Abstract
Exposure to contaminated soils can adversely affect health and the well-being of both humans and animals. Environmental stressors can influence the mobility and toxicity of contaminants, altering their potential impacts. This study aimed to assess the impact on the behavior of offspring from rats exposed during the gestation and lactation period to contaminated and acidified soils. Female Wistar rats were gavaged daily for 42 days with soil leachate from an industrial region known to be contaminated with metals and metalloids, using solvents with different pH values (6.0, 5.2, and 3.6). The offspring were evaluated in behavioral tests including Open Field, Elevated Plus Maze, and Inhibitory Avoidance. Our findings revealed significant statistical differences in all three tests conducted, indicating that the exposed groups exhibited lower exploratory behavior, higher anxiety behavior, and lower memory retention than the control groups. The difference was more pronounced in the soil leachate with acidified solvent, at both pH 5.2 and 3.6, suggesting that the combined effect of both stressors led to synergistic interactions, potentiating their impacts. Elemental analysis revealed elevated levels of neurotoxic metals, including Cr, Cu, and Ni, as well as the metalloid As, with acidification significantly enhancing their bioavailability. Moreover, our results demonstrate that acidification facilitated the mobilization of metals and the metalloid As, increasing their bioavailability and acting synergistically to exacerbate the behavioral impacts of contaminated soils. Special attention should be given to populations living in industrial areas that may be exposed to contaminated soils.
1. Introduction
Soil is a complex environmental matrix its quality can impact the health, well-being, and quality of life of populations residing in its vicinity [1,2]. Its pollution and contamination are directly linked to health impacts on humans and animals, with extensive research focusing on its effects on human health in urban [3,4], industrial [5,6], mining [6,7], and agricultural areas [8,9,10].
Living organisms can be exposed to contaminants present in soils through processes such as leaching, infiltration, runoff, gas volatilization, dispersion, advection, diffusion, and sorption/desorption, due to the close connections of soil with air and water [11]. Consequently, the routes of exposure of soil contaminants to humans include the inhalation of dust or vapor, dermal absorption through the skin, and the direct ingestion of contaminated soil particles, as well as indirect ingestion through food and/or water [11,12]. Among these routes, ingestion predominates as the primary route of exposure to soil contaminants [12].
Soil pollution with chemical substances can occur through both natural processes and anthropogenic activities, with the latter exerting a significant influence in urban and industrial scenarios [13], particularly concerning contamination by metals and metalloids. Additionally, in these areas, the effects of acid rain, potentiated by local pollution, can alter the toxicity of contaminants due to changes in pH [14,15]. Allied to this, variation in soil pH can modulate the mobility and reactivity of contaminants, thereby altering their potential impact on health [14]. In the case of pollution by metallic contaminants, a decrease in pH can also increase their bioavailability [15]. Consequently, the combination of different environmental stressors can magnify the individual detrimental effects of these factors. This highlights the complexity of estimating the impact of soil pollutants, as assessing the particulars of one or a few elements alone is insufficient. It is also essential to investigate their interactions with local environmental factors.
The city of Rio Grande, situated in the state of Rio Grande do Sul, Brazil, is a medium-sized city characterized by a robust industrial complex. Its economy revolves around a diverse range of industries, including fertilizer, petrochemical, food, fish, and metallurgical industries [16], many of which are located near urban zones [17]. This proximity stems from rapid urban and industrial expansion, which directed urban development towards the industrial complex [17]. Within this scenario, a previous study in this region revealed high levels of soil contamination by metals in urban areas near the industrial zone [18]. Adding to this issue, Mirlean et al. (2000) [19] highlighted that 60% of the city’s rainfall manifests as acid rain, with an average pH of 5.2 and a recorded minimum pH of 3.6, indicating widespread contamination and pollution affecting a segment of the city’s population. Moreover, multiple experimental studies have underscored the toxic potential of soils in this region, impacting physiological [20,21], reproductive parameters [22,23], and genotoxic damage [24,25].
In addition to the aforementioned context, epidemiological evidence suggests that maternal exposure to environmental contaminants can significantly influence offspring development [26,27]. Exposure to soil contaminants during critical periods of gestation, as well as pre- and post-natal stages, can disrupt normal physiological processes, impairing fetal development and predisposing offspring to various health complications later in life [28]. Among these contaminants, exposure to metals during gestation and breastfeeding is of particular concern, as these elements can cross the placental barrier and be transmitted through breast milk during lactation [29,30], potentially impacting neural and behavioral development. Studies have highlighted that exposure to different levels of metals, like lead (Pb) and cadmium (Cd), and the metalloid arsenic (As), during gestation and lactation in rat models, can induce oxidative stress in nervous tissue, interfere with mitochondrial function, and alter the regulation of neurotransmitters such as dopamine and serotonin, which are critical for behavior and cognitive function [31,32,33,34,35]. Furthermore, elevated exposure to these elements during developmental periods can lead to epigenetic changes that affect gene expression related to neural development, resulting in behavioral deficits such as anxiety, memory impairment, and intellectual deficits [35,36].
Multiple behavioral tests exist to assess neurotoxic effects, each focusing on different aspects of neurological function. The Open Field test is widely used to evaluate locomotor and exploratory behavior [37], while the Elevated Plus Maze test assesses anxiety-related responses [38]. The Inhibitory Avoidance test investigates memory retention and learning capacity [39]. Together, these methodologies provide a comprehensive approach to evaluating behavioral impairments following early-life exposure to contaminants, offering insight into different neurofunctional domains affected by toxicant exposure.
Given this background, the present study aims to investigate the impacts of maternal consumption of acidified contaminated soils on the behavior of Wistar rat offspring exposed during gestation and lactation until their pre-adult age (24 days). Behavioral assessment will be conducted through established methodologies for rat models, including the Open Field test to investigate exploratory behavior, the Elevated Plus Maze test to evaluate anxiolytic behavior, and the Inhibitory Avoidance test to assess memory retention in the offspring.
