Aerosol Particles in Homes Close to Highways. Indoor Air 2018, 28, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Google. 2024. Available online: https://www.google.com/maps (accessed on 28 February 2025).
- Huang, S.; Lawrence, J.; Kang, C.M.; Li, J.; Martins, M.; Vokonas, P.; Gold, D.R.; Schwartz, J.; Coull, B.A.; Koutrakis, P. Road proximity influences indoor exposures to ambient fine particle mass and components. Environ. Pollut. 2018, 243, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Dancey, C.P.; Reidy, J.; Rowe, R. Statistics for the Health Sciences: A Non-Mathematical Introduction; Sage Office Locations: London, UK, 2012. [Google Scholar]
- Volesky, K.D.; Maki, A.; Scherf, C.; Watson, L.; Van Ryswyk, K.; Fraser, B.; Weichenthal, S.A.; Cassol, E.; Villeneuve, P.J. The influence of three e-cigarette models on indoor fine and ultrafine particulate matter concentrations under real-world conditions. Environ. Pollut. 2018, 243, 882–889. [Google Scholar] [CrossRef]
- Lindson, N.; Butler, A.R.; McRobbie, H.; Bullen, C.; Hajek, P.; Wu, A.D.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2025. [Google Scholar] [CrossRef]
- Martinez, U.; Simmons, V.N.; Sutton, S.K.; Drobes, D.J.; Meltzer, L.R.; Brandon, K.O.; Byrne, M.M.; Harrell, P.T.; Eissenberg, T.; Bullen, C.R.; et al. Targeted smoking cessation for dual users of combustible and electronic cigarettes: A randomised controlled trial. Lancet Public Health 2021, 6, e500–e509. [Google Scholar] [CrossRef] [PubMed]
- Tattan-Birch, H.; Brown, J.; Jackson, S.E.; Jarvis, M.J.; Shahab, L. Secondhand Nicotine Absorption From E-Cigarette Vapor vs Tobacco Smoke in Children. JAMA Netw. Open 2024, 7, e2421246. [Google Scholar] [CrossRef]
- Gorber, S.C.; Schofield-Hurwitz, S.; Hardt, J.; Levasseur, G.; Tremblay, M. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob. Res. 2009, 11, 12–24. [Google Scholar] [CrossRef]
- Shiffman, S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, time-line follow-back, and ecological momentary assessment. Health Psychol. 2009, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.; Brennan, E.; Durkin, S.; Borland, R.; Travers, M.J.; Hyland, A.; Spittal, M.J.; Wakefield, M.A. Secondhand smoke exposure (PM2.5) in outdoor dining areas and its correlates. Tob. Control 2009, 19, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Gurung, G.; Bradley, J.; Delgado-Saborit, J.M. Effects of shisha smoking on carbon monoxide and PM 2.5 concentrations in the indoor and outdoor microenvironment of shisha premises. Sci. Total Environ. 2016, 548–549, 340–346. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef]
- Semple, S.; Apsley, A.; Azmina Ibrahim, T.; Turner, S.W.; Cherrie, J.W. Fine particulate matter concentrations in smoking households: Just how much secondhand smoke do you breathe in if you live with a smoker who smokes indoors? Tob. Control 2014, 24, e205–e211. [Google Scholar] [CrossRef]
- Semple, S.; Latif, N. How Long Does Secondhand Smoke Remain in Household Air: Analysis of PM2.5 Data From Smokers’ Homes. Nicotine Tob. Res. 2014, 16, 1365–1370. [Google Scholar] [CrossRef]
- Gehrig, R.; Buchmann, B. Characterising seasonal variations and spatial distribution of ambient PM10 and PM2.5 concentrations based on long-term Swiss monitoring data. Atmos. Environ. 2003, 37, 2571–2580. [Google Scholar] [CrossRef]
- Huang, F.; Li, X.; Wang, C.; Xu, Q.; Wang, W.; Luo, Y.; Tao, L.; Gao, Q.; Guo, J.; Chen, S.; et al. PM2.5 Spatiotemporal Variations and the Relationship with Meteorological Factors during 2013-2014 in Beijing, China. Sun Q, editor. PLoS ONE 2015, 10, e0141642. [Google Scholar]
- Leaderer, B.P.; Naeher, L.P.; Jankun, T.; Balenger, K.; Holford, T.R.; Toth, C.; Sullivan, J.; Wolfson, J.M.; Koutrakis, P. Indoor, outdoor, and regional summer and winter concentrations of PM10, PM2.5, SO42−, H+, NH4+, NO3−, NH3, and nitrous acid in homes with and without kerosene space heaters. Environ. Heal. Perspect. 1999, 107, 223–231. [Google Scholar]
- Department for Environment Food; Rural Affairs; National Statistics. Concentrations of Particulate Matter (PM10 and PM2.5). 2020. Available online: https://www.gov.uk/government/statistics/air-quality-statistics/concentrations-of-particulate-matter-pm10-and-pm25#temporal-variations-in-concentrations-of-pm25-in-the-uk-2020 (accessed on 28 February 2025).
