Environmental Implications of the Global Prevalence of Hyperthyroidism in Cats from a “One Health” Perspective
Abstract
1. Introduction
1.1. One Health and Sentinel Species
1.2. Cats as Sentinel Species
2. Feline Hyperthyroidism
2.1. Thyroid Hormone and Hyperthyroidism
2.2. Risk Factors
- It has been postulated that wide swings in daily iodine or even low or high intake of iodine may contribute to development of thyroid disease in cats [17,18]. Although circulating free T4 concentrations are acutely affected by varying iodine intake, more prolonged ingestion of high- or low-iodine diets has been shown to have no apparent effect on free T4 levels (reviewed in [5,6]). Therefore, dietary iodine may have a modulatory effect on circulating thyroid hormone.
3. Consideration
3.1. Thyroid Hormone
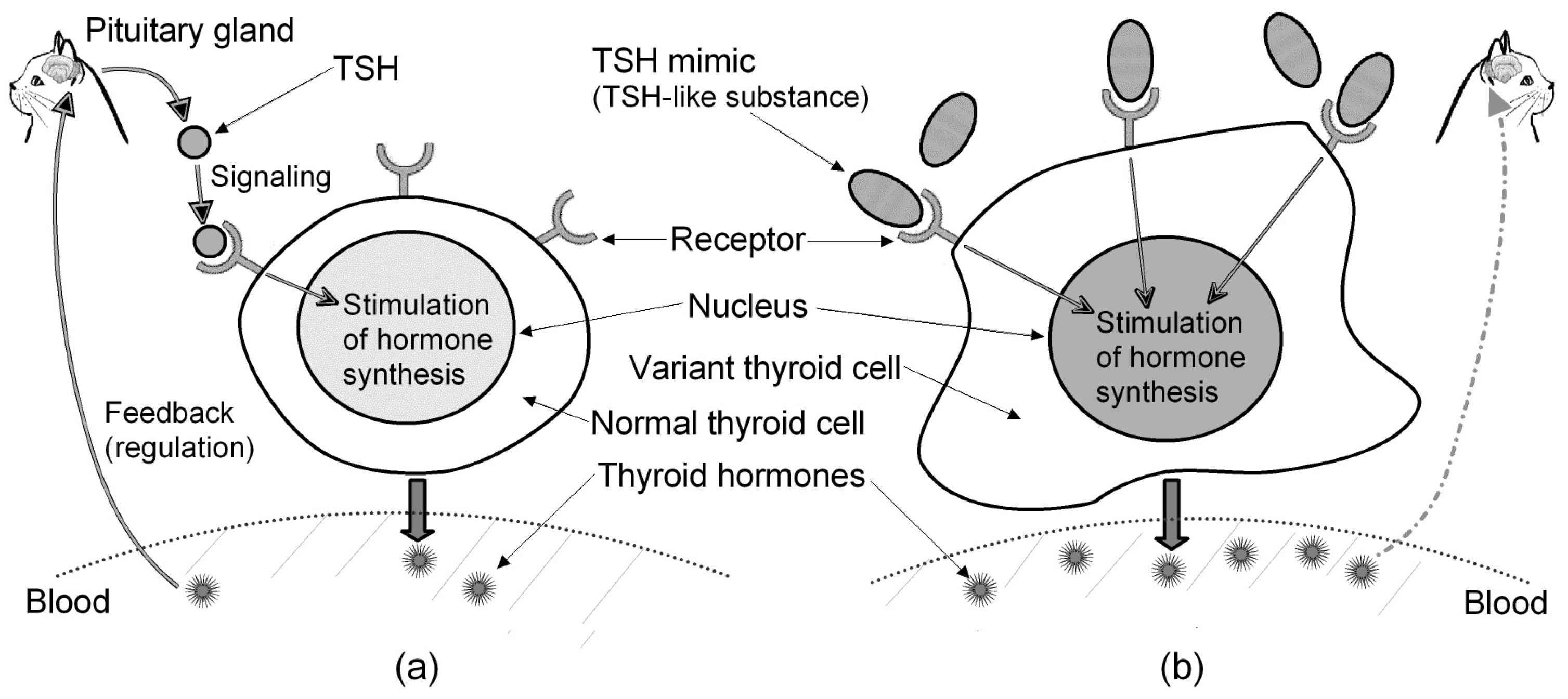
3.1.1. Regulation of Thyroid Hormones
3.1.2. Autoimmunity in Humans and Dogs
3.2. Hypothesis for Peculiar Incidence of Feline Hyperthyroidism
- Receptor similarity between cats and dogs—A comparison between species [28] shows that the feline TSH receptor and the canine TSH receptor are the most closely related, with 96% identity and 97% similarity in amino acid sequence. Despite the receptor similarity, hyperthyroidism is rare in dogs [26], but this is a common in cats [9].