2. Materials and Methods
2.1. Soil Sampling
Soil samples were collected from the vicinity of the industrial district in the city of Rio Grande-RS, Brazil, specifically from the region known as “Coroa do Boi”, which is considered an area of high environmental contamination due to direct emissions from petrochemical and fertilizer industries (32°02′58″ S; 52°05′04″ W—Figure 1). Previous investigations conducted in this area have consistently identified elevated levels of soil contaminants and multiple outcomes [20,21,22,23,24,25]. Soil collection followed the established protocol described by Da Silva Júnior and Vargas (2009) [40].
Figure 1.
Map of the region where the soil sampling site is located.
Surface soil samples were obtained using a plastic shovel, packed into plastic bags, and transported to the laboratory. Soil profile was sampled manually by coring down to depths ranging from 20 to 40 cm below the current groundwater level, with the depth of the holes varying from 0.7 to 1.5 m. Each 5 cm segment of the core was averaged, and representative samples were collected by weight and transported in plastic bags to the laboratory for subsequent analysis.
2.2. Leachate Preparation
Soil samples were dissolved in three different solvents: distilled water (serving as the control group), an acid solvent adjusted to pH 5.2, and an acid solvent adjusted to pH 3.6. The solubilization process involved mixing soil samples with each solvent at a ratio of 1:2 (g/mL). Acidified solvents for the exposed groups were prepared using distilled water and hydrofluoric acid (Êxodo Científica, São Paulo, Brazil) and were freshly prepared prior to administration. The distilled water used in this study had an average pH of 6. The solutions were stirred for 24 h, then centrifuged at 13,000× g for 15 min. Subsequently, the solutions were stored for up to one week. After this maximum duration, the leachate preparation procedure was repeated to ensure consistency and reproducibility.
The selection of pH values of 3.6 and 5.2 for the acid solvents was based on the lowest and average recorded pH values of rainfall in Rio Grande, as documented in previous research [19]. The decision to use hydrofluoric acid to acidify the samples stemmed from the significant contribution of hydrofluoric acid to acid rain in this region. This local phenomenon is attributed to the release of fluoride in high concentrations into the atmosphere, primarily associated with fertilizer industries.
2.3. Animals, Experimental Design and Ethical Approval
Adult Wistar rats, both males and females aged between 3 and 4 months, were obtained from the Central Animal Laboratory of the Federal University of Rio Grande (FURG). These rats were housed in the animal facility at the Instituto de Ciências Biológicas (ICB) of the university, maintaining controlled environmental conditions of temperature (21 ± 3 °C) and photoperiod (12 h light/12 h dark). They were provided with a standard laboratory animal diet (Bio Base, Bio-Tec, Águas Frias, SC, Brazil) and had access to water ad libitum.
For mating, adult females were separated in a ratio of 3 females to 1 male. After 24 h of contact with males, females underwent vaginal swab testing to determine pregnancy. Detection of sperm indicated pregnancy. Vaginal swabs were conducted by washing the vaginal canal with 50 μL of physiological saline, which was then collected using a micropipette. The collected content was immediately smeared onto a slide for examination under light microscopy at ×200 magnification (Olympus CX41, Olympus Corporation, Hachioji, Japan).
Following confirmation of pregnancy, female rats were divided into 6 different groups, each consisting of 18 animals. The female groups received the following treatments: Group 1—distilled water (control group); Group 2—soil leachate with distilled water; Group 3—acidified solvent at pH 3.6; Group 4—soil leachate with acidified solvent at pH 3.6; Group 5—acidified solvent at pH 5.2; and Group 6—soil leachate with acidified solvent at pH 5.2. The male rats used for mating were not exposed to any kind of treatment.
Each female group received 1 mL of the respective treatment via gavage, in adherence to the guidelines established by the United States Environmental Protection Agency [41], which suggests a maximum daily voluntary intake of 10 g of soil during a single geophagy event, based on an individual weighing 70 kg. The rats received the cumulative dose of the daily content via gavage twice a week to minimize the potential gastric mucosa damage from the acidic pH. This procedure was performed from the detection of pregnancy until the conclusion of the lactation period of the rat pups, which lasted approximately 42 days of experimentation.
After weaning, when the pups were 25 days old, the animals were prepared for the behavior tests. This study was conducted in accordance with the guidelines and regulations set forth by the Ethics Committee on Research in the Area of Health of the FURG, with approval obtained under process no. 59/2010-CEPAS/FURG.
2.4. Behavioral Tests
The behavioral tests employed were the Open Field Test, Elevated Plus Maze, and the Inhibitory Avoidance Test. On the first day after the pups reached 24 days of age, the animals underwent the Open Field Test. The following day, they were exposed to the Elevated Plus Maze test and the first phase of the Inhibitory Avoidance Test. It is important to note that all equipment was cleaned with a solution of 70% alcohol and water before each test to prevent behavioral influences from the odor of the previous animal.
2.4.1. Open Field Test
This test was employed to evaluate the locomotor activity and exploratory behavior of the offspring. It was performed as described by Archer (1973) [42] and following the guidelines of Gould et al. (2009) [37]. Animals were placed individually in a box with a floor divided into 12 equal squares. They were positioned in the upper left corner of the floor and observed for 5 min for the number of crossings in the peripheral and central quadrants, the number of times the rodent stood in a bipedal position (rearing), the number of times the animal exhibited self-grooming behavior, the time spent immobile (freezing), and feces elimination (feces). This procedure was conducted in two sessions: a training session and a testing session. All tests were recorded and analyzed via video. As this task is non-aversive, it explores the animal’s habituation to the environment, as well as exploratory and locomotor activity.
2.4.2. Elevated Plus Maze
The Elevated Plus Maze test was used to assess the anxiolytic or anxiogenic responses of the offspring. It was performed according to Pellow et al. (1985) [43] and following guidelines of Walf and Frye (2007) [38]. This assessment involves placing the animal in the central square of a cross-shaped maze with two open and two closed arms, each measuring 50 cm in length by 10 cm in width, elevated 70 cm above the ground (EP 151A, INSIGHT Ltd., Ribeirão Preto, Brazil). The closed arms have side walls measuring 25 cm in height. The parameters assessed in our study included the number of entries and time spent by the offspring in the closed arms, and the number of entries, time spent, and number of head dips by the offspring in the open arms.
