Combined Effect of per- and Polyfluoroalkyl Substances, Toxic Metals, and Essential Elements on Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. PFAS Extraction and Quantitation
2.3. Quantification of Lead, Cadmium, and Mercury Alongside Selenium, Manganese, and Iron
2.4. CKD Biomarkers
2.4.1. Ratio of Urinary Albumin to Creatinine
2.4.2. Estimated Glomerular Filtration Rate (eGFR) and CKD
- Stage 1: eGFR ≥ 90.
- Stage 2: eGFR from 60 and 89.
- Stage 3: eGFR ranging from 30 and 59.
- Stage 4: eGFR ranging from 15 and 29.
- Stage 5: eGFR < 15.
2.5. Selected Covariates and Variables for Analytical Adjustment
2.6. Statistical Analysis
2.6.1. Summary Statistics, Regression, Correlations, and Statistical Testing
2.6.2. Bayesian Kernel Machine Regression
2.6.3. Quantile g-Computation
2.6.4. Weighted Quantile Sum Regression
3. Results
3.1. Demographic, Exposure, and Clinical Characteristics
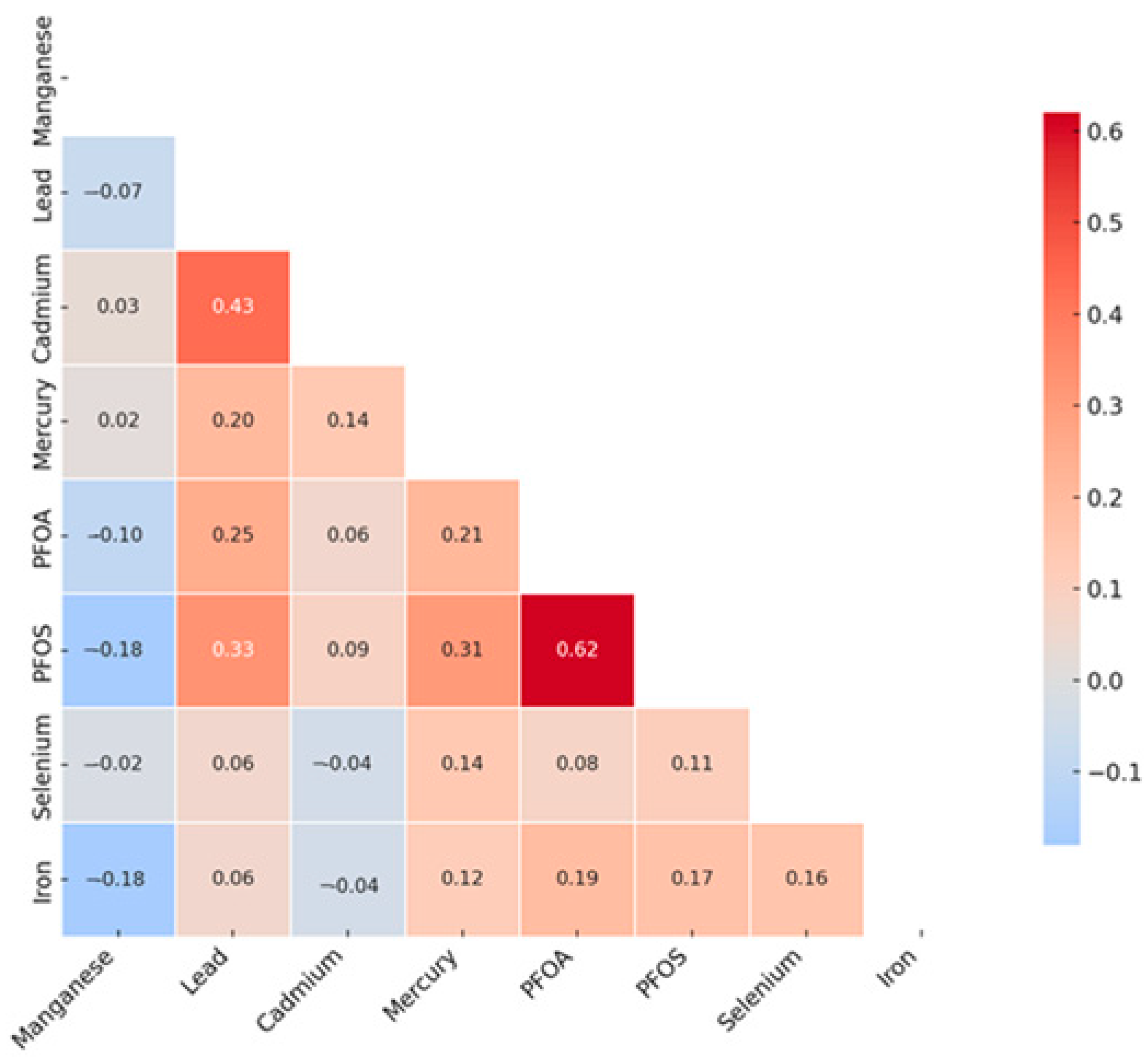
3.2. Assessing Relationships Among Key Variables: Spearman Pearson Correlation Matrix Analysis
3.3. Logistic Regression Analysis of Exposure Variables with CKD
3.4. BKMR
3.4.1. Evaluating PFAS and Metals’ Contributions to CKD Using BKMR
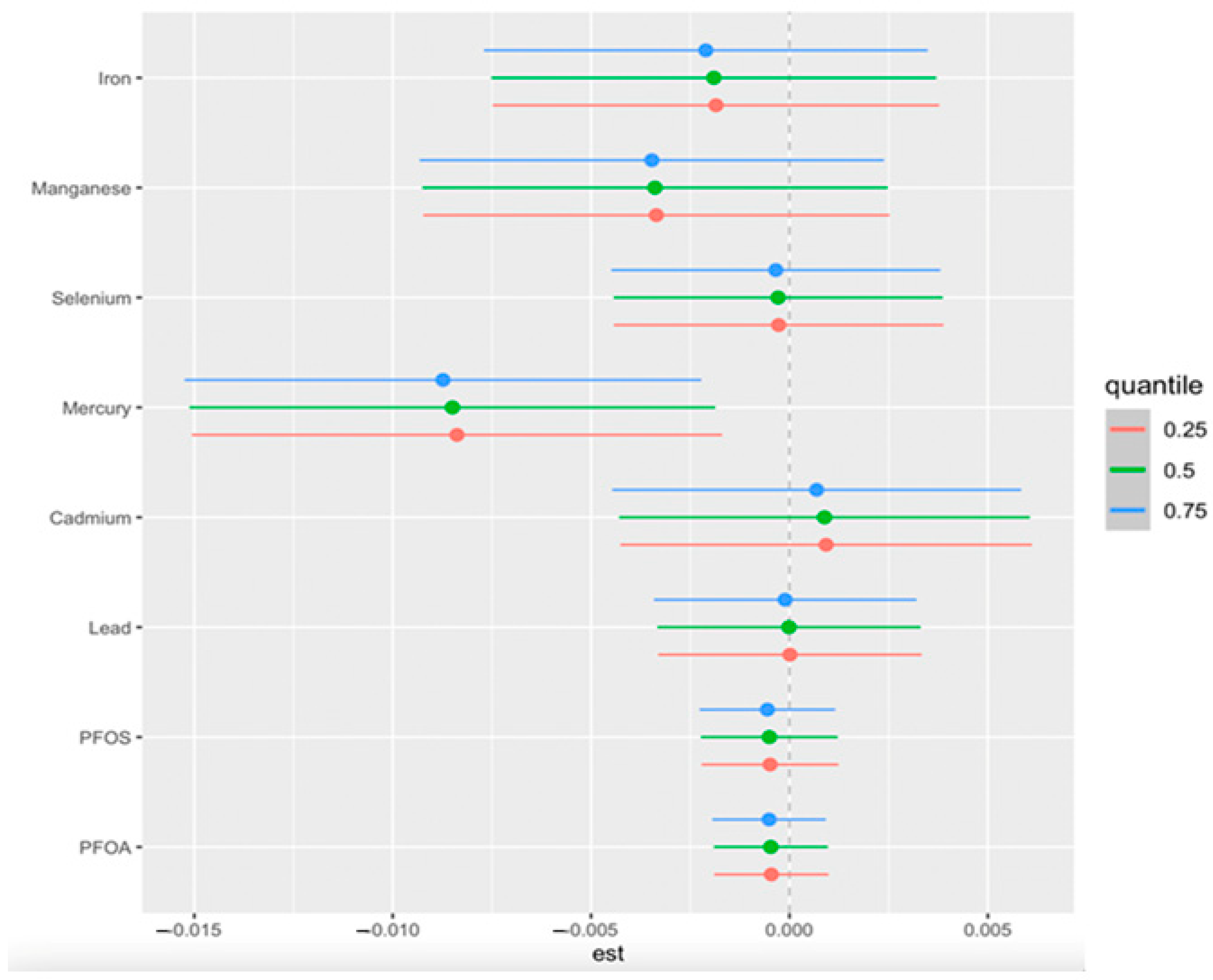
3.4.2. Univariate Analysis of Individual Effects of PFAS, Toxic Metals, and Essential Elements on CKD
3.4.3. Bivariate Exposure–Response Functions with Fixed Quantile Values