- No evidence of autoimmunity in feline hypothyroidism—The data on immunoglobulin G (IgG) obtained from 16 hyperthyroid cats suggest the absence of stimulatory auto-antibodies [28]. Autoimmunity does not seem to be a major trigger of hyperthyroidism in cats.
- Regulation of thyroid hormones—A Portuguese mix cat aged 15 years was diagnosed with hyperthyroidism (a thyroxine (T4) value of 4.74 μg/dL) in 2023, and we examined this cat’s interbrain by using an MRI scan and a cerebrospinal fluid test in 2024. The obtained results suggested no particular problem with the hypothalamus [29]. The sample size is small, but a regulation disorder affecting thyroid hormone concentrations does not seem to be the main cause of feline hyperthyroidism.
- Relation between thyroid hormone and tumor/adenoma—BRAF (gene) is a proto-oncogene that encodes the B-Raf protein, and it is reported that a BRAF mutation can lead to the development of a tumor [30]: (i) BRAF mutations are enriched in human thyroid tumors [30]; (ii) a mice-based study demonstrates the key role of TSH signaling in BRAF-induced initiation of thyroid tumors [31]; (iii) mice with thyroid-specific expression of BRAFV600E develop papillary thyroid cancer at high levels of serum TSH [32]. The above-mentioned Portuguese cat suffering from hyperthyroidism had surgery to remove part of the thyroid in 2024. The removed part was examined in a pathology laboratory, and the examination results showed the thyroid follicular adenoma was a benign tumor [33]. This suggests an association between thyroid hormone levels and adenoma/tumor incidence in cats, and it should be noted that feline thyroid adenoma is often a functional tumor that produces excessive thyroid hormones [34].
- Aging and tumors—Age-related degeneration gives rise to some pathologies such as heart failure, macular degeneration, pulmonary insufficiency, renal failure, and neurodegeneration in mammals [35]. A human-based study (20,033 tumors across 35 tumor-types) shows that age influences both the number of mutations in a tumor and their evolutionary timing [36]. Senility symptoms are usually a universal feature of biological organisms, and one prominent aging feature is a gradual loss of function that occurs at the molecular, cellular and tissue level [35]; therefore, it can be considered that aging facilitates the growth of mutation-related thyroid adenomas, especially in middle-aged to older cats.