- 77van Drooge, B.L.; Marco, E.; Perez, N.; Grimalt, J.O. Influence of electronic cigarette vaping on the composition of indoor organic pollutants, particles, and exhaled breath of bystanders. Environ. Sci. Pollut. Res. 2018, 26, 4654–4666. [Google Scholar] [CrossRef] [PubMed]
- Grana, R.; Benowitz, N.; Glantz, S.A. E-Cigarettes: A Scientific Review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef]
- Ruprecht, A.A.; De Marco, C.; Pozzi, P.; Munarini, E.; Mazza, R.; Angellotti, G.; Turla, F.; Boffi, R. Comparison between Particulate Matter and Ultrafine Particle Emission by Electronic and Normal Cigarettes in Real-life Conditions. Tumori J. 2014, 100, e24–e27. [Google Scholar] [CrossRef]
- Schober, W.; Szendrei, K.; Matzen, W.; Osiander-Fuchs, H.; Heitmann, D.; Schettgen, T.; Jörres, R.A.; Fromme, H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 2014, 217, 628–637. [Google Scholar] [CrossRef]
- Palmisani, J.; Di Gilio, A.; Palmieri, L.; Abenavoli, C.; Famele, M.; Draisci, R.; de Gennaro, G. Evaluation of Second-Hand Exposure to Electronic Cigarette Vaping under a Real Scenario: Measurements of Ultrafine Particle Number Concentration and Size Distribution and Comparison with Traditional Tobacco Smoke. Toxics 2019, 7, 59. [Google Scholar] [CrossRef]
- Savdie, J.; Canha, N.; Buitrago, N.; Almeida, S.M. Passive Exposure to Pollutants from a New Generation of Cigarettes in Real Life Scenarios. Int. J. Environ. Res. Public Health 2020, 17, 3455. [Google Scholar] [CrossRef]
- Wootton, R.E.; Greenstone, H.S.R.; Abdellaoui, A.; Denys, D.; Verweij, K.J.H.; Munafò, M.R.; Treur, J.L. Bidirectional effects between loneliness, smoking and alcohol use: Evidence from a Mendelian randomization study. Addiction 2020, 116, 400–406. [Google Scholar] [CrossRef]
- Paterson, C.A.; Sharpe, R.A.; Taylor, T.; Morrissey, K. Indoor PM2.5, VOCs and asthma outcomes: A systematic review in adults and their home environments. Environ. Res. 2021, 202, 111631. [Google Scholar] [CrossRef]
- Kaunelienė, V.; Meišutovič-Akhtarieva, M.; Martuzevičius, D. A review of the impacts of tobacco heating system on indoor air quality versus conventional pollution sources. Chemosphere 2018, 206, 568–578. [Google Scholar] [CrossRef]
- Russo, E.T.; Hulse, T.E.; Adamkiewicz, G.; Levy, D.E.; Bethune, L.; Kane, J.; Reid, M.; Shah, S.N. Comparison of Indoor Air Quality in Smoke-Permitted and Smoke-Free Multiunit Housing: Findings From the Boston Housing Authority. Nicotine Tob. Res. 2014, 17, 316–322. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; BTinghino Mangiaracina, G.; Marani, A.; Vitali, M.; Protano, C.; Osborn, J.F.; Cattaruzza, M.S. Electronic cigarettes: An evaluation of exposure to chemicals and fine particulate matter (PM). Ann. Ig. 2012, 24, 279–288. [Google Scholar] [PubMed]
- Lee, M.S.; LeBouf, R.F.; Son, Y.S.; Koutrakis, P.; Christiani, D.C. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ. Health 2017, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Tobacco: E-Cigarettes. 2020. Available online: https://www.who.int/news-room/q-a-detail/tobacco-e-cigarettes (accessed on 28 February 2025).