3.4.4. Single-Variable Effects of PFAS, Toxic Metals, and Essential Elements on CKD
3.4.5. Single-Exposure Interaction Terms of PFAS, Essential Elements, and Toxic Metals
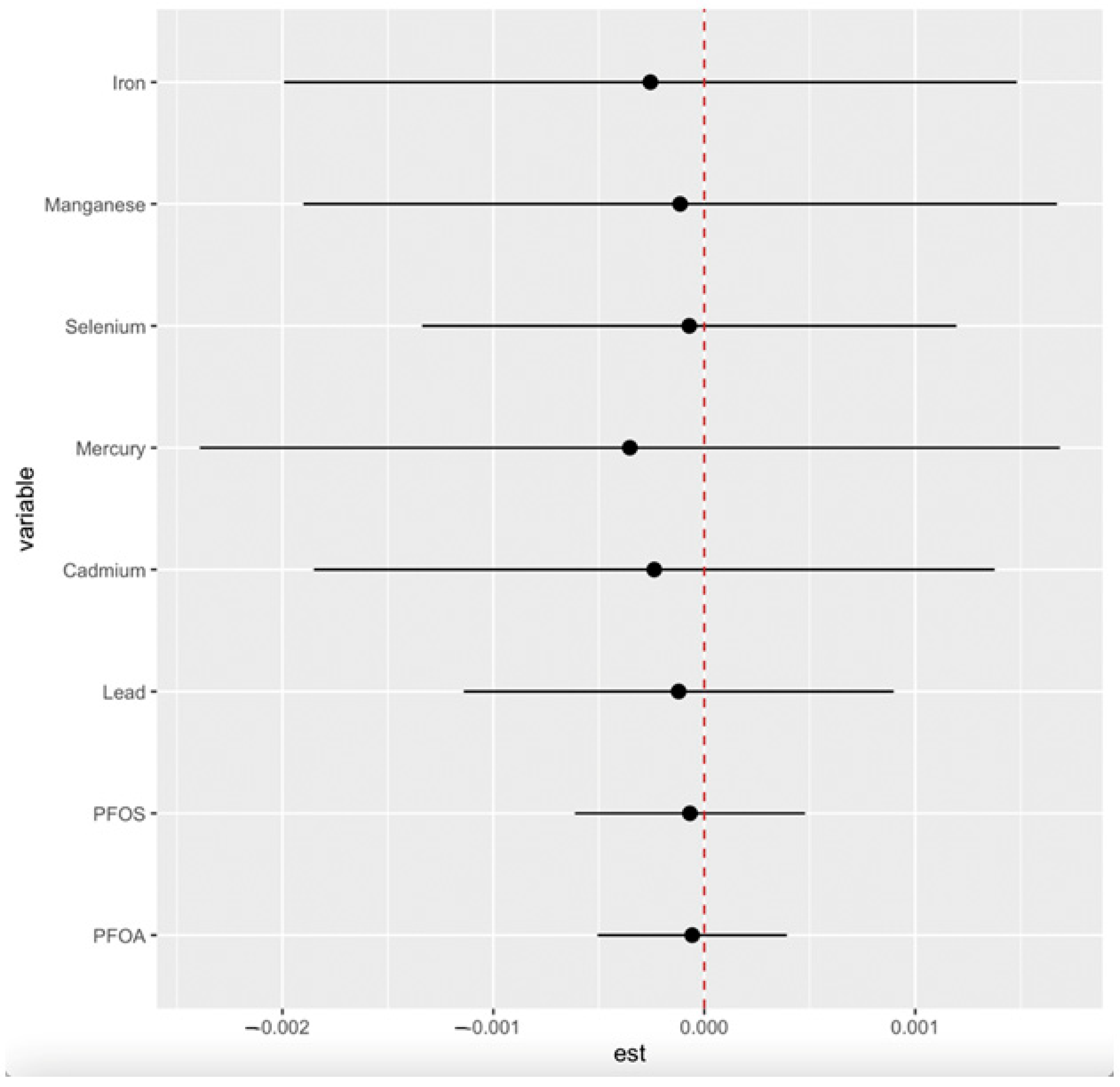
3.4.6. Overall Effects of PFAS, Toxic Metals, and Essential Elements on CKD
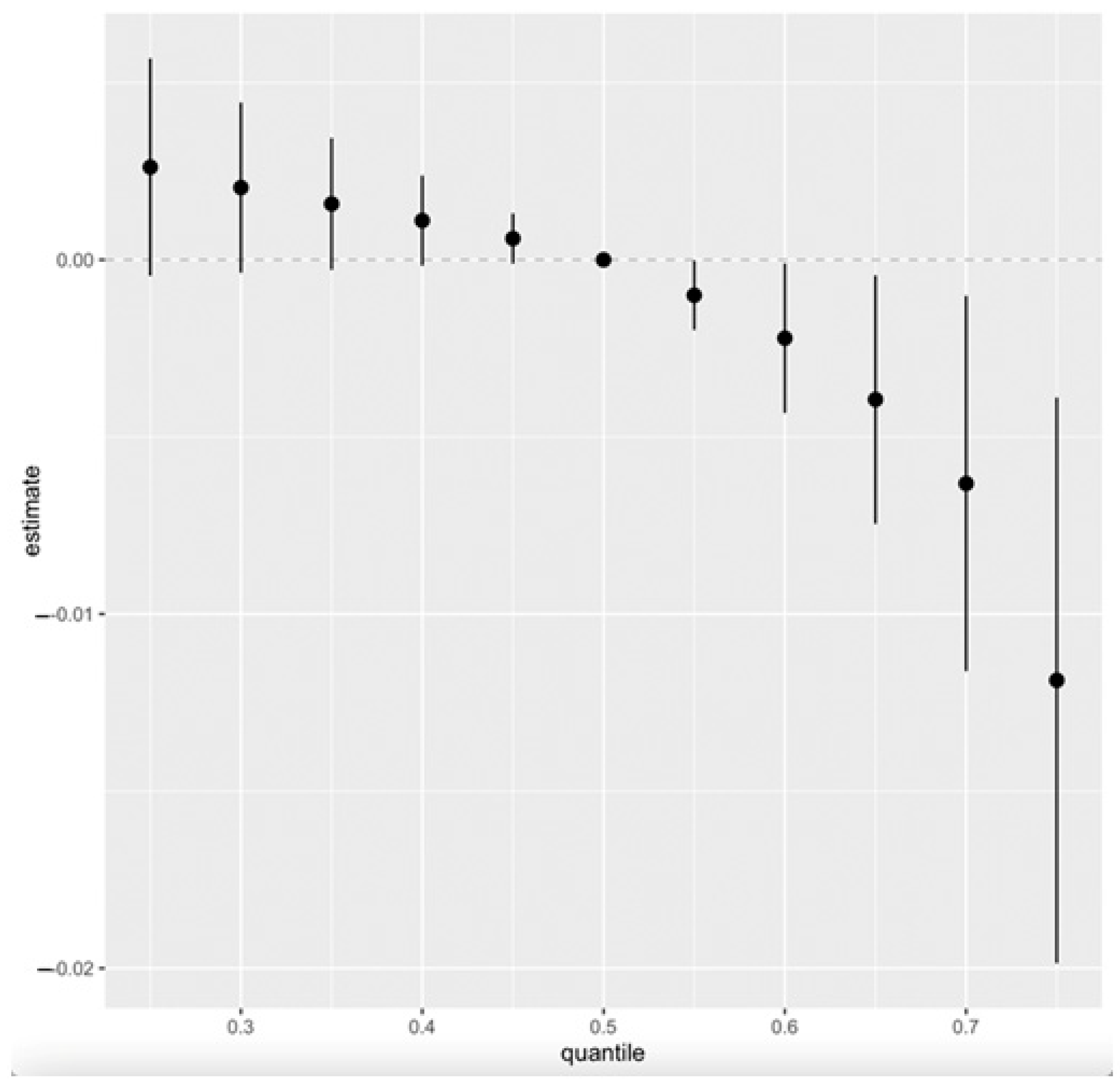
3.5. Quantile g-Computation
3.6. Weighted Quantile Sum (WQS) Regression
4. Discussion
4.1. Environmental Pollutants, Essential Elements, and Kidney Disease: Unraveling Complex Interactions
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Kim, N.H.; Hyun, Y.Y.; Lee, K.B.; Chang, Y.; Ryu, S.; Oh, K.H.; Ahn, C. Environmental heavy metal exposure and chronic kidney disease in the general population. J. Korean Med. Sci. 2015, 30, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, P.; Wang, L.; Li, Q.; Li, X.; Luo, Y. Toxicity of per- and polyfluoroalkyl substances to aquatic vertebrates. Front. Environ. Sci. 2023, 11, 1101100. [Google Scholar] [CrossRef]
- Liu, D.; Yan, S.; Wang, P.; Chen, Q.; Liu, Y.; Cui, J.; Liang, Y.; Ren, S.; Gao, Y. Perfluorooctanoic acid (PFOA) exposure in relation to the kidneys: A review of current available literature. Front. Physiol. 2023, 14, conf 1103141. