Exploitation of Waste Algal Biomass in Northern Italy: A Cost–Benefit Analysis
Abstract
1. Introduction
2. Background
2.1. Algae
2.2. Biomass and Biorefinery
- Phase 1: one feed source/nonflexible main processes/only one product
- Phase 2: one feed source/rather flexible main processes/multitude of products
- Phase 3: various feed sources/flexible main processes/multitude of products
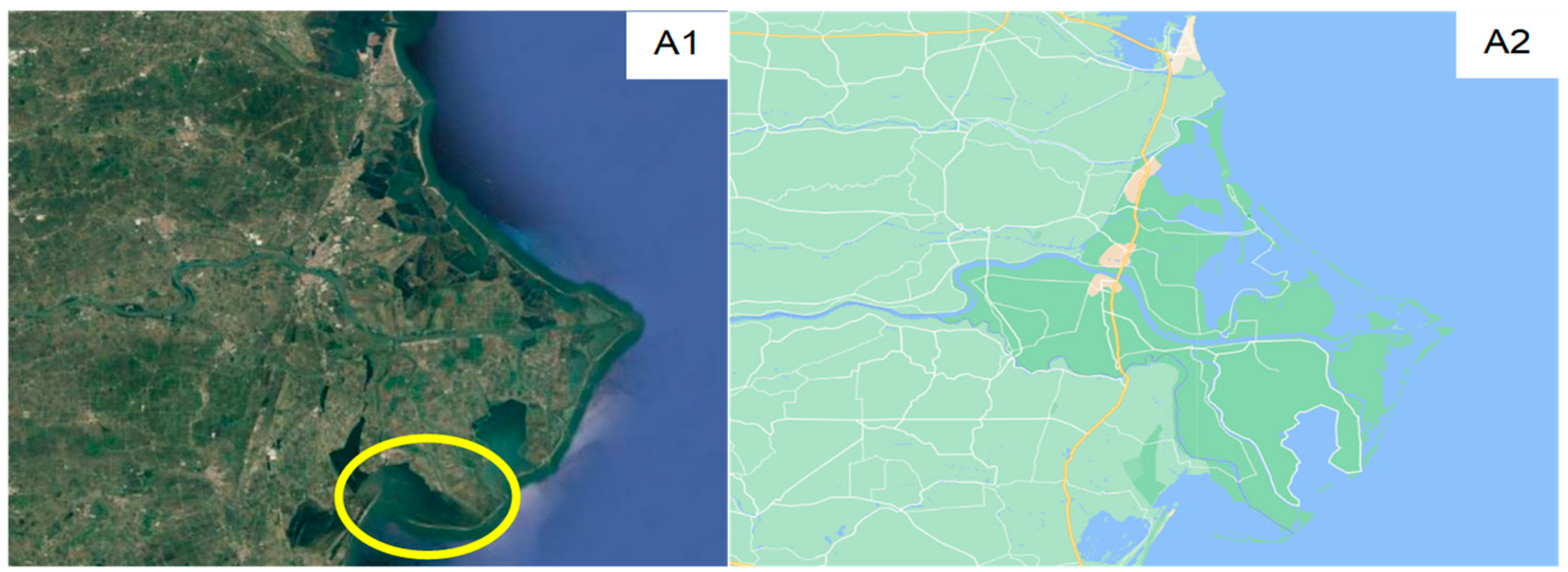
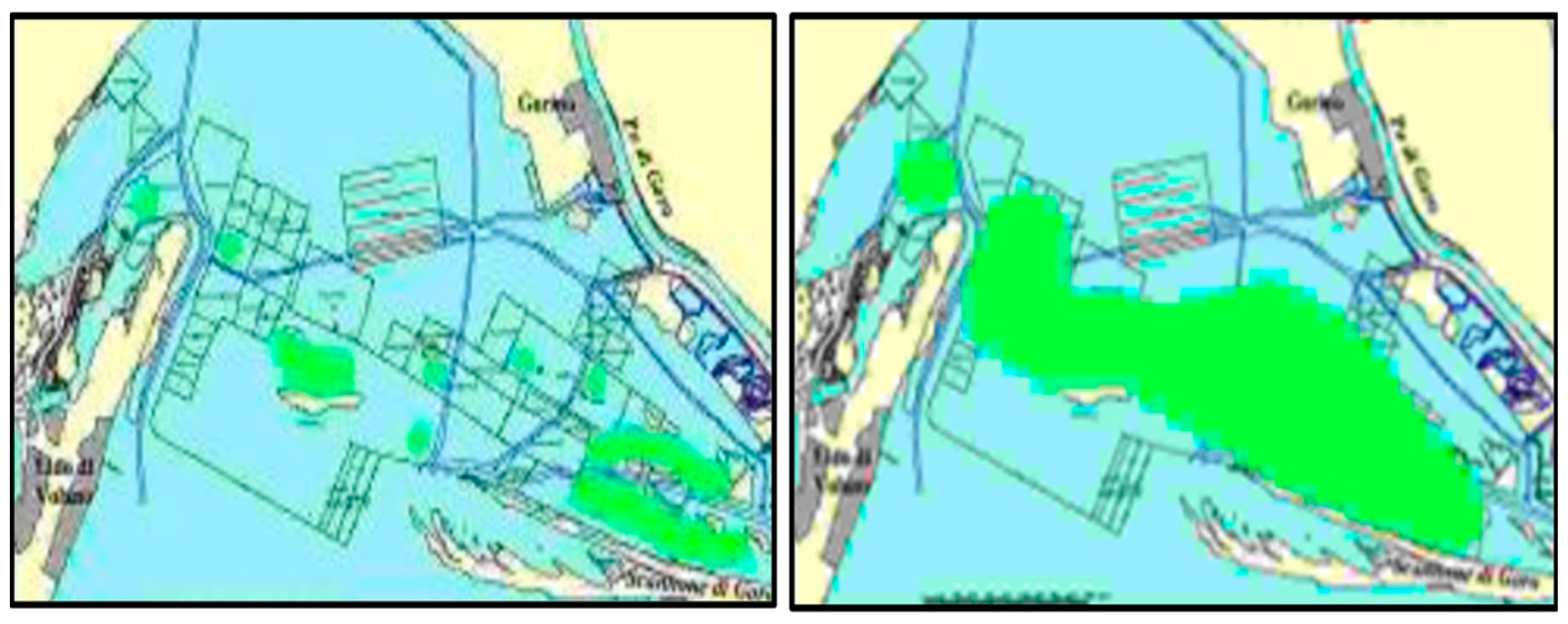
3. The Case Study
4. Methods
4.1. Cost–Benefit Analysis
4.2. Foresight Analysis
4.3. Scenarios
4.3.1. The Baseline, or “Status Quo”
4.3.2. Scenarios’ Common Grounds
4.3.3. Scenarios Description
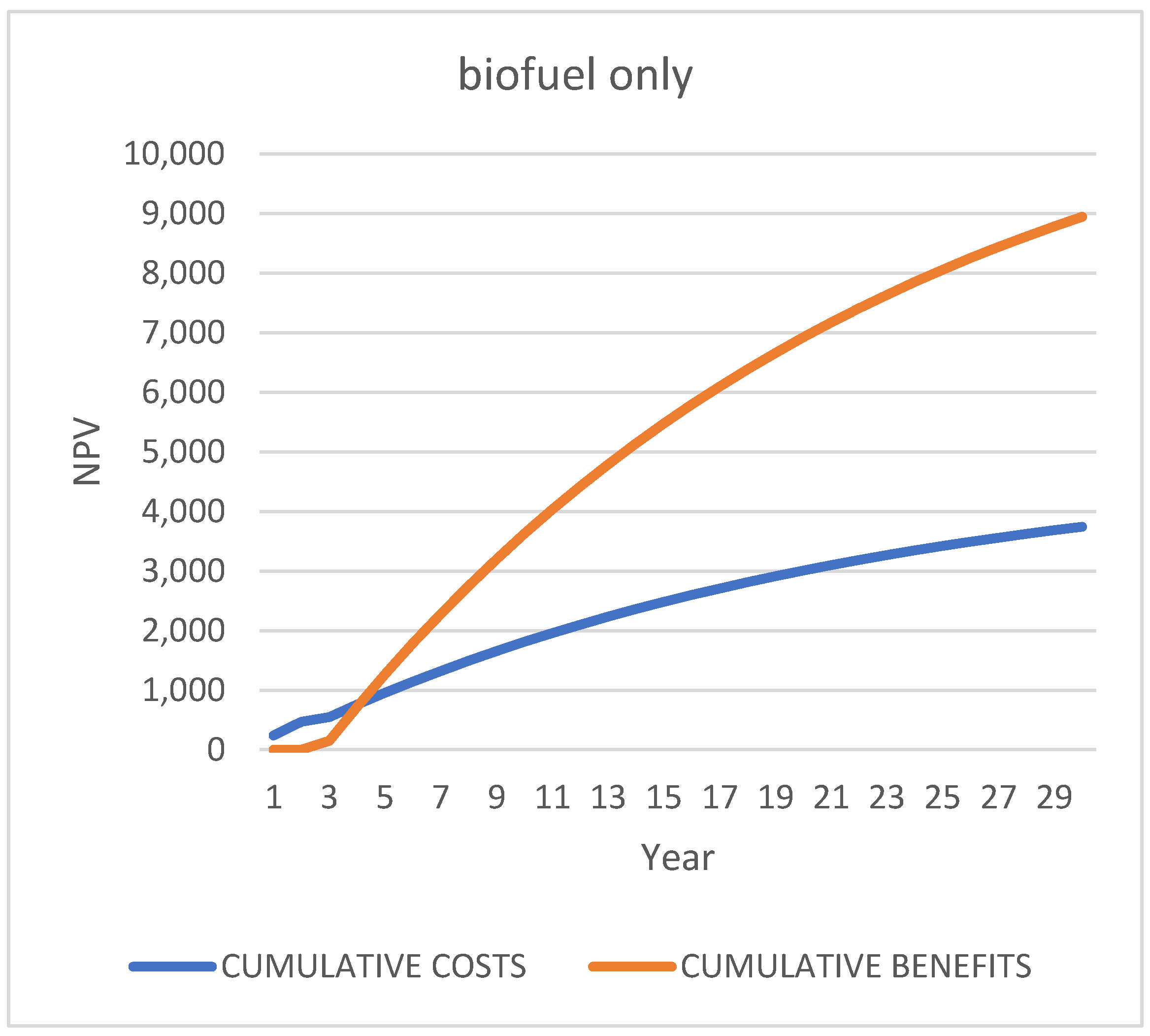
First Scenario [52]: “Biofuels Only”, “1”
Second Scenario [88]: “Biofuels + One Bioproduct” “2 a/b”
Third Scenario [87]: “Multi-Product Biorefinery”, “2 c/d”
Fourth Scenario [86]: “Bioproducts”, “3”
4.3.4. Resume Table
| Scenarios: | 1: Biofuels | 2a–d: Biorefineries | 3: Food/Fodder | |||
