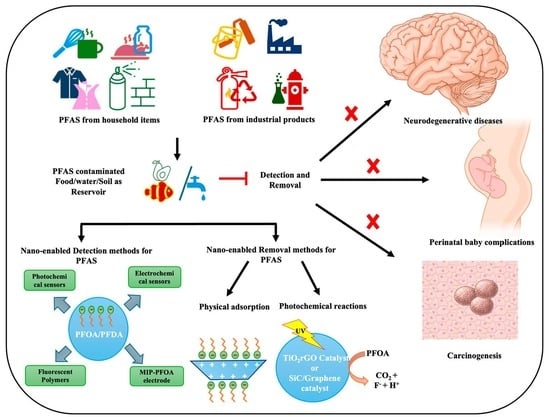
Advanced Nanoformulations for Detection and Removal of Poly- and Perfluoroalkyl Substances (PFAS)
Abstract
:1. Introduction
2. PFAS Exposure and Human Health: Investigating the Link to Cancer, Immune Disorders, and Neurodegeneration
2.1. PFAS Induced Carcinogenesis
2.2. Metabolic and Immune Effects
2.3. Neurodegenerative Disorders
2.4. Pre- and Postnatal PFAS Exposure
3. Nano-Enabled Techniques for the Detection of PFAS
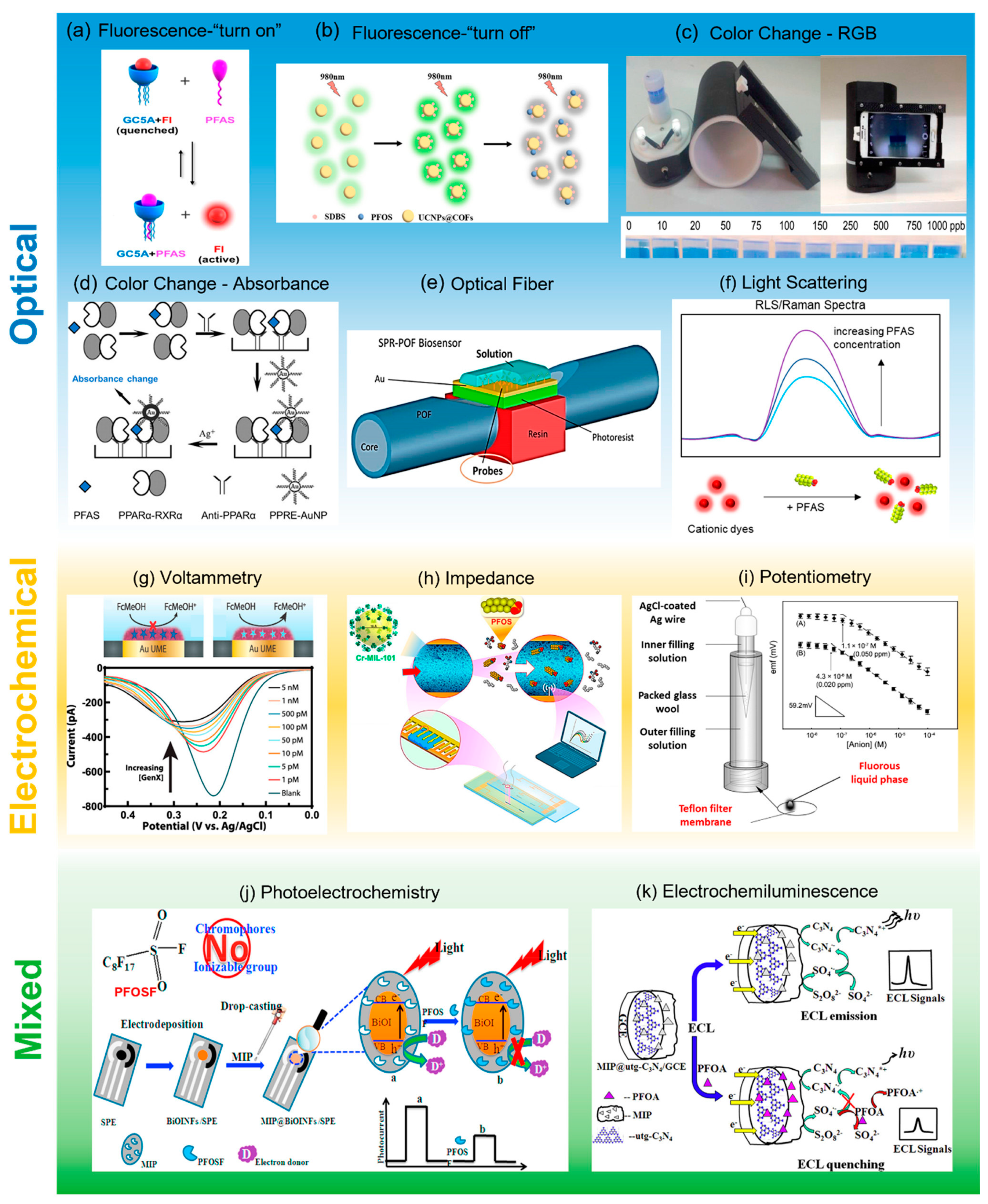
3.1. Nanotechnology-Based Sensors for PFAS Detection
3.1.1. Photo-Electrochemical Sensors
3.1.2. Electrochemical Sensors
3.1.3. Electro-Chemifluorescence Sensors
3.1.4. Living Cell Sensor for PFAS Detection
4. Nano-Enabled Strategies for PFAS Removal
4.1. CNM/TiO2 Composite for PFAS Removal
4.2. Biomimetic Lignocellulosic Nano-Framework
4.3. Photochemical Reactions
4.3.1. TiO2 Based Nanomaterials
4.3.2. Pb-Modified Nanoparticles
4.3.3. Ga2O3-Based Nanomaterials
4.3.4. Titanate Nanotube
4.4. Physical Adsorption
4.4.1. Nanofiltration Membrane Separation
4.4.2. Carbon Nanotubes
4.4.3. Magnetic Iron-Oxide Nanoparticles
4.4.4. Microbial PFAS Degradation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahrens, L.; Taniyasu, S.; Yeung, L.W.Y.; Yamashita, N.; Lam, P.K.S.; Ebinghaus, R. Distribution of Polyfluoroalkyl Compounds in Water, Suspended Particulate Matter and Sediment from Tokyo Bay, Japan. Chemosphere 2010, 79, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Reade, A.; Quinn, T.; Schreiber, J.S. Michigan PFAS 2019 Scientific and Policy Assessment for Addressing Per-and Polyfluoroalkyl Substances (PFAS) in Drinking Water; NRDC: New York, NY, USA, 2019. [Google Scholar]
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide Trends in Tracing Poly- and Perfluoroalkyl Substances (PFAS) in the Environment. TrAC Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.E.; Karvounis, M.; Marinos, G.; Theodorakopoulou, Z.; Aloizou, E.; Petsangourakis, G.; Papakonstantinou, M.; Stoitsis, G. Comprehensive Analysis of PFAS Presence from Environment to Plate. NPJ Sci. Food 2024, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Pinney, S.M.; Biro, F.M.; Fassler, C.S.; Windham, G.C.; Herrick, R.L.; Xie, C.; Kushi, L.H. Exposure to Perfluoroalkyl Substances and Associations with Pubertal Onset and Serum Reproductive Hormones in a Longitudinal Study of Young Girls in Greater Cincinnati and the San Francisco Bay Area. Environ. Health Perspect. 2023, 131. [Google Scholar] [CrossRef]