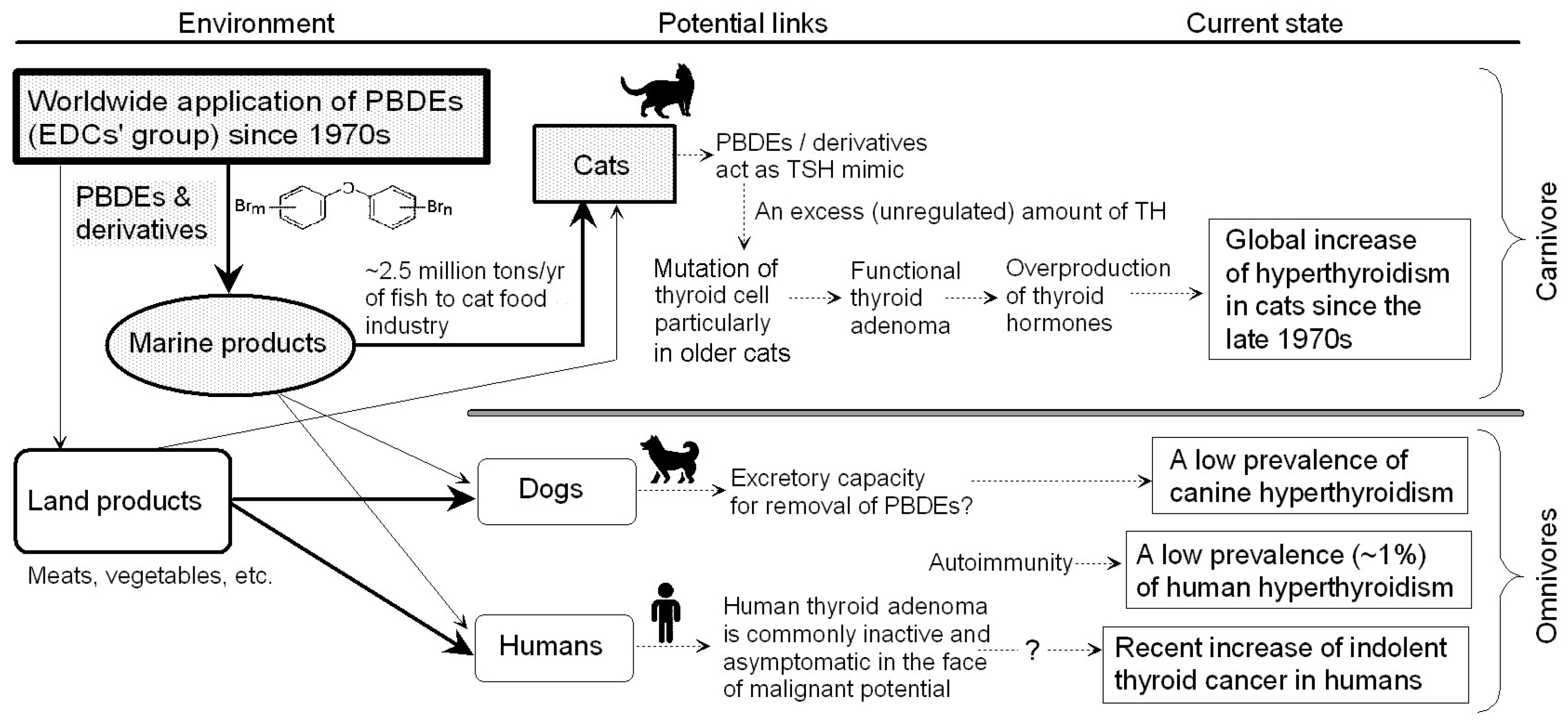
- Prevalence tendency—The prevalence rate of feline hyperthyroidism has been increasing worldwide over the past ~40 years [9]. It is unlikely that cats have had a genetic tendency for hyperthyroidism in this period.
3.3. Endocrine Disrupter (Environmental Hormone)
3.3.1. EDCs’ Features
3.3.2. Choice of PBDEs from Among a Large Number of EDCs
- Timeline—As stated in Section 2, the prevalence of feline hyperthyroidism has steadily increased since the late 1970s. PBDEs are brominated compounds connected by ester bonds between two benzene rings [44]. Due to their low cost and excellent flame retardancy, PBDEs have been widespread as flame retardants since the 1970s [45].
- Endocrine effect on thyroid—PBDEs have been detected across a range of environments and biological organisms [45]. Animal studies show that in rats and mice exposed to BDE-47 (a PBDE congener), there is disturbance of the homeostasis of the thyroid hormone (i.e., higher than 0.7 mg/kg T4 in body weight) [46]. It is suggested that human exposure to PBDEs may lead to subclinical hyperthyroidism, which can cause serious lingering symptoms [47].