|---|---|---|---|---|---|---|
| Phase | I | I | I/II | I | II | II |
| Product/s | Biodiesel, bioethanol, biogas | Bioethanol | bioethanol + alginate | bioethanol | bioethanol + mannitol, alginate, proteins, and soil conditioner | food supplement: laminarin + fucoidan, and fertilizers |
| building time (months) | 6 (planning) + 24 | 6 (planning) + 24 | 6 (planning) + 24 | 36 | 36 | 12 |
| months for startup | 6: 50% gain with 75% variable expenses | 6: 50% gain with 75% variable expenses | 6: 50% gain with 75% variable expenses | 3 | 3 | none (paper assumption) |
| Variable operating costs (M€/Y) | 237.39 | 186.23 | 695.93 | 63.37 | 107.96 | 49.61 |
| Fixed operating cost (M€/Y) | 15.01 | 11.58 | 43.27 | 3.87 | 10.8 | 0.54 |
| Total operating costs (M€/Y) | 252.4 | 197.81 | 739.19 | 67.24 | 117.58 | 50.15 |
| total direct cost (M€) | 298.24 | 257.51 | 514.14 | 181.06 | 361.54 | 3.12 |
| total indirect cost (M€) | 178.88 | 154.48 | 308.43 | 108.66 | 216.86 | 1.87 |
| total capital investments (M€) | 503.05 | 435.94 | 870.37 | 307.09 | 613.13 | 5.4 |
| Total annual sales (M€/Y) | 695.33 | 218.63 | 832.89 | 29.22 | 370.46 | 166.15 |
5. Cost–Benefit Analysis, Results
5.1. NPV
5.2. Fixed Discount Rate
5.3. Variable Discount Rate
6. Foresight Analysis
6.1. Chemical Pollution
6.2. Social Failure
- Suspicious sources of the other biomasses, with dilution purpose, and consequent release of unknown and deadly substances in the atmosphere,
- Suspected structural fragilities and hypothetic plant lack of resilience toward natural disasters and rare phenomena,
- Suspected enormous microbiological and biological damages in case of un-stabilized compost misuse,
- Suspects regarding the “unknown” air emissions (usually air emitting plants are transparent and produce real-time data, with law limits, such as in this incinerator example near the case study’s area: [95]),
- Personal, biased, uncheckable experiences, inconsistent and exacerbated facts, such as: “thirty noisy trucks each 12 min to and from the plant”.