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, C.; Iori, S.; Pietropoli, E.; Bonato, M.; Giantin, M.; Barbarossa, A.; Bardhi, A.; Pilastro, A.; Dacasto, M.; Pauletto, M. Sub-acute exposure of male guppies (Poecilia reticulata) to environmentally relevant concentrations of PFOA and GenX induces significant changes in the testis transcriptome and reproductive traits. Environ. Int. 2024, 187, 108703. [Google Scholar] [CrossRef]
- Bline, A.P.; DeWitt, J.C.; Kwiatkowski, C.F.; Pelch, K.E.; Reade, A.; Varshavsky, J.R. Public Health Risks of PFAS-Related Immunotoxicity Are Real. Curr. Environ. Health Rep. 2024, 11, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Wang, K.; Zelnick, L.R.; Imrey, P.B.; deBoer, I.H.; Himmelfarb, J.; Allon, M.D.; Cheung, A.K.; Dember, L.M.; Roy-Chaudhury, P.; Vazquez, M.A.; et al. Effect of Anti-Hypertensive Medication History on Arteriovenous Fistula Maturation Outcomes. Am. J. Nephrol. 2018, 48, 56–64. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef]
- Kshirsagar, A.V.; Zeitler, E.M.; Weaver, A.; Franceschini, N.; Engel, L.S. Environmental Exposures and Kidney Disease. Kidney360 2022, 3, 2174–2182. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef]
- Guan, D.X.; Yang, C.; Nriagu, J.O. Editorial: The role of essential trace elements in health and disease. Front. Public Health 2023, 11, 1285603. [Google Scholar] [CrossRef]