3.3.3. PBDEs and Pet Foods
3.3.4. Difference in PBDE Intake Between Cats and Dogs
- Cats show a particular partiality to food containing histidine and inosine monophosphate, which are found at high levels in tuna [50]. In the Middle Ages, cats consumed fish scraps left by fishermen in ports [54]. Generally speaking, cats like to eat fish. In fact, domestic cats are eating more fish worldwide than people are. It is estimated that (i) the global cat food industry uses 2.5 million tons/yr of fishes such as sardines, herrings and anchovies; (ii) well-fed U.S. felines consume more than 1.1 million tons of fish; (iii) European felines dine upon 870,000 tons; (iv) Japanese house cats eat 132,000 tons/yr; and (v) Canadian cats account for 111,000 tons/yr of fish consumption [55].
- According to a taste test by the American Kennel Club [56], most dogs prefer beef and pork over chicken and lamb, and they also prefer warm and moist foods over cold and dry foods.
3.3.5. Excretory Ability for Removing PBDEs
3.4. Clinical Relation Between Cats and Humans
3.4.1. Thyroid Adenoma in Humans
- Approximately 7% of people have some sort of abnormal growth on their thyroids [64]. Patients with thyroid nodules account for 19–68% of the general population [65]. Benign thyroid tumors account for most thyroid disease, and gene expression profile suggests that follicular adenomas are similar to both benign and malignant tumors, meaning that some of them have malignant potential [66].
- Epidemiological studies suggest iodine deficiency, radiation, inflammation, genetics, autoimmunity, etc., but the mechanisms of thyroid diseases are not yet clear (reviewed in [67]).
3.4.2. PBDEs and Human Thyroid Disease
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- One Health. Available online: https://www.who.int/news-room/questions-and-answers/item/one-health (accessed on 4 October 2024).
- Bost, P.C.; Strynar, M.J.; Reiner, J.L.; Zweigenbaum, J.A.; Secoura, P.L.; Lindstrom, A.B.; Dye, J.A. U.S. domestic cats as sentinels for perfluoroalkyl substances: Possible linkages with housing, obesity, and disease. Environ. Res. 2016, 151, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Frazzoli, C.; Bocca, B.; Mantovani, A. The one health perspective in trace elements biomonitoring. J. Toxicol. Environ. Health B Crit. Rev. 2015, 18, 344–370. [Google Scholar] [CrossRef]
- Callaway, E. I can haz genomes—Cats claw their way into genetics. Nature 2015, 517, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.E.; Kintzer, P.P.; Cavanagh, P.G.; Fox, P.R.; Ferguson, D.C.; Johnson, G.F. Feline hyperthyroidism: Pretreatment clinical and laboratory evaluation of 131 cases. J. Am. Vet. Med. Assoc. 1981, 183, 103–110. [Google Scholar] [CrossRef]
- Mooney, C.T.; Peterson, M.E. Feline hyperthyroidism. In Manual of Canine and Feline Endocrinology, 4th ed.; Mooney, C.T., Peterson, M.E., Eds.; Small Animal Veterinary Association: Gloucester, UK, 2012; pp. 92–110. [Google Scholar]
- Lucke, V.M. A histological study of thyroid abnormalities in the domestic cat. J. Small Anim. Pract. 1964, 5, 351–358. [Google Scholar] [CrossRef]
- Leav, I.; Schiller, A.L.; Rijnberk, A.; Legg, M.A.; Kinderen, P.J. Adenomas and carcinomas of the canine and feline thyroid. Am. J. Pathol. 1976, 83, 61–122. [Google Scholar]