- Potential Health Effects of PFAS Chemicals | ATSDR. Available online: https://www.atsdr.cdc.gov/pfas/health-effects/index.html (accessed on 9 November 2024).
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Wanninayake, D.; Chen, D.; Nguyen, N.T.; Li, Q. Physicochemical Properties and Interactions of Perfluoroalkyl Substances (PFAS)—Challenges and Opportunities in Sensing and Remediation. Sci. Total Environ. 2023, 905, 166764. [Google Scholar] [CrossRef]
- Ahrens, L.; Bundschuh, M. Fate and Effects of Poly- and Perfluoroalkyl Substances in the Aquatic Environment: A Review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; Dewitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for Grouping Per- and Polyfluoroalkyl Substances (PFAS) to Protect Human and Environmental Health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef]
- Kannan, K.; Corsolini, S.; Falandysz, J.; Fillmann, G.; Kumar, K.S.; Loganathan, B.G.; Mohd, M.A.; Olivero, J.; Van Wouwe, N.; Yang, J.H.; et al. Perfluorooctanesulfonate and Related Fluorochemicals in Human Blood from Several Countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [Google Scholar] [CrossRef]
- Garg, S.; Kumar, P.; Mishra, V.; Guijt, R.; Singh, P.; Dumée, L.F.; Sharma, R.S. A Review on the Sources, Occurrence and Health Risks of per-/Poly-Fluoroalkyl Substances (PFAS) Arising from the Manufacture and Disposal of Electric and Electronic Products. J. Water Process Eng. 2020, 38, 101683. [Google Scholar] [CrossRef]
- Martin, J.W.; Kannan, K.; Berger, U.; De Voogt, P.; Field, J.; Franklin, J.; Giesy, J.P.; Harner, T.; Muir, D.C.G.; Scott, B.; et al. Peer Reviewed: Analytical Challenges Hamper Perfluoroalkyl Research. Environ. Sci. Technol. 2004, 38, 248A–255A. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Kumar, P.; Greene, G.W.; Mishra, V.; Avisar, D.; Sharma, R.S.; Dumée, L.F. Nano-Enabled Sensing of per-/Poly-Fluoroalkyl Substances (PFAS) from Aqueous Systems—A Review. J. Environ. Manag. 2022, 308, 114655. [Google Scholar] [CrossRef]
- Guelfo, J.L.; Higgins, C.P. Subsurface Transport Potential of Perfluoroalkyl Acids at Aqueous Film-Forming Foam (AFFF)-Impacted Sites. Environ. Sci. Technol. 2013, 47, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Developing and Demonstrating Nanosensor Technology to Detect, Monitor, and Degrade Pollutants Request for Applications (RFA) | US EPA. Available online: https://www.epa.gov/research-grants/developing-and-demonstrating-nanosensor-technology-detect-monitor-and-degrade-1 (accessed on 9 November 2024).