2.4.3. Inhibitory Avoidance Test
The step-down Inhibitory Avoidance Test was employed to investigate learning and memory processes in the offspring of rodents. The inhibitory avoidance apparatus used was the EP-104 (INSIGHT Ltd., Ribeirão Preto, Brazil), consisting of a glass and metal box measuring 50 × 25 × 25 cm, with a platform 5 cm high, 8 cm wide, and 25 cm long. In the left corner, a series of aluminum bars, spaced 1 cm apart, constituted the floor of the box and were connected to an electric stimulator.
The evaluation comprised a training and post-training phase. During the training phase, the animal was placed on the platform and, upon descending to the floor, received a low-intensity shock, serving as an aversive stimulus to discourage the animal from executing a specific task. In the post-training phase, conducted 24 h after the training phase, this procedure was repeated, and the time taken for the animal to descend from the platform was recorded. This was performed to assess the animal’s memory retention mechanism. The post-training phase was conducted 24 h after the training phase, as this corresponds to the memory consolidation period for the animal [39].
2.5. Trace Metals and Arsenic Analysis
The quantification of metals and metalloids in soil leachate samples was conducted to assess their potential contribution to the observed behavioral outcomes. Elemental extraction was performed using microwave-assisted digestion. The concentrations of cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), nickel (Ni), and the metalloid arsenic (As) were determined using an atomic absorption spectrophotometer with flame and graphite furnace modes (AA Duo, Agilent Technologies, Santa Clara, California). Mercury (Hg) analysis was performed using a cold-vapor system coupled to an atomic absorption spectrophotometer (GBC 932AA, GBC Scientific Equipment Ltd., Penang, Malaysia) to achieve accurate detection of this element. The limits of detection (LOD) for Cd, Cr, Cu, Pb, Ni, and As were 0.022 µg/L, and the limit of quantification (LOQ) was 0.067 µg/L. For Hg, the LOD was 0.005 µg/L, and the LOQ was 0.016 µg/L. All analyses were conducted in triplicate, and results were expressed in micrograms per cubic meter (µg/L). Standard solutions of each metal and the metalloid As were used for calibration, and quality control was ensured by analyzing certified reference materials alongside the samples.
2.6. Data Analysis
Statistical analysis of the data was conducted using STATA 10 software. Initially, all variables were assessed for normality using the Kolmogorov–Smirnov test and for homogeneity using the Levene test. As all data followed a normal distribution, comparisons of measurements were performed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test to determine specific differences between groups. Results were presented as the mean ± standard deviation of the mean. The statistical significance level was set at 5% (p < 0.05).
3. Results
The offspring investigated in this study exhibited varied results in behavioral assessments. Initially, Table 1 presents the exploratory behavioral outcomes obtained via the Open Field Test, revealing differences among the groups. In general, the exposed groups showed reduced central and peripheral crossings, as well as lower rearing behavior, compared to the control group (Group 1), which may indicate decreased exploratory activity. Freezing time was significantly increased in all exposed groups, suggesting heightened anxiety and fear behavior rather than a suppression of general motor activity. Additionally, grooming behavior was statistically increased in four exposed groups, which may also be associated with stress-related responses. Groups 4 and 6, exposed to acidified solvents at pH 3.6 and 5.2 with soil leachate, respectively, exhibited pronounced alterations across these parameters, even exhibiting significantly different values compared to the other groups in the time spent at the central quadrants. Indicating that the exploratory behavior of the offspring was mainly affected by exposure to acidified soil leachates. Furthermore, feces elimination did not show any statistical difference among the groups, suggesting that this parameter was not notably affected by exposure conditions.

Table 1.
Effects of maternal exposure to soil leachate and acidified solvents on offspring behavior in the Open Field Test (mean ± SD) *.
The behavioral impacts exhibited by the offspring of females exposed to soil leachate and acidified solvents in the Elevated Plus Maze test are shown in Table 2. Notably, in the open arms, all test groups exhibited significantly lower results than the control group (Group 1) in the three parameters assessed: number of head dips, number of entries, and time spent in the open arm, suggesting an increase in anxiety-related behavior across all exposed groups. Specifically, groups exposed to acidified solvent at pH 3.6 and 5.2, with or without soil leachate, showed the lowest results for open arm parameters, also statistically differing from Group 2 in the number of entries. In the closed arms, all exposed groups spent significantly more time compared to the control group, further supporting an anxiety-like response. However, the number of entries was similar among almost all groups, differing only between Groups 2 and 3, suggesting potential variability in how different exposure conditions affect locomotor activity or risk assessment behaviors. This highlights that, while increased anxiety-like behavior was observed across all exposed groups, the magnitude and specific behavioral adjustments varied depending on the exposure condition.

Table 2.
Effects of maternal exposure to soil leachate and acidified solvents on offspring behavior in the Elevated Plus Maze test (mean ± SD) *.