- Czogala, J.; Goniewicz, M.L.; Fidelus, B.; Zielinska-Danch, W.; Travers, M.J.; Sobczak, A. Secondhand Exposure to Vapors From Electronic Cigarettes. Nicotine Tob. Res. 2013, 16, 655–662. [Google Scholar] [CrossRef]
- Soule, E.K.; Maloney, S.F.; Spindle, T.R.; Rudy, A.K.; Hiler, M.M.; Cobb, C.O. Electronic cigarette use and indoor air quality in a natural setting. Tob. Control 2016, 26, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Whitsel, L.P.; Ribisl, K.M.; Bullen, C.; Chaloupka, F.; Piano, M.R.; Robertson, R.M.; McAuley, T.; Goff, D.; Benowitz, N. Electronic Cigarettes. Circulation 2014, 130, 1418–1436. [Google Scholar] [CrossRef]
- Smoking in England. E Cigarettes Latest Trends—Graphs—Smoking in England. 2025. Available online: https://smokinginengland.info/graphs/e-cigarettes-latest-trends (accessed on 28 February 2025).
- Hajek, P.; Phillips-Waller, A.; Przulj, D.; Pesola, F.; Myers Smith, K.; Bisal, N.; Li, J.; Parrott, S.; Sasieni, P.; Dawkins, L.; et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N. Engl. J. Med. 2019, 380, 629–637. [Google Scholar] [CrossRef]
- Farzal, Z.; Perry, M.F.; Yarbrough, W.G.; Kimple, A.J. The Adolescent Vaping Epidemic in the United States—How It Happened and Where We Go From Here. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 885–886. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; Rynard, V.L.; Reid, J.L. Changes in Prevalence of Vaping Among Youths in the United States, Canada, and England from 2017 to 2019. JAMA Pediatr. 2020, 174, 797. [Google Scholar] [CrossRef] [PubMed]
- Stratton, K.R.; Kwan, L.Y.; Eaton, D.L.; And, E. Public Health Consequences of E-Cigarettes; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Wyatt, L.H.; Devlin, R.B.; Rappold, A.G.; Case, M.; Diaz-Sanchez, D. Low levels of fine particulate matter increase vascular damage and reduce pulmonary function in young healthy adults. Part. Fibre Toxicol. 2020, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Gowers, A.; Miller, B.; Stedman, J. Estimating Local Mortality Burdens Associated with Particulate Air Pollution; Public Health England; Centre for Radiation, Chemical and Environmental Hazards: Didcot, UK, 2014.
- Department for Environment Food; Rural Affairs. Modelled Background Pollution Data; Department for Environment, Food and Rural Affairs (Defra): London, UK, 2023. Available online: https://uk-air.defra.gov.uk/data/pcm-data (accessed on 28 February 2025).