- Wee, S.Y.; Aris, A.Z. Environmental impacts, exposure pathways, and health effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef] [PubMed]
- Birch, Q.T.; Birch, M.E.; Nadagouda, M.N.; Dionysiou, D.D. Nano-Enhanced Treatment of per-Fluorinated and Poly-Fluorinated Alkyl Substances (PFAS). Curr. Opin. Chem. Eng. 2022, 35, 100779. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, H.; Wang, S.; Yu, K.; Wang, L. A Portable Molecularly Imprinted Polymer-Modified Microchip Sensor for the Rapid Detection of Perfluorooctanoic Acid. Analyst 2023, 148, 3851–3859. [Google Scholar] [CrossRef]
- Per- and Polyfluoroalkyl Substances (PFAS) | US EPA. Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed on 2 November 2024).
- Yadav, M.; Osonga, F.J.; Sadik, O.A. Unveiling Nano-Empowered Catalytic Mechanisms for PFAS Sensing, Removal and Destruction in Water. Sci. Total Environ. 2024, 912, 169279. [Google Scholar] [CrossRef]
- Pilli, S.; Pandey, A.K.; Pandey, V.; Pandey, K.; Muddam, T.; Thirunagari, B.K.; Thota, S.T.; Varjani, S.; Tyagi, R.D. Detection and Removal of Poly and Perfluoroalkyl Polluting Substances for Sustainable Environment. J. Environ. Manag. 2021, 297, 113336. [Google Scholar] [CrossRef]
- Ahrens, L. Polyfluoroalkyl Compounds in the Aquatic Environment: A Review of Their Occurrence and Fate. J. Environ. Monit. 2011, 13, 20–31. [Google Scholar] [CrossRef]
- SW-846 Test Method 8327: Per-and Polyfluoroalkyl Substances (PFAS) by Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) | US EPA. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-8327-and-polyfluoroalkyl-substances-pfas-liquid-chromatographytandem (accessed on 31 December 2024).
- Rehman, A.U.; Andreescu, D.; Tiwari, S.; Andreescu, S. Rapid Single-Step Detection of Polyfluoroalkyl Substances (PFAS) Using Electropolymerized Phenoxazine Dyes. Anal. Chem. 2024, 96, 17506–17516. [Google Scholar] [CrossRef]
- Karimian, N.; Stortini, A.M.; Moretto, L.M.; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for Trace Analysis of Perfluorooctanesulfonate in Water Based on a Molecularly Imprinted Poly(o-Phenylenediamine) Polymer. ACS Sens. 2018, 3, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Menger, R.F.; Funk, E.; Henry, C.S.; Borch, T. Sensors for Detecting Per- and Polyfluoroalkyl Substances (PFAS): A Critical Review of Development Challenges, Current Sensors, and Commercialization Obstacles. Chem. Eng. J. 2021, 417, 129133. [Google Scholar] [CrossRef]
- van Gerwen, M.; Colicino, E.; Guan, H.; Dolios, G.; Nadkarni, G.N.; Vermeulen, R.C.H.; Wolff, M.S.; Arora, M.; Genden, E.M.; Petrick, L.M. Per- and Polyfluoroalkyl Substances (PFAS) Exposure and Thyroid Cancer Risk. eBioMedicine 2023, 97, 104831. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.A.; Hsu, M.H.; Griffin, K.J.; Raucy, J.L.; Johnson, E.F. Peroxisome Proliferator Activated Receptor-α Expression in Human Liver. Mol. Pharmacol. 1998, 53, 14–22. [Google Scholar] [CrossRef]
- Gilliland, F.D.; Mandel, J.S. Mortality among Employees of a Perfluorooctanoic Acid Production Plant. J. Occup. Med. 1993, 35, 950–954. [Google Scholar] [CrossRef]
- Steenland, K.; Zhao, L.; Winquist, A.; Parks, C. Ulcerative Colitis and Perfluorooctanoic Acid (PFOA) in a Highly Exposed Population of Community Residents and Workers in the Mid-Ohio Valley. Environ. Health Perspect. 2013, 121, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.T.; Sørensen, M.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Perfluorooctanoate and Perfluorooctanesulfonate Plasma Levels and Risk of Cancer in the General Danish Population. JNCI J. Natl. Cancer Inst. 2009, 101, 605–609. [Google Scholar] [CrossRef]
- Barton, K.E.; Zell-Baran, L.M.; DeWitt, J.C.; Brindley, S.; McDonough, C.A.; Higgins, C.P.; Adgate, J.L.; Starling, A.P. Cross-Sectional Associations between Serum PFASs and Inflammatory Biomarkers in a Population Exposed to AFFF-Contaminated Drinking Water. Int. J. Hyg. Environ. Health 2022, 240, 113905. [Google Scholar] [CrossRef]
- Abdellatif, A.G.; Préat, V.; Vamecq, J.; Nilsson, R.; Roberfroid, M. Peroxisome Proliferation and Modulation of Rat Liver Carcinogenesis by 2,4-Dichlorophenoxyacetic Acid, 2,4,5-Trichlorophenoxyacetic Acid, Perfluorooctanoic Acid and Nafenopin. Carcinogenesis 1990, 11, 1899–1902. [Google Scholar] [CrossRef]
- Serum Perfluorooctanoic Acid and Hepatic Enzymes, Lipoproteins, and Cholesterol: A Study of Occupationally Exposed Men—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8732932/ (accessed on 2 November 2024).