- Martinez-Finley, E.J.; Gavin, C.E.; Aschner, M.; Gunter, T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 2013, 62, 65–75. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Davenport, A. Trace Elements in Patients with Chronic Kidney Disease. In Chronic Renal Disease; Academic Press: Cambridge, MA, USA, 2015; pp. 429–439. [Google Scholar]
- Xie, Y.; Liu, F.; Zhang, X.; Jin, Y.; Li, Q.; Shen, H.; Fu, H.; Mao, J. Benefits and risks of essential trace elements in chronic kidney disease: A narrative review. Ann. Transl. Med. 2022, 10, 1400. [Google Scholar] [CrossRef]
- Goyer, R. Toxic Effects of Metals. In Casarett and Doull’s Toxicology; Amdur, M.O., Doull, J.D., Klaassen, C.D., Eds.; Pergamon Press: New York, NY, USA, 1991; pp. 623–680. [Google Scholar]
- Haruna, I.; Obeng-Gyasi, E. Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2024, 21, 468. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Laboratory Procedure Manual. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://wwwn.cdc.gov/nchs/data/nhanes/public/2007/labmethods/cot_e_met.pdf&ved=2ahUKEwj5ldTrsp-NAxWoWGwGHRgXFIcQFnoECBEQAQ&usg=AOvVaw3sSpLrjgFJTn-Emn3DmP8B (accessed on 12 December 2023).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/index.html (accessed on 16 December 2023).
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Estimating Glomerular Filtration Rate. Available online: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate/estimating#the-mdrd-equation (accessed on 16 December 2023).