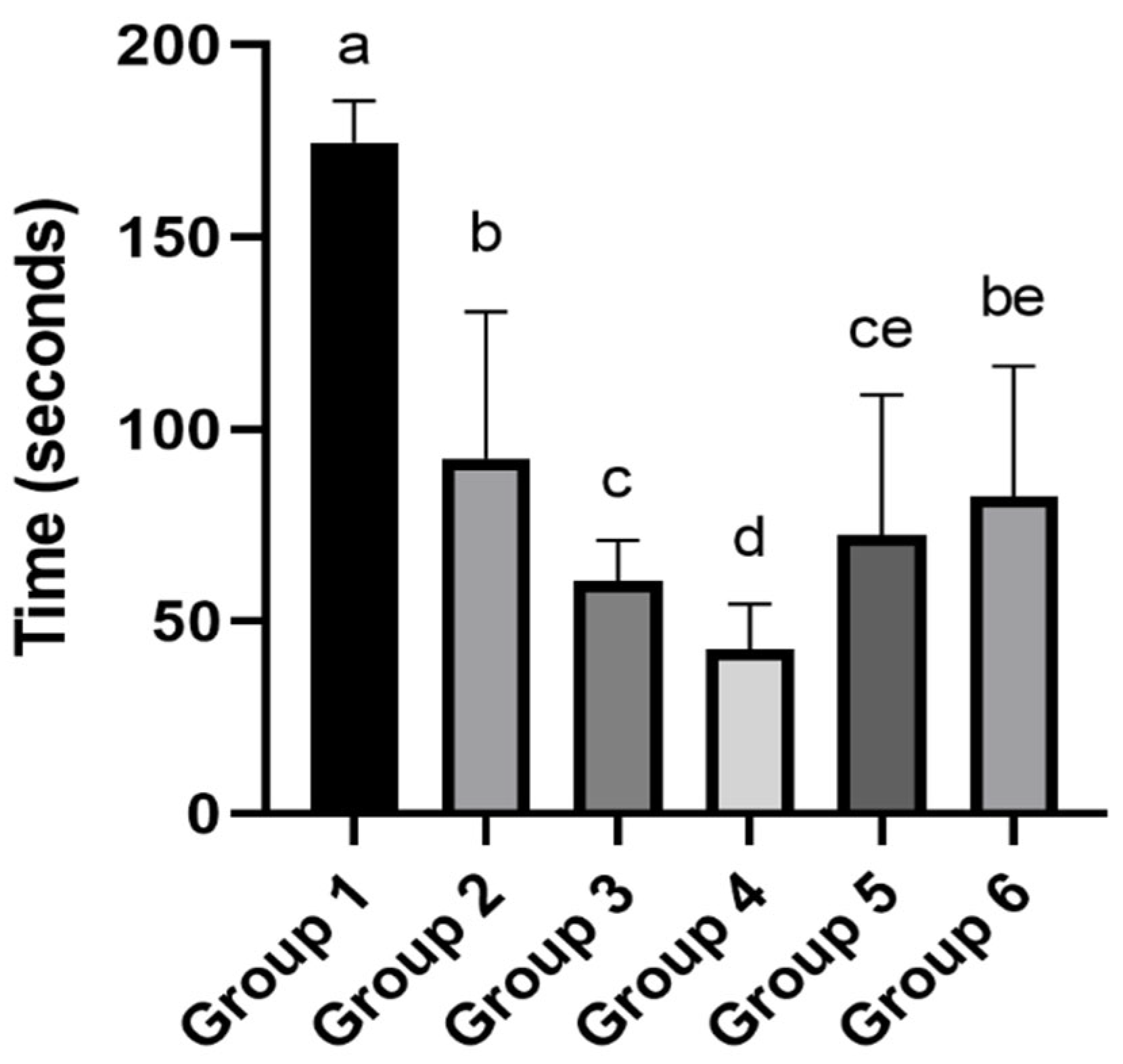
The results for the Inhibitory Avoidance test are illustrated in Figure 2. It is possible to observe that the offspring from the control group exhibited markedly different behavior compared to all other groups. They took significantly longer to descend from the platform. This longer latency is typically associated with better memory retention regarding the aversive stimulus; however, it is important to consider that performance in this test can also be influenced by other factors, such as differences in motivation, pain sensitivity, or general activity levels [44]. Interestingly, Groups 3 and 4 displayed faster descent times, with Group 4, the test with acidified solvent at pH 3.62 and soil leachate, showing the poorest performance, differing statistically from all other groups.
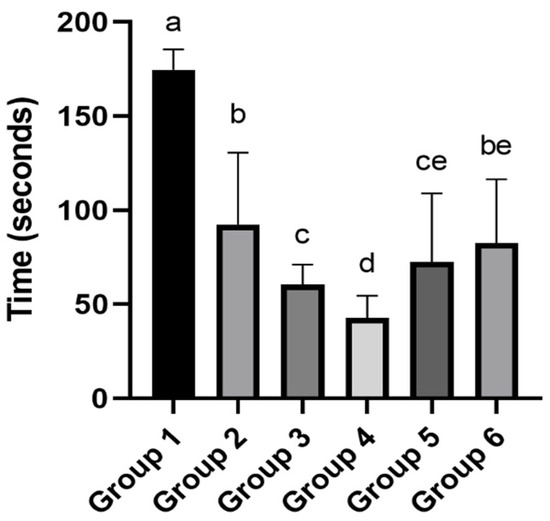
Figure 2.
Effects of maternal exposure to soil leachate and acidified solvents on offspring behavior in the Inhibitory Avoidance Test. Different letters indicate significant differences (p value < 0.05), as determined by one-way ANOVA followed by Tukey’s post hoc test. Treatments: Group 1—Water (control); Group 2—Water + Soil leachate; Group 3—Solvent at pH 3.6; Group 4—Solvent at pH 3.6 + Soil leachate; Group 5—Solvent at pH 5.2; Group 6—Solvent at pH 5.2 + Soil leachate.
Metal and metalloid concentrations in soil leachate samples revealed notable variations across treatments (Table 3). Cr and Cu were consistently detected in all soil leachate samples, with higher concentrations observed in samples acidified to pH 3.6 and 5.2, indicating enhanced solubilization of these metals under acidic conditions. Notably, As was only detected in the acidified soil leachate at pH 3.6, with a concentration of 30.84 µg/L, while Ni was significantly elevated in the same treatment, highlighting the potential synergistic effects of acidification on metal and metalloid bioavailability. Cd and Hg were not detected in any treatment, suggesting their negligible presence or low bioavailability in the tested samples.

Table 3.
Concentrations of metals and metalloids quantified in the soil leachate samples.
When comparing these findings with the behavioral outcomes, a pattern emerges. The groups exposed to acidified soil leachates (Groups 4 and 6) consistently exhibited pronounced behavioral alterations in all behavior assessments. These effects were most evident in Group 4 (pH 3.6 + soil leachate), which also showed the highest solubilization of As, Ni, Cu, and Pb. Given that these elements are known neurotoxicants capable of disrupting neurotransmitter balance, increasing oxidative stress, and impairing neurodevelopment, it is plausible that their heightened bioavailability contributed to the observed behavioral impairments.
4. Discussion
Our study provides a thorough examination of the impacts of maternal exposure to contaminated soils, exacerbated by acidification, on the behavior of rat offspring exposed during their gestational and lactation periods. The methodology employed in this investigation is noteworthy for its holistic approach, considering the interplay among environmental factors, the documented soil contamination in the study site, and the prevalence of acid rain in the region. This approach takes into account how these factors collectively influence the bioavailability and toxicity of contaminants within the studied soil.