- World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen. Global Update 2005. Dioxide and Sulfur Dioxide; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Neuberger, M. Feinstaub und akutes Koronarsyndrom. (Fine particulates and the acute coronary syndrome). Univers. Inn. Med. 2008, 1, 47–49. [Google Scholar]
- Osborne, N.J.; Thornton, C.R.; Sharpe, R.A. Indoor Fungal Exposure and Allergic Respiratory Disease. Curr. Allergy Asthma Rep. 2015, 15, 71. [Google Scholar] [CrossRef]
- Bertholon, J.F.; Becquemin, M.H.; Roy, M.; Roy, F.; Ledur, D.; Annesi Maesano, I.; Dautzenberg, B. Comparison of the aerosol produced by electronic cigarettes with conventional cigarettes and the shisha [translated from French]. Rev. Mal. Respir. 2013, 30, 752–757. [Google Scholar] [CrossRef]
- McNeill, A.; Etter, J.F.; Farsalinos, K.; Hajek, P.; le Houezec, J.; McRobbie, H. A critique of a World Health Organization-commissioned report and associated paper on electronic cigarettes. Addiction 2014, 109, 2128–2134. [Google Scholar] [CrossRef]
| Variables | Study Participants | ||||
|---|---|---|---|---|---|
| N | % | Mean | Range | SD | |
| Proportion Males | 54 | 33 | |||
| Proportion Females | 110 | 67 | |||
| Mean adult age (≥18 years) | 162 | 57 | 22–92 | 15 | |
| Smoking status | |||||
| Never-smoker | 43/164 | 26 | |||
| Ex-smoker | 65/164 | 40 | |||
| Ex < 5 a day | 14/65 | 21 | |||
| Ex 5–15 a day | 18/65 | 28 | |||
| Ex > 15 a day | 31/65 | 48 | |||
| Ex (quantity not reported) | 2/65 | 3 | |||
| Current smoker | 56/164 | 34 | |||
| No smoking or vaping indoors | 105/164 | 64 | |||
| Exclusive smoking indoors | 33/164 | 20 | |||
| Smoking and vaping indoors | 16/164 | 10 | |||
| Exclusive vaping indoors | 10/164 | 6 | |||
| Respiratory Health | |||||
| Emphysema or chronic bronchitis | 15/164 | 6 | |||
| Emphysema or chronic bronchitis in never-smoker | 2/43 | 5 | |||
| Emphysema or chronic bronchitis in ex-smoker | 7/65 | 11 | |||
| Emphysema or chronic bronchitis in current smoker | 6/56 | 11 | |||
| Adults with wheeze or dry cough in ≤12 months | 56/164 | 34 | |||
| Seen a doctor in ≤ 12 months for: | |||||
| Asthma | 43/164 | 26 | |||
| Allergy | 32/164 | 20 | |||
| Education | |||||
| Secondary education (11–16 years of age) | 112/162 | 69 | |||
| Secondary/further education (16–18 years of age) | 41/162 | 25 | |||
| Undergraduate university education | 8/162 | 5 | |||
| Postgraduate university education | 1/162 | 1 | |||
| Employment status | |||||
| Employed | 31/163 | 19 | |||
| Actively looking for work | 5/163 | 3 | |||