- Mendivil, C.O.; Gnecco-Gonzalez, S.; Herrera-Parra, L.J.; Hernandez Vargas, J.A.; Ramirez-Garcia, N.; Acuna-Merchan, L. MDRD is the eGFR equation most strongly associated with 4-year mortality among patients with diabetes in Colombia. BMJ Open Diabetes Res. Care 2023, 11, e003495. [Google Scholar] [CrossRef]
- National Kidney Foundation. Chronic Kidney Disease (CKD). Available online: https://www.kidney.org/atoz/content/about-chronic-kidney-disease (accessed on 20 February 2024).
- Babekir, A.; Mostafa, S.; Obeng-Gyasi, E. The Association of Toxoplasma gondii IgG Antibody and Chronic Kidney Disease Biomarkers. Microorganisms 2022, 10, 115. [Google Scholar] [CrossRef]
- American Kidney Fund. Stages of Kidney Disease (CKD). Available online: https://www.kidneyfund.org/all-about-kidneys/stages-kidney-disease (accessed on 31 December 2023).
- Wu, W.; Zhang, K.; Jiang, S.; Liu, D.; Zhou, H.; Zhong, R.; Zeng, Q.; Cheng, L.; Miao, X.; Tong, Y.; et al. Association of co-exposure to heavy metals with renal function in a hypertensive population. Environ. Int. 2018, 112, 198–206. [Google Scholar] [CrossRef]
- Yang, K.; Chen, C.; Brockman, J.; Shikany, J.M.; He, K. Low- and moderate- levels of arsenic exposure in young adulthood and incidence of chronic kidney disease: Findings from the CARDIA Trace Element Study. J. Trace Elem. Med. Biol. 2021, 63, 126657. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Xie, Z.; Tan, J.; Fang, G.; Ji, H.; Miao, M.; Tian, Y.; Hu, H.; Cao, W.; Liang, H.; Yuan, W. Associations between prenatal exposure to perfluoroalkyl substances and neurobehavioral development in early childhood: A prospective cohort study. Ecotoxicol. Environ. Saf. 2022, 241, 113818. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Schmidt, S. Quantile g-computation: A new method for analyzing mixtures of environmental exposures. Environ. Health Perspect. 2020, 128, 104004. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 2020, 128, 047004. [Google Scholar] [CrossRef]
- Tanner, E.; Bornehag, C.; Gennings, C. Repeated holdout validation for weighted quantile sum regression. MethodsX 2019, 6, 2855–2860. [Google Scholar] [CrossRef]
- R Core Team. R. A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 16 January 2024).
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borne, Y.; Engstrom, G.; et al. Blood Lead Levels and Decreased Kidney Function in a Population-Based Cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, H.; Zhao, J.; Li, B.; Qu, L.; Liu, S.; Zhang, P.; Chai, Z. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ. Health Perspect. 2006, 114, 297–301. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Ginsberg, G.; Lin, J.-W.; Sonawane, B. Mercury exposure and omega-3 fatty acid intake in relation to renal function in the US population. Int. J. Hyg. Environ. Health 2014, 217, 465–472. [Google Scholar] [CrossRef]