In general, our results demonstrated that maternal exposure to contaminated soils was capable of impacting the behavioral development of their offspring. The behavioral tests of Open Field, Elevated Plus Maze, and Inhibitory Avoidance showed significant statistical differences, indicating that the exposed groups exhibited lower exploratory behavior, higher anxiety behavior, and lower memory retention than the control group, respectively. These deleterious effects were more pronounced in groups exposed to acidified contaminated soils, indicating that acidification enhanced the toxicity and bioavailability of contaminants. Metal and metalloid quantification further supported this finding, with the acidification notably increasing the concentration of Cr, Cu, Ni, Pb, and As, particularly at pH 3.6, suggesting that the heightened bioavailability of these neurotoxic metals may have contributed to the observed behavioral impairments.
For our behavioral toxicological research, we opted to employ a combination of well-established tests recommended for assessing rat behavior, including the open field, elevated plus maze, and inhibitory avoidance tests [37,39]. The open field test serves as a valuable tool for evaluating alterations in exploratory and locomotor capabilities [37], while the elevated plus maze is a classical protocol used to gauge anxiety levels [38]. Additionally, the inhibitory avoidance test enables the assessment of memory retention in rats [39]. By utilizing these complementary approaches, we aimed to gain a plural understanding of how the exposure to the contaminated soils may impact multiple behavioral aspects of the exposed animals. However, it is important to recognize that each test has inherent limitations, and potential overlaps or interactions between the behavioral domains assessed could influence the outcomes. Nevertheless, we sought to minimize any external interference or cross-test influence by carefully adhering to standardized experimental protocols and ensuring appropriate conditions throughout the behavioral assessments.
In this aspect, numerous studies have demonstrated a connection between metal exposure and behavioral impairments in animal models, including rats [27,45], with some specifically highlighting the impacts of maternal exposure during gestation and lactation on offspring behavior and neurodevelopment [31,32,33,34]. However, research investigating the behavioral effects of exposure to environmental samples (aside from drinking water) remains limited [29,46,47]. Notably, some of the limited research in this area has been conducted by our research group. For example, Da Silva Júnior et al. (2013) [21] reported that exposure to soil via gavage led to reduced locomotor and exploratory activity, along with impaired short-term memory in adult rats. The authors noted that certain neurotoxic substances, such as arsenic and lead, may contribute to these alterations, although the effects are often attributed to the combination of contaminants present in the soil. Additionally, in a study evaluating the behavioral impacts of acute exposure to contaminated soils in adult rats, using soil from the same region as this study, Da Silva Júnior et al. (2018) [46] observed increased fear and anxiety in the exposed animals.
Pre- and postnatal exposure to toxicants can lead to adverse developmental outcomes in offspring, occurring in both humans and other mammals [27,28]. This exposure to toxic agents can occur directly in the womb and/or later through breastfeeding [29]. Thus, the impact of environmental contaminants on maternal-fetal health is a recognized problem, especially during the period of development and growth. In this regard, emissions from industrial regions, often situated close to urban areas, warrant attention. This is particularly significant as they are responsible for the release of metallic contaminants, many of which are found in high concentrations in the soil of these areas [3,4]. Additionally, acid rain is another issue related to the pollution of industrial areas, and its contact with soil contaminated by metals and metalloids can increase their bioavailability and, depending on the compound, enhance their toxicity [15]. Consequently, this increases the risk to human health, primarily that of children and pregnant women who are particularly susceptible to the negative effects of these contaminants.
Our analysis of soil leachates from this industrial region revealed substantial levels of neurotoxic metals, such as Cr, Ni, and Cu, which are known to disrupt neural function. Acidification further increased the bioavailability of these metals, intensifying their potential impacts on offspring development. Additionally, the detection of arsenic under highly acidic conditions (pH 3.6) underscores the critical role of acid rain in enhancing the mobility of hazardous elements, further compounding the risks associated with soil contamination.
Further studies conducted in the study site have also demonstrated the toxic potential of the contaminated soils. Da Silva Júnior et al. (2013) [21] and Da Silva Júnior and Muccillo-Baisch (2013) [20] showed toxic effects on physiological parameters in male adult Wistar rats. The former demonstrated an increase in TBARS and protein carbonyl levels, as well as a relative weight increase in the kidneys, while the latter indicated that exposure to contaminated soil led to alterations in renal parameters, specifically an increase in plasma creatinine and total protein in urine. Garcia et al. (2015, 2018) [22,23] demonstrated how exposure to this soil also impacted reproductive parameters in the offspring of female Wistar rats, with significant changes in birth weight, weight gain during growth, developmental length, incisor eruption, ear opening, organ weight, swimming behavior, and other developmental characteristics. Garcia et al. (2016, 2017) [24,25] showed that maternal exposure to these contaminated soils could cause deleterious mutagenic and genotoxic effects in their offspring, respectively. However, regarding the evaluation of behavioral impacts of exposure to the contaminated soils of this region, only the previously mentioned study by Da Silva Júnior et al. (2018) [46] was conducted. In this context, it is important to highlight that there has been no previous investigation into the behavioral impacts on the offspring of female rats exposed to contaminated soils during pregnancy and the lactation period in industrial regions.
In environmental toxicology, it is important to recognize that organisms are often subjected to a myriad of stressors simultaneously, rather than being exposed to single, isolated factors [48,49]. This multifaceted exposure can include various environmental contaminants, such as heavy metals, industrial chemicals, and pesticides, alongside other stressors like acid rain, climate change, and habitat degradation [48]. Our study highlights this complexity, as acidification significantly increased the solubilization and bioavailability of metals and the metalloid As in soil leachates. These elements, known for their neurotoxic properties, likely contributed to the synergistic effects observed in behavioral impairments.