- Peterson, M.E.; Broome, M.R. Hyperthyroid cats on longterm medical treatment show a progressive increase in the prevalence of large thyroid tumours, intrathoracic thyroid masses and suspected thyroid carcinoma. Proceedings of 22nd ECVIM-CA Congress, Maastricht, The Netherlands, 6–8 September 2012. [Google Scholar]
- Peterson, M.E.; Johnson, J.G.; Andrews, L.K. Spontaneous hyperthyroidism in the cat. In Proceedings of the American College of Veterinary Internal Medicine, Seattle, WA, USA, 6 June 1979. [Google Scholar]
- Holzworth, J.; Theran, P.; Carpenter, J.L.; Harpster, N.K.; Todoroff, R.J. Hyperthyroidism in the cat: Ten cases. J. Am. Vet. Med. Assoc. 1980, 176, 345–353. [Google Scholar] [CrossRef]
- Domínguez, A.P.; Tostado, R.S.; Bernabe, L.F.; Corredor, A.P.; Prat, J.P. Prevalence of feline hyperthyroidism in a laboratory-based sample of 27,888 cats in Spain. J. Feline Med. Surg. 2024, 26, 1098612X241303304. [Google Scholar] [CrossRef]
- Feline Hyperthyroid Treatment Center. The Evolution of Feline Hyperthyroidism; Feline Hyperthyroid Treatment Center: Shoreline, WA, USA, 2017. [Google Scholar]
- Hyperthyroidism in Cats. Available online: https://www.bluecross.org.uk/advice/cat/health-and-injuries/hyperthyroidism-overactive-thyroid (accessed on 4 October 2024).
- Hayashi, T.; Imakumano, H.; Ohta, M.; Sakai, S.; Takumi, A.; Niizuma, A. The Illustrated Encyclopedia of Cat, 2nd ed.; Kodansha Scientific: Tokyo, Japan, 2011. [Google Scholar]
- Yoshida, K.; Asakura, S.; Sudo, T.; Hashizume, T.; Sugano, S. Parathyroid position and surgery in feline hyperthyroid. J. Vet. Med. 2018, 71, 761–765. [Google Scholar]
- Köhler, I.; Ballhausen, B.D.; Stockhaus, C.; Hartmann, K.; Wehner, A. Prävalenz und Risikofaktoren der felinen Hyperthyreose in einer Klinikpopulation in Süddeutschland (Prevalence of and risk factors for feline hyperthyroidism among a clinic population in Southern Germany). Tieraerztl. Ausg. K. H. 2016, 44, 149–157. (In German) [Google Scholar] [CrossRef]
- Mahony, O. Feline Hyperthyroidism. Clin. Brief 2012, 11, 19–22. [Google Scholar]
- Neumann, S.; Raaka, B.M.; Gershengorn, M.C. Human TSH receptor ligands as pharmacological probes with potential clinical application. Expert Rev. Endocrinol. Metab. 2009, 4, 669–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, M.A. Endocrine System. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Elsevier: Saint Louis, MO, USA, 2017; pp. 682–723. [Google Scholar]
- Estrada, J.M.; Soldin, D.; Buckey, T.M.; Burman, K.D.; Soldin, O.P. Thyrotropin isoforms: Implications for thyrotropin analysis and clinical practice. Thyroid 2014, 24, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Ariyasu, H.; Takeshima, K.; Iwakura, H.; Akamizu, T. Comprehensive research on thyroid diseases associated with autoimmunity: Autoimmune thyroid diseases, thyroid diseases during immune-checkpoint inhibitors therapy, and immunoglobulin-G4-associated thyroid diseases. Endoc. J. 2019, 66, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Hyperthyroid—Overview. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/hyperthyroidism (accessed on 14 October 2024).
- Tywanek, E.; Michalak, A.; Świrska, J.; Zwolak, A. Autoimmunity, New Potential Biomarkers and the Thyroid Gland—The Perspective of Hashimoto’s Thyroiditis and Its Treatment. Int. J. Mol. Sci. 2024, 25, 4703. [Google Scholar] [CrossRef]
- What is the Canine Hyperthyroid? Available online: https://petpet.work/archives/3388 (accessed on 15 October 2024).