- Jones, P.; Hu, W.; De Coen, W.; Newsted, J.; Giesy, J. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem. 2003, 22, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Teng, M.; Zhao, X.; Li, Y.; Sun, J.; Zhao, W.; Ruan, Y.; Leung, K.M.Y.; Wu, F. Insight into the binding model of per- and polyfluoroalkyl substances to proteins and membranes. Environ. Int. 2023, 175, 107951. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Li, Y.; Cao, H.; Huang, A.; Zhuang, Y.; Zhang, C.; Hu, G.; Mao, Y.; Luo, J.; et al. The protection of selenium against cadmium-induced mitophagy via modulating nuclear xenobiotic receptors response and oxidative stress in the liver of rabbits. Environ. Pollut. 2021, 285, 117301. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [PubMed]
- Sabath, E.; Robles-Osorio, M.L. Medio ambiente y riñón: Nefrotoxicidad por metales pesados. Nefrología 2012, 32, 279–286. [Google Scholar] [CrossRef]
- Kuo, P.F.; Huang, Y.T.; Chuang, M.H.; Jiang, M.Y. Association of low-level heavy metal exposure with risk of chronic kidney disease and long-term mortality. PLoS ONE 2024, 19, e0315688. [Google Scholar] [CrossRef]
- Choi, S.H.; Choi, K.H.; Won, J.U.; Kim, H. Impact of multi-heavy metal exposure on renal damage indicators in Korea: An analysis using Bayesian Kernel Machine Regression. Medicine 2023, 102, e35001. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X. Abnormal Iron and Lipid Metabolism Mediated Ferroptosis in Kidney Diseases and Its Therapeutic Potential. Metabolites 2022, 12, 58. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.; Li, J. Exploring the association between two groups of metals with potentially opposing renal effects and renal function in middle-aged and older adults: Evidence from an explainable machine learning method. Ecotoxicol. Environ. Saf. 2024, 269, 115812. [Google Scholar] [CrossRef]
- Wu, M.; Hou, W.; Qin, R.; Wang, G.; Sun, D.; Geng, Y.; Du, Y. Comparative mathematical modeling of causal association between metal exposure and development of chronic kidney disease. Front. Endocrinol. 2024, 15, 1362085. [Google Scholar] [CrossRef]


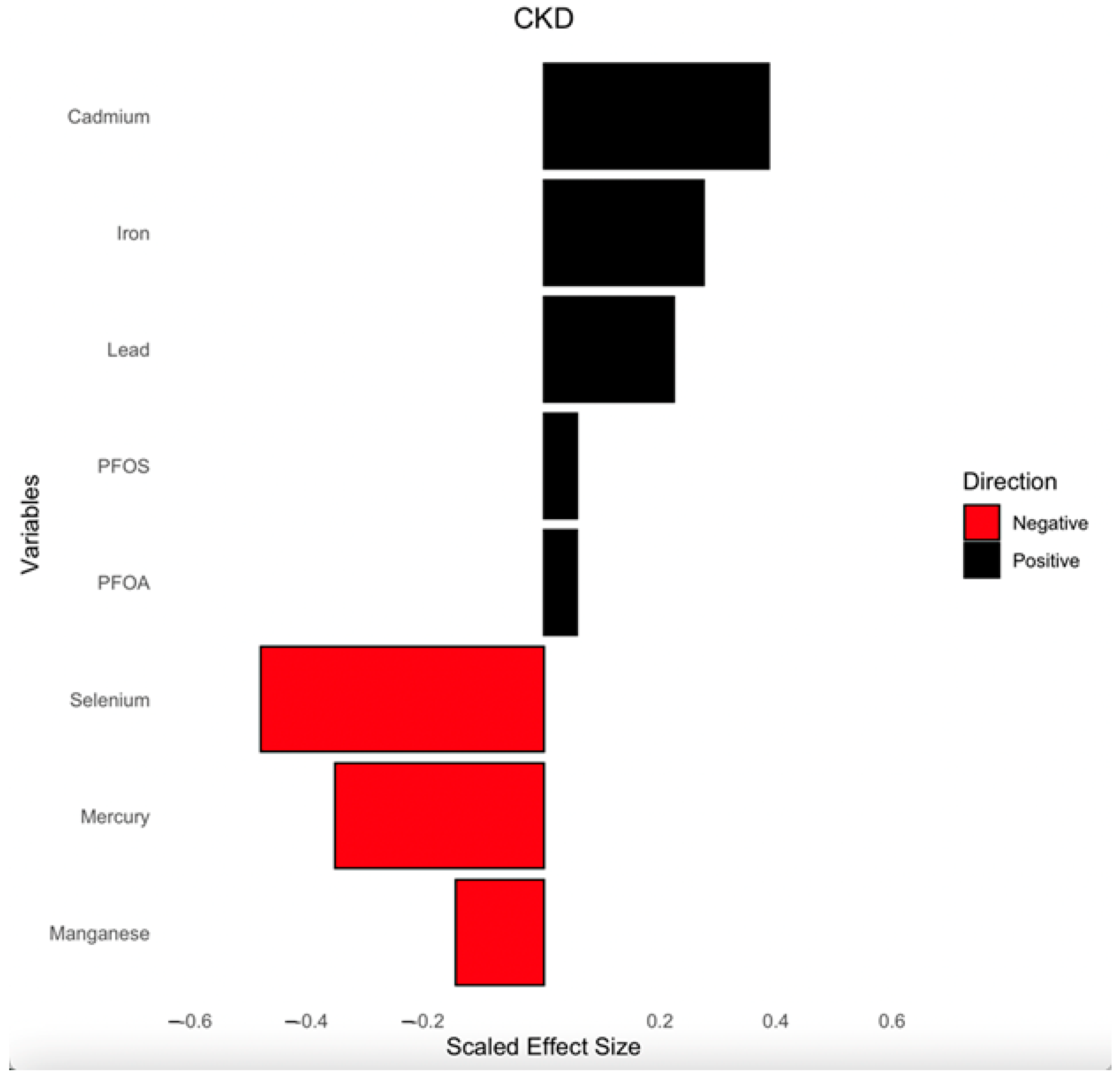
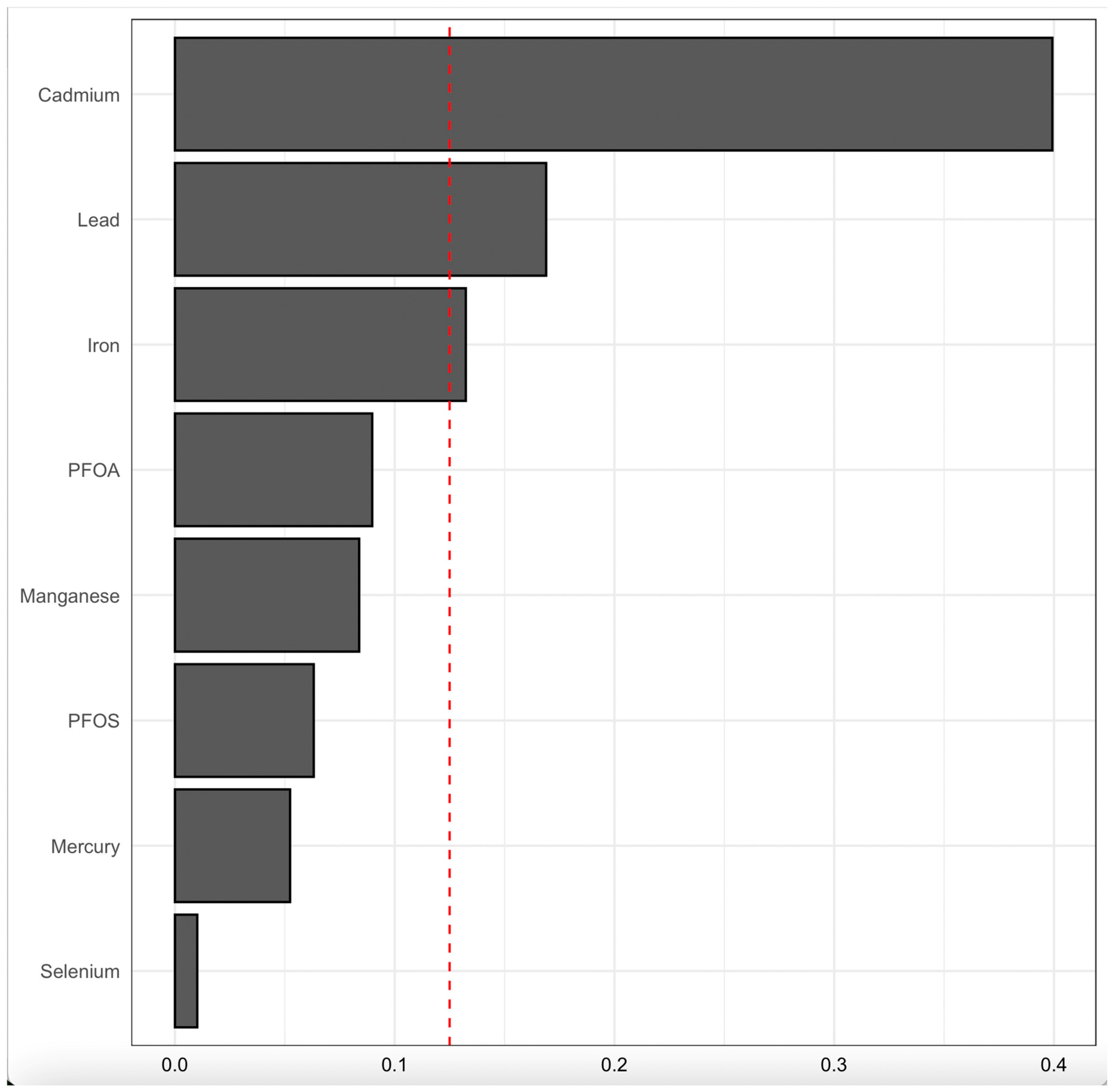
| Variable | Total | CKD | Non-CKD | p-Value |
|---|---|---|---|---|
| Total | 5800 | 1071(18.47) | 4729(81.53) | <0.0001 |
| Gender | ||||
| Male | 2814(48.52) | 508(47.43) | 2306(48.76) | 0.4515 |
| Female | 2986(51.48) | 563(52.57) | 2423(51.24) | |
| Age (yrs) | ||||