For instance, in our study on maternal exposure to contaminated soils, we acknowledged the intricate interplay between different stressors. While the primary focus of our study is on the toxicity of soil contaminants, it is essential to consider how other environmental factors, such as acid rain in our case, exacerbate the effects of contamination. Acid rain, which in our study area typically results from the deposition of hydrogen fluoride, sulfur dioxide, and/or nitrogen oxides emitted from industrial activities [19], can alter soil pH levels, thereby influencing the bioavailability and mobility of toxic contaminants like heavy metals [15]. This alteration in soil chemistry can intensify the toxicity of contaminants, amplifying their adverse effects on organisms exposed to contaminated soils [50]. Additionally, our experimental design included groups exposed solely to acidified solvents, allowing us to distinguish the specific effects of acidification from those caused by the combined exposure to contaminated soil and acidification.
In this context, our research observed significant differences in all exposure tests, both for soil leachate and acidified solvents (acidified water). However, it was also noticeable that this difference was more pronounced in the soil leachate with acidified solvent at both pH levels tested. This enhanced effect may be attributed to the interaction between the acidified solvent and the contaminants present in the soil leachate, where acidification increases the solubility and bioavailability of metals and other contaminants in the soil, potentially increasing their release and uptake by the organisms [14,15]. When combined with the complex mixture of contaminants in the soil leachate, this increased bioavailability may lead to synergistic interactions, in which the combined effects of acidification and soil contaminants are greater than the sum of their individual impacts. Consequently, it is possible to suggest that this synergy contributed to the behavioral effects observed in the offspring of the investigated rat model.
This set of information further underscores the challenges in assessing environmental risks to human health and living organisms. In addition to environmental stressors, socio-economic factors also play a significant role in shaping the overall risk landscape [48,49,50]. Communities residing in industrial areas, often drawn to these regions due to their proximity to work and lower living costs, may face disproportionate exposure to environmental pollutants due to their proximity to industrial facilities [5,6,50], as observed in the direct exposure to the soil tested. Moreover, contamination by metals in urban soils, including those found in public green spaces [51], has increasingly drawn attention and should be given proper consideration. Therefore, an integrated approach that considers the combined influence of environmental stressors and socio-economic factors is crucial not only for addressing the complexity of multiple stressors but also for generating information that should be taken into account in the development of management strategies to safeguard human health and environmental quality in industrialized regions.
Furthermore, the results of this study are relevant to understanding potential risks to human populations, especially vulnerable groups such as infants and young children. The developmental stage of rat offspring at weaning (approximately 21 days of age) is often considered analogous to early childhood in humans, making this model appropriate for evaluating neurodevelopmental impacts [52,53]. Additionally, while direct extrapolation between species is complex, the gestational and lactational exposure periods used in this study reflect critical windows of neurodevelopment that are comparable to prenatal and early postnatal stages in humans [52,53]. In human populations, chronic exposure to contaminated soils, particularly in industrial and urban areas, may occur through multiple pathways, including ingestion, inhalation, and dermal contact [54]. Given that young children frequently engage in hand-to-mouth behaviors and have a higher soil ingestion rate per body weight than adults [55], their risk of exposure to metal contaminants is significantly elevated. Thus, our results also highlight the importance of evaluating real-world exposure scenarios in contaminated environments, as early-life exposure to metals and metalloids has been linked to cognitive and behavioral impairments in human epidemiological studies.
Based on the findings of the aforementioned behavioral tests, it is evident that, in general, the animals in the non-exposed group displayed typical behavior characterized by exploration, adaptability, reduced anxiety, and enhanced memory retention. Considering these are offspring, their natural curiosity likely drove increased exploration of novel environments. Consequently, it is reasonable to infer that groups exhibiting decreased exploration and elevated anxiety levels, as evidenced by the Open Field and Elevated Plus Maze test parameters, respectively, may have been influenced by the presence of pollutants in the contaminated soil. The same trend is apparent in the results of the Inhibitory Avoidance test, where deficits in memory retention were observed.
In addition to all the information presented, it is important to acknowledge the possible limitations of our study. The potential influence of confounding variables on the observed behavioral effect, such as individual variability in maternal metabolism, differences in maternal care, and possible indirect effects of soil contaminants on maternal health, could have contributed to the offspring’s neurodevelopmental outcomes. Additionally, stress responses induced by the exposure procedure itself cannot be ruled out as influencing behavioral results. It is also important to recognize the inherent limitations of the behavioral tests used, as overlapping behavioral domains may interfere with the interpretation of outcomes. Furthermore, while acidification increased the bioavailability of certain metals and metalloids, interactions between multiple contaminants may also have contributed to the observed effects. Future studies should explore these aspects in greater detail, including assessments of maternal physiological responses and additional biochemical analyses in offspring to better understand the underlying mechanisms.
Thus, this study demonstrates that maternal exposure to contaminated and acidified soils can negatively impact offspring behavior in a controlled animal model. While direct extrapolation of these findings to human populations should be made with caution due to species differences and experimental limitations, the results provide important information into potential health risks to individuals residing in industrial areas. It is imperative to recognize that soils with varying compositions, stemming from different sources of pollutants, can elicit distinct health repercussions. Consequently, investigations into exposure to soils from industrial and urban regions should be expanded and further explored to delineate outcomes in diverse contexts and varying degrees of contamination. Such research initiatives are essential, given that exposure to contaminated soils represents a significant environmental health concern and necessitates serious consideration in the formulation of public health policies.
5. Conclusions
In conclusion, our findings demonstrate the impact of maternal exposure to contaminated soils, particularly when potentiated by acidification, on the behavioral development of offspring rats. Through a multi-faceted assessment utilizing a diverse set of behavioral tests, we identified that maternal exposure to contaminated soil negatively affected the offspring’s exploratory behavior, anxiety levels, and memory retention. Moreover, the observed behavioral impairments were exacerbated in groups exposed to acidified soils. These results highlight the critical need for further research into the effects of environmental contaminants on developmental outcomes and underscore the importance of implementing proactive measures to mitigate the risks posed by soil contamination, particularly in industrialized regions.
Author Contributions
E.M.G.: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Roles/Writing—original draft; Writing—review and editing. R.A.T.: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Roles/Writing—original draft; Writing—review and editing. L.d.S.F.: Conceptualization; Formal analysis; Investigation; Writing—review and editing. G.M.G.V.d.A.: Conceptualization; Formal analysis; Investigation; Writing—review and editing. G.d.O.S.: Conceptualization; Formal analysis; Investigation; Writing—review and editing. V.M.d.S.: Conceptualization; Formal analysis; Investigation; Writing—review and editing. A.L.M.-B.: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing—review and editing. F.M.R.d.S.J.: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Research Productivity scholarships to FMRSJ (grant 307791/2023-8), and the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) for the researcher scholarship to FMRSJ (grant 21/2551-0001981-6).