| Retired | 64/163 | 39 | |||
| Long-term sick or disabled | 41/163 | 25 | |||
| Looking after home or family | 14/163 | 9 | |||
| Student/Training | 4/163 | 2 | |||
| Other | 4/163 | 2 | |||
| Variables | Study Participants | ||||
|---|---|---|---|---|---|
| N | % | Mean | Range | SD | |
| Index of Multiple Deprivation (IMD) | |||||
| 10% most deprived | 83 | 51 | |||
| 10% to 20% | 19 | 12 | |||
| 20% to 30% | 18 | 11 | |||
| 30% to 40% | 42 | 26 | |||
| 40% to 50% | 0 | 0 | |||
| 50% to 60% | 2 | 1 | |||
| Presence of pets in the house | |||||
| Any | 96/164 | 59 | |||
| Cat | 47/164 | 29 | |||
| Dog | 50/164 | 30 | |||
| Mean household occupancy and household occupancy | 164 | 1.92 | 1–6 | 1.1 | |
| 1-single occupancy | 75 | 46 | |||
| 2-double occupancy | 51 | 31 | |||
| 3-triple occupancy | 20 | 12 | |||
| 4+ multiple occupancy | 18 | 11 | |||
| Property age | |||||
| Pre 1930 | 7/162 | 4 | |||
| 1930–1959 | 45/162 | 28 | |||
| 1960–1979 | 51/162 | 31 | |||
| 1980–1999 | 31/162 | 19 | |||
| 2000+ | 28/162 | 17 | |||
| Property type | |||||
| Flats (own entrance or communal entrance) | 93/155 | 60 | |||
| Non-standard constructed house | 5/155 | 3 | |||
| Mid-terrace | 17/155 | 11 | |||
| End terrace | 20/155 | 13 | |||
| Semi-detached | 20/155 | 13 | |||
| Monitoring Period * | Study Participants | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither Smoking nor Vaping Indoors n = 105 | Exclusively Smoke Indoors n = 33 | Exclusively Vape Indoors n = 10 | Smoke and Vape Indoors n = 16 | |||||||||||||
| Mean/ Median | Range | SD | Mean/ Median | Range | SD | Mean/ Median | Range | SD | Mean/ Median | Range | SD | |||||
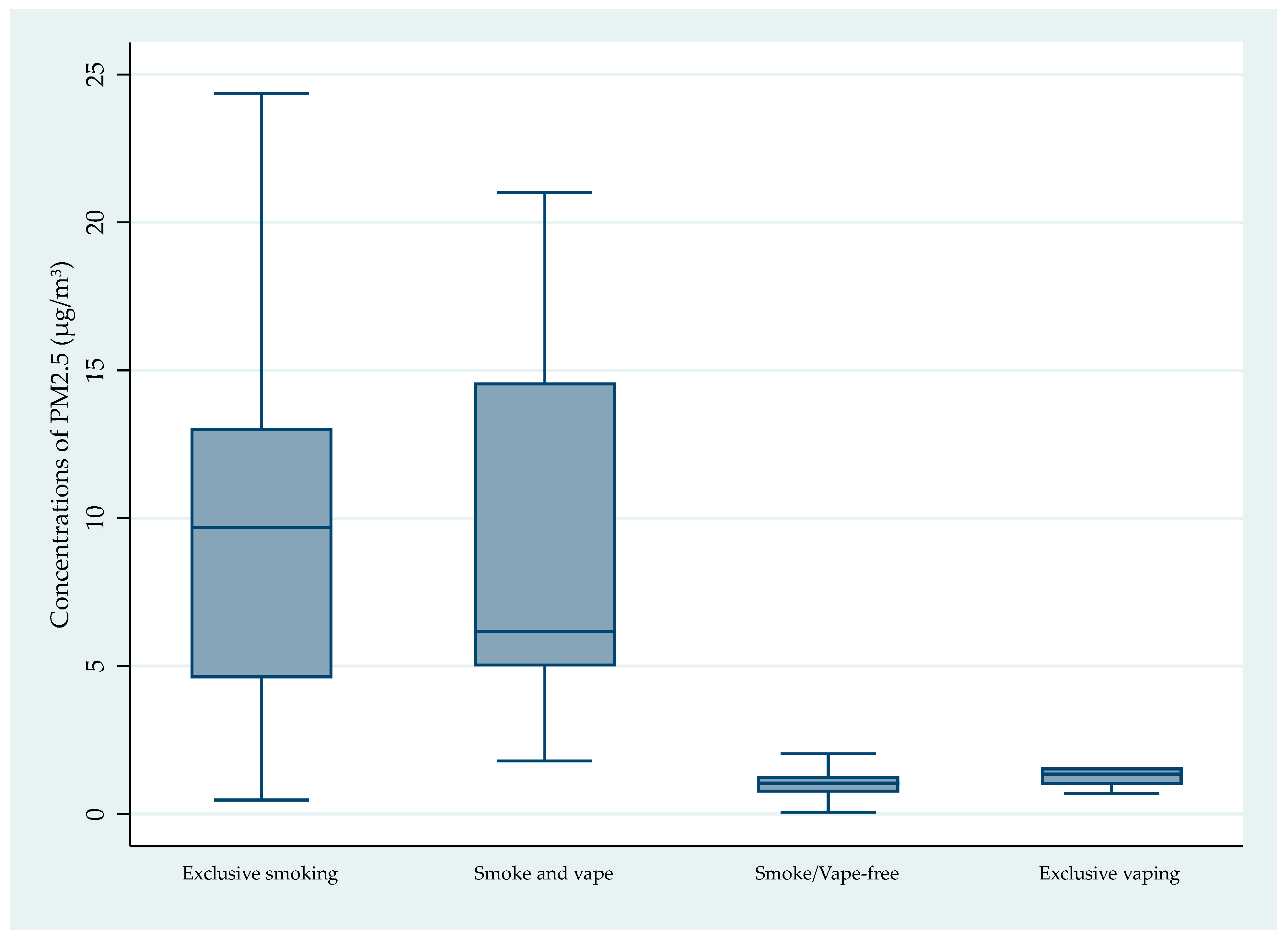
| Annual | 1.28/1.04 | 0.07– 7.28 | 1.14 | 14.07/ 9.67 | 0.48– 96.58 | 17.3 | 2.00/ 1.35 | 0.70– 5.65 | 1.65 | 9.18/ 6.17 | 1.79– 21.01 | 6.21 | ||||
| Winter (December 2018–February 2019) | 1.42/1.07 | 0.07–9.57 | 1.37 | 17.49/12.39 | 0.39– 99.73 | 19.30 | 2.28/ 1.70 | 0.72– 7.51 | 2.00 | 11.42/ 8.16 | 1.43– 28.77 | 8.59 | ||||
| Spring (March 2019–May 2019) | 1.37/1.05 | 0.06–8.04 | 1.17 | 14.87/ 10.14 | 0.51– 94.71 | 18.04 | 2.07/ 1.31 | 0.71– 7.20 | 1.97 | 10.18/ 7.02 | 1.20– 27.63 | 8.30 | ||||
| Summer (June 2019–August 2019) | 1.09/0.90 | 0.05–9.72 | 1.04 | 10.73/ 5.78 | 0.65– 94.40 | 17.03 | 1.63/ 1.05 | 0.63– 6.08 | 1.65 | 6.20/ 5.09 | 1.27– 15.93 | 4.00 | ||||
| Autumn (September 2019–November 2019) | 1.28/0.89 | 0.09–14.8 | 1.73 | 12.84/ 8.06 | 0.36– 96.67 | 17.23 | 2.05/ 1.27 | 0.49– 5.98 | 1.97 | 8.92/ 7.67 | 1.99– 20.45 | 6.06 | ||||