| 0–20 | 1037(17.88) | 93(8.68) | 944(19.96) | <0.0001 |
| 21–40 | 1462(25.21) | 105(9.80) | 1357(28.70) | |
| 41–60 | 1601(27.60) | 248(23.16) | 1353(28.61) | |
| 61–80 | 1700(29.31) | 625(58.36) | 1075(22.73) | |
| BMI | ||||
| <18.5 (Underweight) | 199(3.43) | 33(3.08) | 166(3.51) | <0.0001 |
| 18.5–24.9 (Normal) | 1616(27.86) | 242(22.60) | 1374(29.05) | |
| 25–29.9 (Overweight) | 1717(29.60) | 293(27.36) | 1424(30.11) | |
| ≥30 (Obese) | 2202(37.97) | 480(44.82) | 1722(36.41) | |
| Blank | 66(1.14) | 23(2.15) | 43(0.91) | |
| Ethnicity | ||||
| Mexican American | 858(14.79) | 132(12.32) | 726(15.35) | <0.0001 |
| Other Hispanic | 535(9.22) | 87(8.12) | 448(9.47) | |
| Non-Hispanic White | 1988(34.28) | 445(41.55) | 1543(32.63) | |
| Non-Hispanic Black | 1283(22.12) | 231(21.57) | 1052(22.25) | |
| Non-Hispanic Asian | 803(13.84) | 111(10.36) | 692(14.63) | |
| Other Race—Including Multiracial | 333(5.74) | 65(6.07) | 268(5.67) | |
| Annual income | ||||
| <USD 25,000 | 1534(26.45) | 337(31.47) | 1197(25.31) | <0.0001 |
| USD 25,000–74,999 | 2189(37.74) | 425(39.68) | 1764(37.30) | |
| USD 75,000–99,999 | 542(9.34) | 79(7.38) | 463(9.79) | |
| ≥UDS100,000 | 1022(17.62) | 141(13.17) | 881(18.63) | |
| Missing | 513(8.84) | 89(8.31) | 424(8.97) | |
| Alcohol | ||||
| Yes | 4244(73.17) | 820(76.56) | 3424(72.40) | <0.0001 |
| No | 509(8.78) | 109(10.18) | 400(8.46) | |
| Blank | 1047(18.05) | 142(13.26) | 905(19.14) | |
| Smoking | ||||
| Yes | 2046(35.28) | 465(43.42) | 1581(33.43) | <0.0001 |
| No | 3017(52.02) | 531(49.58) | 2486(52.57) | |
| Missing | 737(12.71) | 75(7.00) | 662(14.00) | |
| Hypertension (Taking Medication) | ||||
| Yes | 1675(28.88) | 604(56.40) | 1071(22.65) | <0.0001 |
| No | 171(2.95) | 22(2.05) | 149(3.15) | |
| Refused | 1(0.02) | 0(0.00) | 1(0.02) | |
| Did not know | 2(0.03) | 1(0.09) | 1(0.02) | |
| Missing | 3951(68.12) | 444(41.46) | 3507(74.16) | |
| Diabetes | ||||
| Yes | 777(13.40) | 350(32.68) | 427(9.03) | <0.0001 |
| No | 4856(83.72) | 689(64.33) | 4167(88.12) | |
| Borderline | 164(2.83) | 32(2.99) | 132(2.79) | |
| Did not know | 3(0.05) | 0.00) | 3(0.06) | |
| Kidney Failing | ||||
| Yes | 187(3.22) | 133(12.42) | 54(1.14) | <0.0001 |
| No | 4632(79.86) | 841(78.52) | 3791(80.16) | |
| Did not know | 8(0.14) | 6(0.56) | 2(0.04) | |
| Missing | 973(16.78) | 91(8.50) | 882(18.65) | |
| eGFR stage | ||||
| Stage 1 | 3206(55.28) | 339(31.65) | 2867(60.63) | <0.0001 |
| Stage 2 | 2117(36.50) | 255(23.81) | 1862(39.37) | |
| Stage 3 | 435(7.50) | 435(40.62) | 0(0.00) | |
| Stage 4 | 33(0.57) | 33(3.08) | 0(0.00) | |
| Stage 5 | 9(0.16) | 9(0.84) | 0(0.00) | |
| ACR (mg/g) | 5.23(8.28, 15.88) | 48.05(17.72, 123.30) | 6.63(4.57, 10.56) | <0.0001 |