- Liu, H.S.; Wen, L.L.; Chu, P.L.; Lin, C.Y. Association among Total Serum Isomers of Perfluorinated Chemicals, Glucose Homeostasis, Lipid Profiles, Serum Protein and Metabolic Syndrome in Adults: NHANES, 2013–2014. Environ. Pollut. 2018, 232, 73–79. [Google Scholar] [CrossRef]
- Dewitt, J.C.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of Perfluorinated Compounds: Recent Developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Lam, T.K.Y.; Tang, H.C.; Ho, T.C.; Wan, H.T.; Wong, C.K.C. PFOS-Elicited Metabolic Perturbation in Liver and Fatty Acid Metabolites in Testis of Adult Mice. Front. Endocrinol. 2023, 14, 1302965. [Google Scholar] [CrossRef]
- Brown-Leung, J.M.; Cannon, J.R. Neurochemical Mechanisms of Perfluoroalkyl Substances (PFAS) Neurotoxic Action. Adv. Neurotoxicol. 2023, 10, 367–398. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef] [PubMed]
- van Larebeke, N.; Koppen, G.; Decraemer, S.; Colles, A.; Bruckers, L.; Den Hond, E.; Govarts, E.; Morrens, B.; Schettgen, T.; Remy, S.; et al. Per- and Polyfluoroalkyl Substances (PFAS) and Neurobehavioral Function and Cognition in Adolescents (2010–2011) and Elderly People (2014): Results from the Flanders Environment and Health Studies (FLEHS). Environ. Sci. Eur. 2022, 34, 98. [Google Scholar] [CrossRef]
- Delcourt, N.; Pouget, A.M.; Grivaud, A.; Nogueira, L.; Larvor, F.; Marchand, P.; Schmidt, E.; Bizec, B.L. First Observations of a Potential Association Between Accumulation of Per- and Polyfluoroalkyl Substances in the Central Nervous System and Markers of Alzheimer’s Disease. J. Gerontol. Ser. A 2024, 79, glad208. [Google Scholar] [CrossRef]
- Ríos-Bonilla, K.M.; Aga, D.S.; Lee, J.; König, M.; Qin, W.; Cristobal, J.R.; Atilla-Gokcumen, G.E.; Escher, B.I. Neurotoxic Effects of Mixtures of Perfluoroalkyl Substances (PFAS) at Environmental and Human Blood Concentrations. Environ. Sci. Technol. 2024, 58, 16774–16784. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Gugliandolo, E.; Cuzzocrea, S.; Crupi, R.; Britti, D. Current Review of Increasing Animal Health Threat of Per- and Polyfluoroalkyl Substances (PFAS): Harms, Limitations, and Alternatives to Manage Their Toxicity. Int. J. Mol. Sci. 2023, 24, 11707. [Google Scholar] [CrossRef]
- Needham, L.L.; Grandjean, P.; Heinzow, B.; Jørgensen, P.J.; Nielsen, F.; Sjödin, A.; Patterson, D.G.; Turner, W.E.; Weihe, P. Partition of Environmental Chemicals between Maternal and Fetal Blood and Tissues. Environ. Sci. Technol. 2011, 45, 1121–1126. [Google Scholar] [CrossRef]
- In Utero PFAS Exposure and Childhood Leukemia—NCI. Available online: https://dceg.cancer.gov/news-events/news/2023/pfas-childhood-leukemia (accessed on 11 November 2024).