Institutional Review Board Statement
This study was conducted in accordance with the guidelines and regulations set forth by the Ethics Committee on Research in the Area of Health of the FURG, with approval obtained under process no. 59/2010-CEPAS/FURG.
Data Availability Statement
The data that support the findings of this study will be made available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Universidade Federal do Rio Grande—FURG.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1178622120934441. [Google Scholar] [CrossRef]
- Qing, X.; Yutong, Z.; Shenggao, L. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015, 120, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Penteado, J.O.; Brum, R.d.L.; Ramires, P.F.; Garcia, E.M.; dos Santos, M.; Júnior, F.M.R.d.S. Health risk assessment in urban parks soils contaminated by metals, Rio Grande city (Brazil) case study. Ecotoxicol. Environ. Saf. 2021, 208, 111737. [Google Scholar] [CrossRef]
- Krishna, A.K.; Mohan, K.R. Distribution, correlation, ecological and health risk assessment of heavy metal contamination in surface soils around an industrial area, Hyderabad, India. Environ. Earth Sci. 2016, 75, 411. [Google Scholar] [CrossRef]
- Bi, C.; Zhou, Y.; Chen, Z.; Jia, J.; Bao, X. Heavy metals and lead isotopes in soils, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China. Sci. Total Environ. 2018, 619, 1349–1357. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and health risk assessment of heavy metals in China’s lead–zinc mine tailings: A meta–analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L.J.E.I. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef]
- Carré, F.; Caudeville, J.; Bonnard, R.; Bert, V.; Boucard, P.; Ramel, M. Soil contamination and human health: A major challenge for global soil security. In Global Soil Security; Springer: Cham, Switzerland, 2017; pp. 275–295. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.X.; Ren, Y.; Luo, X.-S.; Zhu, Y.G. Urban soil and human health: A review. Eur. J. Soil Sci. 2018, 69, 196–215. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, Y.; Luo, G.; Chen, X.; Yang, X.; Hall, M.H.; Guo, R.; Wang, H.; Cui, J.; et al. Heavy metal contamination of urban soil in an old industrial city (Shenyang) in Northeast China. Geoderma 2013, 192, 50–58. [Google Scholar] [CrossRef]
- Kim, R.-Y.; Yoon, J.-K.; Kim, T.-S.; Yang, J.E.; Owens, G.; Kim, K.-R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2021, 73, e13203. [Google Scholar] [CrossRef]
- IBGE. Instituto Brasileiro de Geografia e Estatística. Cidades e Estados do Brasil. 2022. Available online: https://cidades.ibge.gov.br (accessed on 14 May 2024).
- Fragomeni, L.P.d.M.; Roisenberg, A.; Mirlean, N. Poluição por mercúrio em aterros urbanos do período colonial no extremo sul do Brasil. Quim. Nova 2010, 33, 1631–1635. [Google Scholar] [CrossRef]
- Garcia, F.A.d.P.; Mirlean, N.; Baisch, P.R. Marcadores metálicos como avaliação do impacto crônico de emissões petroquímicas em zona urbana. Quim. Nova 2010, 33, 716–720. [Google Scholar] [CrossRef]
- Mirlean, N.; Vanz, A.; Baisch, P. Níveis e origem da acidificação das chuvas na região do Rio Grande, RS. Quim. Nova 2000, 23, 590–593. [Google Scholar] [CrossRef]
- da Silva, F.M.R., Jr.; Muccillo-Baisch, A.L. Alterations in some renal parameters of rats induced by aqueous soil extracts. Toxicol. Environ. Chem. 2013, 95, 1030–1036. [Google Scholar] [CrossRef]
- Júnior, F.M.R.d.S.; Silva, P.F.; Garcia, E.M.; Klein, R.D.; Peraza-Cardoso, G.; Baisch, P.R.; Vargas, V.M.F.; Muccillo-Baisch, A.L. Toxic effects of the ingestion of water-soluble elements found in soil under the atmospheric influence of an industrial complex. Environ. Geochem. Health 2012, 35, 317–331. [Google Scholar] [CrossRef]
- Garcia, E.M.; Junior, F.M.R.d.S.; Soares, M.C.F.; Muccillo-Baisch, A.L. Developmental effects of parental exposure to soil contaminated with urban metals. Sci. Total Environ. 2015, 520, 206–212. [Google Scholar] [CrossRef]
- Garcia, E.M.; Júnior, F.M.R.d.S.; Baisch, P.R.M.; Soares, M.C.F.; Muccillo-Baisch, A.L. Effect of mixing two environmental stressors, pH and metal contaminants, on offspring of rats exposed during gestation and lactation. Environ. Sci. Pollut. Res. 2018, 25, 35555–35561. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.M.; Junior, F.M.R.d.S.; Muccillo-Baisch, A.L. Mutagenic effect of contaminated soil on the offspring of exposed rats. Acta Sci. Health Sci. 2016, 38, 19–22. [Google Scholar] [CrossRef][Green Version]
- Garcia, E.M.; Junior, F.M.R.d.S.; Tavella, R.A.; Cruz, C.G.; Baisch, P.R.M.; Muccillo-Baisch, A.L. Genotoxicity in the offspring of rats exposed to contaminated and acidified experimentally soils. Water Air Soil Pollut. 2017, 228, 254. [Google Scholar] [CrossRef]
- Cummings, J.A.; Clemens, L.G.; Nunez, A.A. Mother counts: How effects of environmental contaminants on maternal care could affect the offspring and future generations. Front. Neuroendocrinol. 2010, 31, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Roig, M.D.; Pascal, R.; Cahuana, M.J.; García-Algar, O.; Sebastiani, G.; Andreu-Fernández, V.; Martínez, L.; Rodríguez, G.; Iglesia, I.; Ortiz-Arrabal, O.; et al. Environmental exposure during pregnancy: Influence on prenatal development and early life: A comprehensive review. Fetal Diagn. Ther. 2021, 48, 245–257. [Google Scholar] [CrossRef]