| Variables | Study Participants | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither Smoking nor Vaping Indoors n = 105 | Exclusively Smoke Indoors n = 33 | Exclusively Vape Indoors n = 10 | Smoke and Vape Indoors n = 16 | |||||||||||||
| N | Mean/ Median | Range | SD | N | Mean/ Median | Range | SD | N | Mean/ Median | Range | SD | N | Mean/ Median | Range | SD | |
| Gas heating | 99 | 1.26 | 0.07–7.28 | 1.15 | 31 | 14.49 | 0.48–96.58 | 18.05 | 10 | 1.96 | 0.70– 5.65 | 1.65 | 16 | 9.18 | 1.79– 21.01 | 6.21 |
| Electric heating | 6 | 1.56 | 0.59–3.58 | 1.05 | 2 | 7.55 | 5.26–9.85 | 3.24 | 0 | N/A | 0 | 0 | 0 | N/A | 0 | 0 |
| 0–18 h spent indoors | 27 | 1.33 | 0.06–7.28 | 1.31 | 7 | 12.01 | 0.48– 35.36 | 14.33 | 3 | 0.99 | 0.69– 1.33 | 0.32 | 1 | 5.14 | N/A | N/A |
| >18 h spent indoors | 78 | 1.26 | 0.50–6.92 | 1.01 | 26 | 14.62 | 0.81–96.58 | 18.56 | 7 | 2.42 | 1.38–4.81 | 1.95 | 15 | 9.45 | 1.79–21.01 | 6.54 |
| Opened front window weekly | 81 | 1.25 | 0.07–7.28 | 1.10 | 27 | 13.05 | 1.03–35.36 | 9.59 | 8 | 2.23 | 0.89–5.65 | 1.88 | 15 | 8.73 | 1.79–21.01 | 6.37 |
| Not opened window weekly | 24 | 1.38 | 0.51–6.92 | 1.31 | 6 | 18.66 | 0.48–96.58 | 38.30 | 2 | 1.64 | 0.90– 2.38 | 1.05 | 1 | 15.94 | N/A | N/A |
| Vacuumed 0–10 times a month | 55 | 1.19 | 0.07–6.91 | 1.21 | 19/32 | 14.80 | 0.48–96.58 | 22.19 | 4 | 2.06 | 0.69–4.81 | 1.86 | 6 | 10.37 | 2.73–19.19 | 6.92 |
| Vacuumed 11–20 times a month | 18 | 1.25 | 0.60–2.89 | 0.58 | 3/ 32 | 16.41 | 10.64–35.23 | 11.43 | 2 | 1.10 | 0.99–1.22 | 0.16 | 1 | 6.16 | N/A | N/A |
| Vacuumed >21 times a month | 32 | 1.44 | 0.46–7.28 | 1.27 | 10/32 | 12.89 | 5.71–27.13 | 6.31 | 4 | 2.37 | 0.89–5.65 | 2.21 | 9 | 8.71 | 1.80–21.01 | 6.70 |
| Any pet indoors | 59 | 1.32 | 0.46– 7.28 | 1.01 | 19 | 9.71 | 0.81– 28.1 | 6.92 | 7 | 2.32 | 0.69– 5.65 | 2.02 | 11 | 8.21 | 1.79– 21.01 | 6.39 |
| No pets present | 46 | 1.23 | 0.06– 6.91 | 1.31 | 14 | 19.98 | 0.48– 96.58 | 25.07 | 3 | 1.23 | 0.99– 1.38 | 0.21 | 5 | 11.30 | 2.73– 18.44 | 6.63 |
| Single occupancy | 47 | 1.31 | 0.45– 7.28 | 1.36 | 22 | 17.12 | 0.48– 96.58 | 20.27 | 2 | 3.07 | 1.33– 4.82 | 2.47 | 4 | 8.80 | 1.79– 19.19 | 7.60 |
| Multiple occupancy | 58 | 1.25 | 0.07– 6.92 | 0.95 | 11 | 7.97 | 0.81– 29.6 | 7.97 | 8 | 1.73 | 0.69– 5.65 | 1.61 | 12 | 9.30 | 1.96– 21.01 | 6.35 |
| House is ventilated | 41 | 1.29 | 0.53– 6.92 | 1.05 | 9 | 15.00 | 1.16– 29.58 | 10.79 | 3 | 2.57 | 1.33– 4.82 | 1.95 | 9 | 10.47 | 1.79– 21.01 | 7.26 |