- Oh, J.; Shin, H.M.; Kannan, K.; Busgang, S.A.; Schmidt, R.J.; Schweitzer, J.B.; Hertz-Picciotto, I.; Bennett, D.H. Childhood Exposure to Per- and Polyfluoroalkyl Substances and Neurodevelopment in the CHARGE Case-Control Study. Environ. Res. 2022, 215 Pt 2, 114322. [Google Scholar] [CrossRef]
- Dalsager, L.; Christensen, N.; Halekoh, U.; Timmermann, C.A.G.; Nielsen, F.; Kyhl, H.B.; Husby, S.; Grandjean, P.; Jensen, T.K.; Andersen, H.R. Exposure to Perfluoroalkyl Substances during Fetal Life and Hospitalization for Infectious Disease in Childhood: A Study among 1,503 Children from the Odense Child Cohort. Environ. Int. 2021, 149, 106395. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, C.; Frömel, T.; Knepper, T.P. HPLC–MS/MS Methods for the Determination of 52 Perfluoroalkyl and Polyfluoroalkyl Substances in Aqueous Samples. Anal. Bioanal. Chem. 2017, 409, 1643–1655. [Google Scholar] [CrossRef]
- Cao, S.; Xie, Z.; Xiao, G.; Sun, X.; Diao, H.; Zhou, X.; Yue, Z. Photoelectrochemical Sensors Based on Heterogeneous Nanostructures for in Vitro Diagnostics. Biosens. Bioelectron. X 2022, 11, 100200. [Google Scholar] [CrossRef]
- Zang, Y.; Lei, J.; Ju, H. Principles and Applications of Photoelectrochemical Sensing Strategies Based on Biofunctionalized Nanostructures. Biosens. Bioelectron. 2017, 96, 8–16. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Fang, T.; Zhang, L.; Gong, J. Disposable Photoelectrochemical Sensing Strip for Highly Sensitive Determination of Perfluorooctane Sulfonyl Fluoride on Functionalized Screen-Printed Carbon Electrode. Talanta 2018, 181, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Li, J.; Feng, H.; Cai, J.; Yuan, L.; Wang, N.; Cai, Q. Molecularly Imprinted Polymer Modified TiO2 Nanotube Arrays for Photoelectrochemical Determination of Perfluorooctane Sulfonate (PFOS). Sens. Actuators B Chem. 2014, 190, 745–751. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Reddy, K.R.; Aminabhvi, T.M. Graphene-Clay-Based Hybrid Nanostructures for Electrochemical Sensors and Biosensors. In Graphene-Based Electrochemical Sensors for Biomolecules: A Volume in Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–274. [Google Scholar] [CrossRef]
- Li, S.; Piletsky, S.A.; Ge, Y.; Lunec, J. Molecularly Imprinted Sensors. Overview and Applications. In Molecularly Imprinted Sensors: Overview and Applications; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–370. [Google Scholar] [CrossRef]
- Clark, R.B.; Dick, J.E. Electrochemical Sensing of Perfluorooctanesulfonate (PFOS) Using Ambient Oxygen in River Water. ACS Sens. 2020, 5, 3591–3598. [Google Scholar] [CrossRef]
- Sahu, S.P.; Kole, S.; Arges, C.G.; Gartia, M.R. Rapid and Direct Perfluorooctanoic Acid Sensing with Selective Ionomer Coatings on Screen-Printed Electrodes under Environmentally Relevant Concentrations. ACS Omega 2022, 7, 5001–5007. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Ma, C.; Zhu, J.J. Carbon-Based Dots for Electrochemiluminescence Sensing. Mater. Chem. Front. 2020, 4, 369–385. [Google Scholar] [CrossRef]
- Ma, W.; Han, D.; Zhou, M.; Sun, H.; Wang, L.; Dong, X.; Niu, L. Ultrathin G-C3N4/TiO2 Composites as Photoelectrochemical Elements for the Real-Time Evaluation of Global Antioxidant Capacity. Chem. Sci. 2014, 5, 3946–3951. [Google Scholar] [CrossRef]