- Mallozzi, M.; Bordi, G.; Garo, C.; Caserta, D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res. Part C Embryo Today Rev. 2016, 108, 224–242. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Sinkiewicz-Darol, E.; Gadzała-Kopciuch, R. The impact of environmental pollution on the quality of mother’s milk. Environ. Sci. Pollut. Res. 2019, 26, 7405–7427. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Graziano, A.; Monte, G.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharm. Sci. 2013, 17, 2198–2206. [Google Scholar]
- Andersson, H.; Petersson-Grawé, K.; Lindqvist, E.; Luthman, J.; Oskarsson, A.; Olson, L. Low-level cadmium exposure of lactating rats causes alterations in brain serotonin levels in the offspring. Neurotoxicol. Teratol. 1997, 19, 105–115. [Google Scholar] [CrossRef]
- Molina, R.M.; Phattanarudee, S.; Kim, J.; Thompson, K.; Wessling-Resnick, M.; Maher, T.J.; Brain, J.D. Ingestion of Mn and Pb by rats during and after pregnancy alters iron metabolism and behavior in offspring. Neurotoxicology 2011, 32, 413–422. [Google Scholar] [CrossRef]
- Araujo-Padilla, X.; Briseño-Bugarín, J.; López-Luna, A.; de la Torre, J.A.F. Effects of Cadmium exposure on lactating mice and rats: A systematic review of breastfeeding experiments. Appl. Sci. 2022, 12, 11412. [Google Scholar] [CrossRef]
- De Marco, M.; Halpern, R.; Barros, H.M. Early behavioral effects of lead perinatal exposure in rat pups. Toxicology 2005, 211, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Htway, S.-M.; Suzuki, T.; Kyaw, S.; Nohara, K.; Win-Shwe, T.-T. Effects of maternal exposure to arsenic on social behavior and related gene expression in F2 male mice. Environ. Health Prev. Med. 2021, 26, 34. [Google Scholar] [CrossRef]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The open field test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.A.; Ghisoni, K.; Latini, A.; Quevedo, J.; Tasca, C.I.; Prediger, R.D. Lithium and valproate prevent olfactory discrimination and short-term memory impairments in the intranasal 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) rat model of Parkinson’s disease. Behav. Brain Res. 2012, 229, 208–215. [Google Scholar] [CrossRef]
- Júnior, F.M.R.d.S.; Rocha, J.A.V.; Vargas, V.M.F. Extraction parameters in the mutagenicity assay of soil samples. Sci. Total Environ. 2009, 407, 6017–6023. [Google Scholar] [CrossRef]
- USEPA. United States Environmental Protection Agency. Exposure Factors Handbook Chapter 5. Office of Health and Environmental Assessment, US Environmental Protection Agency. 2017. Available online: https://www.epa.gov/expobox/exposure-factors-handbook-chapter-5 (accessed on 14 May 2024).
- Archer, J. Tests for emotionality in rats and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Detrait, E.R.; Hanon, É.; Dardenne, B.; Lamberty, Y. The inhibitory avoidance test optimized for discovery of cognitive enhancers. Behav. Res. Methods 2009, 41, 805–811. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Okori, S.O.; Ijomone, O.K.; Ebokaiwe, A.P. Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain. Drug Chem. Toxicol. 2018, 41, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Júnior, F.M.R.d.S.; Pinto, E.A.; da Silveira, T.B.; Garcia, E.M.; de Oliveira, A.M.N.; Muccillo-Baisch, A.L. Feet in danger: Short exposure to contaminated soil causing health damage—An experimental study. Environ. Sci. Pollut. Res. 2018, 25, 8669–8675. [Google Scholar] [CrossRef]
- Martinez, C.S.; Alterman, C.D.; Peçanha, F.M.; Vassallo, D.V.; Mello-Carpes, P.B.; Miguel, M.; Wiggers, G.A. Aluminum exposure at human dietary levels for 60 days reaches a threshold sufficient to promote memory impairment in rats. Neurotox. Res. 2016, 31, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, M.; Bindesbøl, A.-M.; Oostingh, G.J.; Duschl, A.; Scheil, V.; Köhler, H.-R.; Loureiro, S.; Soares, A.M.; Ferreira, A.L.; Kienle, C.; et al. Interactions between effects of environmental chemicals and natural stressors: A review. Sci. Total Environ. 2010, 408, 3746–3762. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Baxter, J.W.; Hatfield, C.A.; Howarth, R.W.; Jones, C.G.; Lovett, G.M.; Wigand, C. Understanding effects of multiple stressors: Ideas and challenges. In Successes, Limitations, and Frontiers in Ecosystem Science; Springer: New York, NY, USA, 1998; pp. 416–431. [Google Scholar] [CrossRef]
- Thomsen, M.; Faber, J.H.; Sorensen, P.B. Soil ecosystem health and services—Evaluation of ecological indicators susceptible to chemical stressors. Ecol. Indic. 2012, 16, 67–75. [Google Scholar] [CrossRef]
- Golia, E.E.; Emmanouil, C.; Charizani, A.; Koropouli, A.; Kungolos, A. Assessment of Cu and Zn contamination and associated human health risks in urban soils from public green spaces in the city of Thessaloniki, Northern Greece. Euro-Mediterr. J. Environ. Integr. 2023, 8, 517–525. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106, 1–16. [Google Scholar] [CrossRef]
- Zeiss, C.J. Comparative milestones in rodent and human postnatal central nervous system development. Toxicol. Pathol. 2021, 49, 1368–1373. [Google Scholar] [CrossRef]
- Shukla, P.; Srivastava, P.; Mishra, A. Routes of Exposure (Inhalation, Ingestion, Dermal Contact) of Heavy Metals and Their Implications for Human Health. In Heavy Metal Contamination in the Environment; CRC Press: Boca Raton, FL, USA, 2024; pp. 114–123. [Google Scholar]
- Xue, J.; Zartarian, V.; Moya, J.; Freeman, N.; Beamer, P.; Black, K.; Tulve, N.; Shalat, S. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. Int. J. 2007, 27, 411–420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).