| No, house is not ventilated | 64 | 1.27 | 0.07– 7.28 | 1.21 | 24 | 13.72 | 0.48– 96.58 | 19.71 | 7 | 1.75 | 0.69– 5.65 | 1.74 | 7 | 7.51 | 4.82– 19.19 | 5.18 |
| <100 m from main road | 9 | 1.03 | 0.51– 3.58 | 0.98 | 6 | 11.91 | 0.48– 35.36 | 12.43 | 1 | 1.42 | N/A | N/A | 1 | 19.19 | N/A | N/A |
| >100–500 m from main road | 64 | 1.27 | 0.45– 6.92 | 1.11 | 18 | 17.24 | 0.81– 96.58 | 21.7 | 6 | 1.80 | 0.70– 1.57 | 1.51 | 6 | 8.95 | 4.82– 18.44 | 5.10 |
| >500–1800 m from main road | 32 | 1.36 | 0.07– 7.28 | 1.28 | 9 | 9.17 | 1.03– 31.50 | 9.23 | 3 | 2.59 | 0.89– 5.65 | 2.66 | 9 | 8.21 | 1.79– 21.01 | 6.87 |
| Hypothesis | Z | p | p Order ** |
|---|---|---|---|
| HO: Exclusive vaping (10) = Smoke and vape free (105) | 1.926 | 0.0542 | 0.685 |
| HO: Exclusive smoking (33) = Exclusive vaping (10) | 3.450 | 0.0006 * | 0.864 |
| HO: Smoking and vaping (16) = Exclusive vaping (10) | 3.742 | 0.0002 * | 0.944 |
| HO: Exclusive smoking (33) = Smoke and vape free (105) | 7.190 | <0.0001 * | 0.916 |
| HO: Smoking and vaping (16) = Smoke and vape free (33) | 6.052 | <0.0001 * | 0.971 |
| HO: Exclusive smoking (105) = Smoking and vaping (16) | 0.682 | 0.4951 | 0.561 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, G.D.; Menneer, T.; Sharpe, R.A. Preliminary Study Using Sensor Measurements in Selected Homes in Cornwall, England, over a One-Year Period Confirms Increased Indoor Exposure from Second-Hand Smoking but Not from Second-Hand Vaping. Pollutants 2025, 5, 34. https://doi.org/10.3390/pollutants5040034
Walsh GD, Menneer T, Sharpe RA. Preliminary Study Using Sensor Measurements in Selected Homes in Cornwall, England, over a One-Year Period Confirms Increased Indoor Exposure from Second-Hand Smoking but Not from Second-Hand Vaping. Pollutants. 2025; 5(4):34. https://doi.org/10.3390/pollutants5040034
Chicago/Turabian StyleWalsh, Gareth David, Tamaryn Menneer, and Richard Alan Sharpe. 2025. "Preliminary Study Using Sensor Measurements in Selected Homes in Cornwall, England, over a One-Year Period Confirms Increased Indoor Exposure from Second-Hand Smoking but Not from Second-Hand Vaping" Pollutants 5, no. 4: 34. https://doi.org/10.3390/pollutants5040034
APA StyleWalsh, G. D., Menneer, T., & Sharpe, R. A. (2025). Preliminary Study Using Sensor Measurements in Selected Homes in Cornwall, England, over a One-Year Period Confirms Increased Indoor Exposure from Second-Hand Smoking but Not from Second-Hand Vaping. Pollutants, 5(4), 34. https://doi.org/10.3390/pollutants5040034