- Shadwick, S.R.; Ridgway, M.D.; Kubier, A. Thyrotoxicosis in a dog induced by the consumption of feces from a levothyroxine-supplemented housemate. Can. Vet. J. 2013, 54, 987–989. [Google Scholar]
- Wiersinga, W.M.; Roppe, K.G.; Effeamidis, G. Hyperthyroidism: Aetiology, pathogenesis, diagnosis, management, complications, and prognosis. Lancet Diabetes Endocrinol. 2023, 11, 282–298. [Google Scholar] [CrossRef]
- Nguyen, L.Q.; Arseven, O.K.; Gerber, H.; Stein, B.S.; Jameson, J.L.; Kopp, P. Cloning of the Cat TSH Receptor and Evidence Against an Autoimmune Etiology of Feline Hyperthyroidism. Endocrinology 2002, 143, 395–402. [Google Scholar] [CrossRef]
- Iwata, K. Veterinary Neurology Report No. 01912; Neuro Vets: Kyoto, Japan, 2024. [Google Scholar]
- Smiech, M.; Leszczynski, P.; Kono, H.; Wardell, C.; Taniguchi, H. Emerging BRAF Mutations in Cancer Progression and Their Possible Effects on Transcriptional Networks. Genes 2020, 11, 1342. [Google Scholar] [CrossRef]
- Franco, A.T.; Malaguarnera, R.; Refetoff, S.; Liao, X.; Lundsmith, E.; Kimura, S.; Pritchard, C.; Marais, R.; Davies, T.F.; Weinstein, L.S.; et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 1615–1620. [Google Scholar] [CrossRef]
- Zou, M.; Baitei, E.Y.; Al-Rijjal, R.A.; Parhar, R.S.; Al-Mohanna, F.A.; Kimura, S.; Pritchard, C.; Binessa, H.A.; Alzahrani, A.S.; Al-Khalaf, H.H.; et al. TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer. Oncogene 2016, 35, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Okada, K. Pathology Report No. 15732-02; North Lab: Sapporo, Japan, 2024. [Google Scholar]
- Scott-Moncrieff, J.C. Feline hyperthyroidism. In Canine and Feline Endocrinology, 4th ed.; Feldman, E.C., Nelson, R.W., Reusch, C., Scott-Moncrieff, J.C., Eds.; Elsevier Saunders: Saint Louis, MO, USA, 2015; pp. 137–187. [Google Scholar]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Haider, S.; Paul, C.; Boutros, P.C. Age influences on the molecular presentation of tumours. Nat. Commun. 2022, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.E.; Ward, C.R. Etiopathologic Finding of Hyperthyroidism in Cats. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 633–645. [Google Scholar] [CrossRef]
- Gunn-Moore, D. Feline Endocrinopathies. Vet. Clin. Small Anim. 2005, 35, 171–210. [Google Scholar] [CrossRef]
- Peterson, M. Hyperthyroidism in Cats: What’s causing this epidemic of thyroid disease and can we prevent it? J. Feline Med. Surg. 2012, 14, 804–818. [Google Scholar] [CrossRef]
- Bender, D.A. A Dictionary of Food and Nutrition; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Monneret, C. Endocrine disruptors/perturbateurs endocriniens—What is an endocrine disruptor? C. R. Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Vendenburg, L.M. Nonmonotonic responses in endocrine disruption. In Endocrine Disruption and Human Health, 2nd ed.; Darbre, P.D., Ed.; Academic Press: Amsterdam, The Netherlands, 2022; pp. 141–163. [Google Scholar]