- Zheng, Z.; Yu, H.; Geng, W.C.; Hu, X.Y.; Wang, Y.Y.; Li, Z.; Wang, Y.; Guo, D.S. Guanidinocalix[5]Arene for Sensitive Fluorescence Detection and Magnetic Removal of Perfluorinated Pollutants. Nat. Commun. 2019, 10, 5762. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.M.; Berger, B.W. A Genetically-Encoded Biosensor for Direct Detection of Perfluorooctanoic Acid. Sci. Rep. 2023, 13, 15186. [Google Scholar] [CrossRef]
- Sunantha, G.; Vasudevan, N. A Method for Detecting Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Water Samples Using Genetically Engineered Bacterial Biosensor. Sci. Total Environ. 2021, 759, 143544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumar, V.; Sugumar, V.; Umesh, M.; Sondhi, S.; Chakraborty, P.; Kaur, K.; Thomas, J.; Kamaraj, C.; Maitra, S.S. A Comprehensive Review on the Need for Integrated Strategies and Process Modifications for Per- and Polyfluoroalkyl Substances (PFAS) Removal: Current Insights and Future Prospects. Case Stud. Chem. Environ. Eng. 2024, 9, 100623. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Da, Y.; Yu, J.; Long, B.; Zhang, P.; Bakker, C.; McCarl, B.A.; Yuan, J.S.; Dai, S.Y. Sustainable Environmental Remediation via Biomimetic Multifunctional Lignocellulosic Nano-Framework. Nat. Commun. 2022, 13, 4368. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium Dioxide Photocatalysis: Present Situation and Future Approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Chen, M.J.; Lo, S.L.; Lee, Y.C.; Huang, C.C. Photocatalytic Decomposition of Perfluorooctanoic Acid by Transition-Metal Modified Titanium Dioxide. J. Hazard. Mater. 2015, 288, 168–175. [Google Scholar] [CrossRef]
- Schindler, M.; Santosh, M.; Dotto, G.; Silva, L.F.O.; Hochella, M.F. A Review on Pb-Bearing Nanoparticles, Particulate Matter and Colloids Released from Mining and Smelting Activities. Gondwana Res. 2022, 110, 330–346. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, D.; Liang, Y. Nanotechnology in Remediation of Water Contaminated by Poly- and Perfluoroalkyl Substances: A Review. Environ. Pollut. 2019, 247, 266–276. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, P. Photocatalytic Decomposition of Perfluorooctanoic Acid with β-Ga2O3 Wide Bandgap Photocatalyst. Catal. Commun. 2009, 10, 1184–1187. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton Catalysts: A Review of Recent Advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Franke, V.; Ullberg, M.; McCleaf, P.; Wålinder, M.; Köhler, S.J.; Ahrens, L. The Price of Really Clean Water: Combining Nanofiltration with Granular Activated Carbon and Anion Exchange Resins for the Removal of Per- and Polyfluoralkyl Substances (PFASs) in Drinking Water Production. ACS ES T Water 2021, 1, 782–795. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Bruno, R.; Pardo, E.; Armentano, D. Reverse Osmosis and Nanofiltration Membranes for Highly Efficient PFASs Removal: Overview, Challenges and Future Perspectives. Dalton. Trans. 2021, 50, 5398–5410. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F. Adsorption of Perfluorooctane Sulfonate (PFOS) onto Metal Oxides Modified Biochar. Environ. Technol. Innov. 2020, 19, 100816. [Google Scholar] [CrossRef]
- Chaudhary, M.; Sela-Adler, M.; Ronen, A.; Nir, O. Efficient PFOA Removal from Drinking Water by a Dual-Functional Mixed-Matrix-Composite Nanofiltration Membrane. NPJ Clean Water 2023, 6, 77. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef]