| SCr (mg/dL) | 0.82(0.68, 0.98) | 0.98(0.75, 1.25) | 0.80(0.67, 0.93) | <0.0001 |
| Variable | Total | CKD | Non-CKD | p-Value |
| PFOA (ng/mL) | 1.37(0.87, 2.07) | 1.47(0.87, 2.27) | 1.37(0.87, 1.97) | 0.072 |
| PFOS (ng/mL) | 4.20(2.40, 7.80) | 5.20(2.70, 9.60) | 4.00(2.30, 7.40) | 0.0004 |
| Pb (µg/dL) | 0.76(0.46, 1.30) | 1.07(0.66, 1.66) | 0.77(0.46, 1.30) | <0.0001 |
| Cd (µg/L) | 0.22(0.12, 0.42) | 0.35(0.20, 0.60) | 0.25(0.15, 0.47) | <0.0001 |
| Hg (µg/L) | 0.51(0.20, 1.12) | 0.65(0.34, 1.33) | 0.62(0.31, 1.34) | 0.1579 |
| Mn (µg/L) | 9.71(7.75, 12.13) | 9.24(7.28, 11.45) | 9.57(7.68, 12.03) | <0.0001 |
| Se (µg/L) | 184.30(169.50, 200.90) | 187.90(171.40, 205.80) | 188.30(174.3, 204.00) | 0.5270 |
| Fe (µg/dL) | 82.00(61.00, 106.00) | 78.00(59.00, 99.00) | 83.00(62.00, 108.00) | <0.0001 |
| Exposures | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| PFOA | 1.370 | 1.016–1.848 | 0.039 * |
| PFOS | 0.940 | 0.894–0.987 | 0.013 * |
| Lead | 1.065 | 0.918–1.235 | 0.408 |
| Cadmium | 2.161 | 1.111–4.203 | 0.023 * |
| Mercury | 0.923 | 0.765–1.115 | 0.408 |
| Selenium | 0.998 | 0.984–1.012 | 0.791 |
| Manganese | 1.032 | 0.940–1.133 | 0.512 |
| Iron | 1.000 | 0.989–1.010 | 0.934 |
| Exposures | PIP |
|---|---|
| PFOA | 0.701 |
| PFOS | 0.612 |
| Lead | 0.662 |
| Cadmium | 0.668 |
| Mercury | 0.908 |
| Selenium | 0.616 |
| Manganese | 0.754 |
| Iron | 0.668 |
| Exposures | Group | groupPIP | condPIP |
|---|---|---|---|
| PFOA | 1 | 0.251 | 0.578 |
| PFOS | 1 | 0.251 | 0.422 |
| Lead | 2 | 0.684 | 0.029 |
| Cadmium | 2 | 0.684 | 0.116 |
| Mercury | 2 | 0.684 | 0.855 |
| Selenium | 3 | 0.702 | 0.025 |
| Manganese | 3 | 0.702 | 0.140 |
| Iron | 3 | 0.702 | 0.835 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haruna, I.; Obeng-Gyasi, E. Combined Effect of per- and Polyfluoroalkyl Substances, Toxic Metals, and Essential Elements on Chronic Kidney Disease. Pollutants 2025, 5, 12. https://doi.org/10.3390/pollutants5020012
Haruna I, Obeng-Gyasi E. Combined Effect of per- and Polyfluoroalkyl Substances, Toxic Metals, and Essential Elements on Chronic Kidney Disease. Pollutants. 2025; 5(2):12. https://doi.org/10.3390/pollutants5020012
Chicago/Turabian StyleHaruna, Issah, and Emmanuel Obeng-Gyasi. 2025. "Combined Effect of per- and Polyfluoroalkyl Substances, Toxic Metals, and Essential Elements on Chronic Kidney Disease" Pollutants 5, no. 2: 12. https://doi.org/10.3390/pollutants5020012
APA StyleHaruna, I., & Obeng-Gyasi, E. (2025). Combined Effect of per- and Polyfluoroalkyl Substances, Toxic Metals, and Essential Elements on Chronic Kidney Disease. Pollutants, 5(2), 12. https://doi.org/10.3390/pollutants5020012