- Schug, T.T.; Johnson, A.F.; Birnbaum, L.S.; Colborn, T.; Guillette, L.J.; Crews, D.P.; Collins, T.; Soto, A.M.; Vom Saal, F.S.; McLachlan, J.A.; et al. Minireview: Endocrine disruptors—Past lessons and future directions. Mol. Endocrinol. 2016, 30, 833–847. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Yang, H.; Kong, L.; Ma, H.; Zhu, Y.; Zhao, X.; Yang, T.; Liu, W. Advanced understanding of the polybrominated diphenyl ethers (PBDEs): Insights from total environment to intoxication. Toxicology 2024, 509, 153959. [Google Scholar] [CrossRef]
- Andrade, A.J.M.; Kuriyama, S.N.; Akkoc, Z.; Talsness, C.E.; Chahoud, I. Effects of developmental low dose PBDE 47 exposure on thyroid hormone status and serum concentrations of FSH and inhibin B in male rats. In Proceedings of the 24th International Symposium on Halogenated Environmental Organic Pollutants and POPs (Organohal Compounds, 66, 3858–3863), Berlin, Germany, 6–10 September 2004. [Google Scholar]
- Czerska, M.; Zieliński, M.; Kamińska, J.; Ligocka, D. Effects of polybrominated diphenyl ethers on thyroid hormone, neurodevelopment and fertility in rodents and humans. Int. J. Occup. Med. Environ. Health 2013, 26, 498–510. [Google Scholar] [CrossRef]
- Guo, W.; Gardner, S.; Yen, S.; Petreas, M.; Park, J.S. Temporal Changes of PBDE Levels in California House Cats and a Link to Cat Hyperthyroidism. Environ. Sci. Technol. 2016, 50, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, H.; Li, J.; Shan, Z.; Teng, W.; Teng, X. The Correlation between Polybrominated Diphenyl Ethers (PBDEs) and Thyroid Hormones in the General Population: A Meta-Analysis. PLoS ONE 2015, 10, e0126989. [Google Scholar] [CrossRef]
- Guruge, K.S.; Seike, N.; Yamanaka, N.; Miyazaki, S. Polychlorinated dibenzo-p-dioxins, -dibenzofurans, and biphenyls in domestic animal food stuff and their fat. Chemosphere 2005, 58, 883–889. [Google Scholar] [CrossRef]
- Shimasaki, M.; Mizukawa, H.; Takaguchi, K.; Saengtienchai, A.; Ngamchirttakul, A.; Pencharee, D.; Khidkhan, K.; Ikenaka, Y.; Nakayama, S.M.M.; Ishizuka, M.; et al. Contamination Status of Pet Cats in Thailand with Organohalogen Compounds (OHCs) and Their Hydroxylated and Methoxylated Derivatives and Estimation of Sources of Exposure to These Contaminants. Animals 2022, 12, 3520. [Google Scholar] [CrossRef]
- Mizukawa, H.; Nomiyama, K.; Nakatsu, S.; Iwata, H.; Yoo, J.; Kubota, A.; Yamamoto, M.; Ishizuka, M.; Ikenaka, Y.; Nakayama, S.M.M.; et al. Organohalogen Compounds in Pet Dog and Cat: Do Pets Biotransform Natural Brominated Products in Food to Harmful Hydroxlated Substances? Environ. Sci. Tech. 2016, 50, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Hagen-Plantinga, E.A.; Hendriks, W.H. Dietary nutrient profiles of wild wolves—Insights for optimal dog nutrition? Br. J. Nutr. 2015, 113, S40–S54. [Google Scholar] [CrossRef]
- Grimm, D. Why do cats love tuna so much? Scientists may finally know. Science 2023, 381, 935. [Google Scholar] [CrossRef] [PubMed]
- Cats a Bigger Danger to Fish Stocks. Available online: https://www.reuters.com/article/us-fishing-cats (accessed on 20 October 2024).
- Accounting for Taste. Available online: https://www.akc.org/expert-advice/nutrition/accounting-taste-probing-mysteries-dogs-find-delicious (accessed on 20 October 2024).