- Song, X.L.; Lv, H.; Liao, K.C.; Wang, D.D.; Li, G.M.; Wu, Y.Y.; Chen, Q.Y.; Chen, Y. Application of Magnetic Carbon Nanotube Composite Nanospheres in Magnetic Solid-Phase Extraction of Trace Perfluoroalkyl Substances from Environmental Water Samples. Talanta 2023, 253, 123930. [Google Scholar] [CrossRef]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A Concentrate-and-Destroy Technique for Degradation of Perfluorooctanoic Acid in Water Using a New Adsorptive Photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Berhanu, A.; Mutanda, I.; Taolin, J.; Qaria, M.A.; Yang, B.; Zhu, D. A Review of Microbial Degradation of Per- and Polyfluoroalkyl Substances (PFAS): Biotransformation Routes and Enzymes. Sci. Total Environ. 2023, 859, 160010. [Google Scholar] [CrossRef] [PubMed]
- Wackett, L.P. Pseudomonas: Versatile Biocatalysts for PFAS. Environ. Microbiol. 2022, 24, 2882–2889. [Google Scholar] [CrossRef] [PubMed]
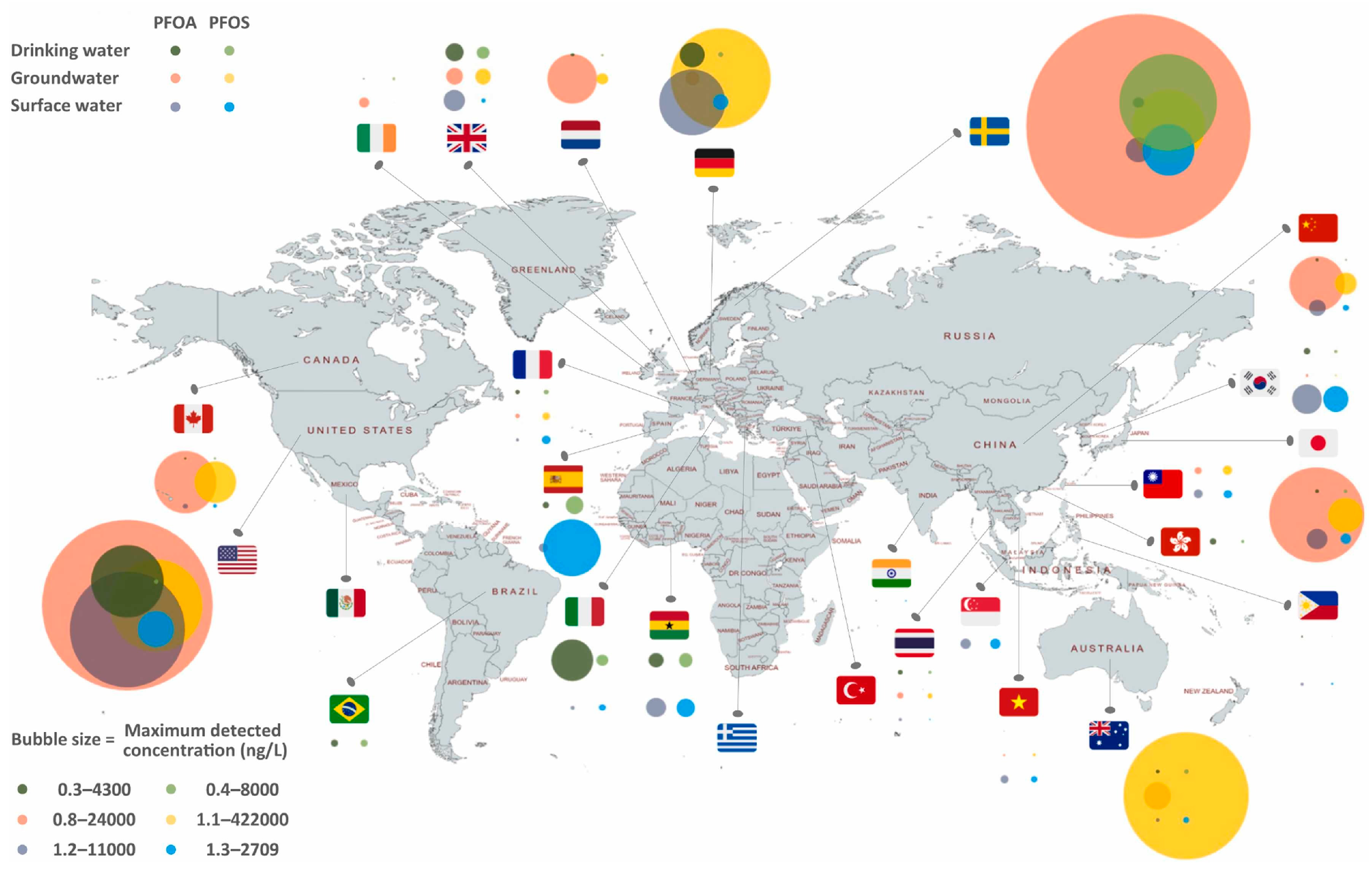
| Country/State | Concentration | Regulatory Guidelines |
|---|---|---|
| Global Average Concentration | 100 ng L−1 | Elevated levels, particularly in coastal regions and riverbanks near industrial urban centers [19]. |
| Coastal Saltwater (Laizhou Bay, China) | 475 ng L−1 | Reported concentrations pose potential health risks to coastal populations [19]. |
| U.S. EPA Drinking Water Limit | 4 ng L−1 (9.658 × 10−12 M) | Strict threshold recommended to ensure potable water safety [20]. |
| Swedish National Food Administration Limit | 90 ng L−1 (2.17 × 10−10 M) | Higher action level relative to U.S. EPA standards [20]. |
| European Drinking Water Directive Limit | 100 ng L−1 (2.41 × 10−10 M) | Emphasizes public health protection through stringent regulatory measures [20]. |
| Freshwater Acute Benchmarks (U.S. States) | 4.47 mg L−1 (Texas)–20 mg L−1 (Florida) | State-derived acute benchmarks for short-term exposure’s impacts on aquatic life [21]. |
| U.S. EPA Acute Freshwater Criteria | 3.1 mg L−1 | Slightly lower threshold than state-specific benchmarks for acute toxicity [21]. |
| Freshwater Chronic Benchmarks (U.S. States) | 0.22 mg L−1 (Australia/New Zealand)–2.27 mg L−1 (Texas) | State-specific guidelines addressing the risks of long-term exposure to aquatic ecosystems [21]. |
| U.S. EPA Chronic Freshwater Criteria | 0.10 mg L−1 | More conservative approach to chronic toxicity compared to state standards [21]. |
| Method | Applications | Detection Limit | Selectivity and Sensitivity |
|---|---|---|---|
| Photo-electrochemical Sensors | Detection of PFOA and PFOS using nanohybrids and metal oxides. | LOD: 86 ng/mL for PFOS detection. | High sensitivity, enhanced surface area, selective detection [53]. |
| Electrochemical Sensors | Quantitative and qualitative PFOS and PFOA detection. | LOD: 3.4 pM for PFOS, 6.51 ppb for PFOA. | Fast detection, low-cost, portable, suitable for field testing [55]. |
| Electro-Chemifluorescence Sensors | Detection of PFOA and PFOS using luminescence and molecular probes. | LOD: 0.01 μg/L for PFOA. | Highly sensitive and selective detection at low concentrations [58]. |
| Surface-Enhanced Raman Spectroscopy (SERS) | PFAS detection through signal enhancement using nanoparticles. | LOD: 10 ng/L for PFOA. | Highly specific, non-destructive, and fast analysis [59]. |
| Graphene-Based Sensors | Direct detection of PFAS using graphene nanostructures. | LOD: 1.2 ng/L for PFOS. | High surface-to-volume ratio, chemical stability, and fast response [60]. |
| Molecularly Imprinted Polymers (MIPs) | Selective PFAS detection via molecular recognition templates. | LOD: 5.4 nM for PFOA. | Reusable, cost-effective, and high selectivity [62]. |
| Fluorescent Nanoparticles | Detection of PFAS based on fluorescence-quenching or enhancement. | LOD: 15 ng/L for PFOS. | High sensitivity, multiplex detection, and portable nature [63]. |
| Method | Mechanism of Action | Removal Efficiency | Advantages |
|---|---|---|---|
| CNM/TiO2 Composite | Photochemical degradation through hydroxyl radicals generated via UV light reactions. | >90% degradation of PFOA under UV exposure. | High efficiency, rapid degradation, reusable material [54]. |
| Biomimetic Lignocellulosic Framework | Adsorption and in situ bioremediation using fungus Irpex lacteus. | High adsorption capacity and biodegradation. | Low-cost, eco-friendly, promotes sustainability [65]. |
| Photochemical Reactions (TiO2-based) | UV-induced photocatalysis using metal-doped TiO2 nanoparticles. | 12.5–32.5 times higher efficiency compared to standard TiO2. | Improved photoactivity, enhanced electron transfer, reusable [66]. |
| Pb-Modified Nanoparticles | Electron trap mechanism reduces recombination, boosting degradation. | 32.5 times faster degradation rate than pure TiO2 systems. | Enhanced electron–hole separation, higher hydroxyl radical generation [67,68]. |
| Ga2O3-Based Nanomaterials | Photocatalysis using wide-band-gap (4.8 eV) semiconductor. | High degradation rate for PFOA in aqueous environments. | Stable under UV light, superior conduction band position [69,70]. |
| Titanate Nanotubes | Adsorption and photocatalytic degradation under UV light. | >90% surface concentration reduction with 62% mineralization. | Reusable without chemicals, stable across multiple cycles [71]. |
| Nanofiltration Membrane Separation | Pressure-driven separation for PFAS removal. | >98% removal efficiency in water treatment systems. | Scalable, high efficiency, effective for short-chain PFAS [73]. |
| Carbon Nanotubes (CNTs) | Adsorption and photocatalysis using high-surface-area nanostructures. | >90% removal efficiency for PFAS. | High adsorption capacity, scalable, and reusable [78]. |
| Magnetic Iron-Oxide Nanoparticles | Magnetic separation combined with adsorption for PFAS removal. | >95% removal of PFAS, including short-chain variants. | Reusable, selective adsorption, easy separation [79]. |
| Microbial PFAS Degradation | Enzymatic defluorination and biodegradation by microbes. | Effective for specific PFAS compounds. | Biodegradable, environmentally friendly [81]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, J.; Narayan, M. Advanced Nanoformulations for Detection and Removal of Poly- and Perfluoroalkyl Substances (PFAS). Pollutants 2025, 5, 10. https://doi.org/10.3390/pollutants5020010
Kumar J, Narayan M. Advanced Nanoformulations for Detection and Removal of Poly- and Perfluoroalkyl Substances (PFAS). Pollutants. 2025; 5(2):10. https://doi.org/10.3390/pollutants5020010
Chicago/Turabian StyleKumar, Jyotish, and Mahesh Narayan. 2025. "Advanced Nanoformulations for Detection and Removal of Poly- and Perfluoroalkyl Substances (PFAS)" Pollutants 5, no. 2: 10. https://doi.org/10.3390/pollutants5020010
APA StyleKumar, J., & Narayan, M. (2025). Advanced Nanoformulations for Detection and Removal of Poly- and Perfluoroalkyl Substances (PFAS). Pollutants, 5(2), 10. https://doi.org/10.3390/pollutants5020010