- Xu, C.; Li, C.Y.T.; Kong, A.N.T. Induction of Phase I, II and III Drug Metabolism/Transport by Xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Beusekom, C.D.; Fink-Gremmels, J.; Schrickx, J.A. Comparing the glucuronidation capacity of the feline liver with substrate-specific glucuronidation in dogs. J. Vet. Pharmacol. Ther. 2014, 37, 18–24. [Google Scholar] [CrossRef]
- Savides, M.C.; Oehme, F.W.; Nash, S.L.; Leipold, H.W. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicol. Appl. Pharmacol. 1984, 74, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Espe, S. Malacards—The Human Disease Database. J. Med. Libr. Assoc. 2018, 106, 140–141. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chang, J.; Jia, B.; Liu, J. The Blood Biomarkers of Thyroid Cancer. Cancer Manag. Res. 2020, 12, 5431–5438. [Google Scholar] [CrossRef]
- Kim, J.; Gosnell, J.E.; Roman, S.A. Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol. 2020, 16, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Thyroid Adenoma. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562252/ (accessed on 25 October 2024).
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Arora, N.; Scognamiglio, T.; Zhu, B.; Fahey, T.J. Do benign thyroid nodules have malignant potential? An evidence-based review. World J. Surg. 2008, 32, 1237–1246. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, L.; Xie, M.; Li, H.; Zhang, Q. Exposure to Polybrominated Diphenyl Ethers and Thyroid Disease: A Systematic Review and Meta-analysis of Epidemiological Studies. Curr. Epidemiol. Rep. 2024, 11, 20–31. [Google Scholar] [CrossRef]
- Linares, V.; Bellés, M.; Domingo, J.L. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 2015, 89, 335–356. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Meng, T.; Jiali Cheng, J.; Tang, Z.; Yin, H.; Zhang, M. Global distribution and trends of polybrominated diphenyl ethers in human blood and breast milk: A quantitative meta-analysis of studies published in the period 2000–2019. J. Environ. Manage 2021, 280, 111696. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Futatsuka, M. Minamata Disease from the viewpoint of occupational health. J. Occup. Health 1998, 40, 1–8. [Google Scholar] [CrossRef]
- Harada, M. The Global Lessons of Minamata Disease—An Introduction to Minamata Studies. In Taking Life and Death Seriously Bioethics from Japan (Advances in Bioethics. Volume 8); Takahashi, T., Ed.; Emerald Group Publishing: Leeds, UK, 2005; pp. 299–335. [Google Scholar]
- Harada, M. Minamata Disease; Kumamoto Nichinichi Shinbun Culture and Information Center: Kumamoto, Japan, 2004. [Google Scholar]
- Harada, M. Minamata Disease Incident—History and Modernity; Open Research Center for Minamata Studies (Kumamoto Gakuen University): Kumamoto, Japan, 2021. [Google Scholar]

| Country | Prevalence |
|---|---|
| USA | Up to 10% of cats older than 10 years (reviewed in [9]) |
| UK | 11.9% of cats older than 9 years (reviewed in [9]) |
| Germany | 11.4% of cats older than 8 years (reviewed in [9]) |
| Japan | 8.9% of cats older than 9 years (reviewed in [9]) |
| Spain | 6.2% overall (n = 27,888) and 7.9% of cats older than 10 years [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, R.; Costa, R.P.R.d.; Ferreira, C.S.S. Environmental Implications of the Global Prevalence of Hyperthyroidism in Cats from a “One Health” Perspective. Pollutants 2025, 5, 8. https://doi.org/10.3390/pollutants5010008
Kikuchi R, Costa RPRd, Ferreira CSS. Environmental Implications of the Global Prevalence of Hyperthyroidism in Cats from a “One Health” Perspective. Pollutants. 2025; 5(1):8. https://doi.org/10.3390/pollutants5010008
Chicago/Turabian StyleKikuchi, Ryunosuke, Rosário Plácido Roberto da Costa, and Carla Sofia Santos Ferreira. 2025. "Environmental Implications of the Global Prevalence of Hyperthyroidism in Cats from a “One Health” Perspective" Pollutants 5, no. 1: 8. https://doi.org/10.3390/pollutants5010008
APA StyleKikuchi, R., Costa, R. P. R. d., & Ferreira, C. S. S. (2025). Environmental Implications of the Global Prevalence of Hyperthyroidism in Cats from a “One Health” Perspective. Pollutants, 5(1), 8. https://doi.org/10.3390/pollutants5010008