- Solution to HABs issue,
- Rise of new activities for bio products commercialization,
- Openings for more workplaces,
- Increased circularity of resources and carbon cycle with lesser “new fertilizer” bought.
- Renewable production of energy and heat for local consumption.
6.3. SWOT Analyses
6.3.1. General SWOT Analysis for Goro’s Lagoon Case Study

6.3.2. Targeted SWOT Analysis for “Biofuel only” Scenario
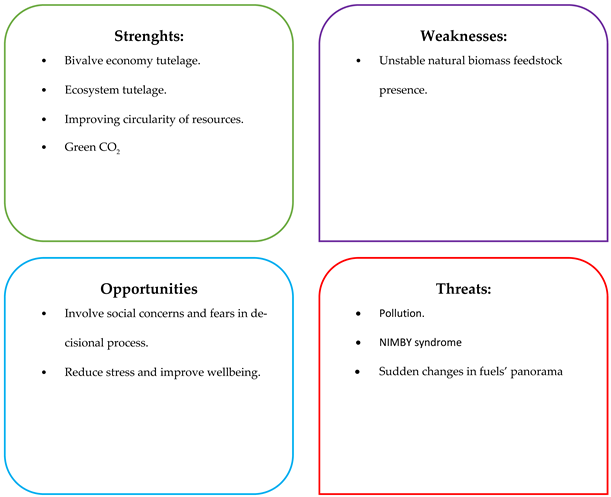
6.3.3. Targeted SWOT Analysis for Biorefinery Multiproduct” Scenario
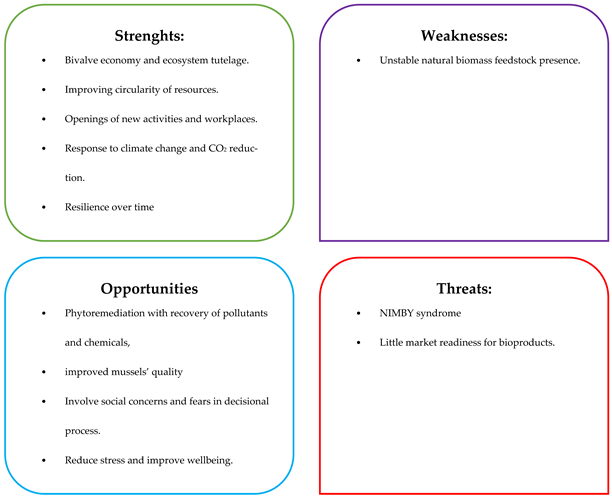
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Scopus Research by Year | 2012 | 2021 | 2022 |
|---|---|---|---|
| Algae and biodiesel | 794 | 4114 | 4226 |
| Algae and bioethanol | 112 | 765 | 793 |
| Algae and biogas | 110 | 1112 | 1164 |
| Algae and biohydrogen | 66 | 375 | 393 |
| Algae and biobutanol | 9 | 76 | 77 |
- Additional References for algal biomass usage
- Landfill Building Costs and Demolition Expenditure
| LANDFILL, Typical Construction Costs | ||
|---|---|---|
| Task | Minimum | Maximum |
| Clear and Grub | 397 | 1190 |
| Site Survey | 1983 | 3173 |
| Excavation | 39,659 | 130,875 |
| Perimeter Berm | 3966 | 6345 |
| Clay Liner | 12,691 | 64,248 |
| Geomembrane | 9518 | 13,881 |
| Geocomposite | 13,088 | 17,450 |
| Granular Soil | 19,036 | 25,382 |
| Leachate System | 3173 | 40,452 |
| QA/QC | 29,744 | 39,659 |
| TOTAL | 133,255 | 306,962 |
| LANDFILL, Closure Care and Maintenance Costs | ||
|---|---|---|
| Task | Minimum | Maximum |
| Final grades survey | 1190 | 2380 |
| Gas management layer | 9518 | 12,691 |
| Compacted caly cap | 10,311 | 20,226 |
| Geomembrane cap | 7139 | 9122 |
| Geocomposite | 13,088 | 17,450 |
| Cover and vegetative soil | 5156 | 10,311 |
| Seed, much, fertilize | 397 | 793 |
| Gas management system | 11,501 | 13,881 |
| Run-off control system | 1983 | 2776 |
| QA/QC | 29,744 | 39,659 |
| Total | 90,026 | 129,289 |
| LANDFILL, Post-Closure Maintenance Costs | ||
|---|---|---|
| Task | Minimum | Maximum |
| Security and fencing | 35,693 | 71,387 |
| Final cap and cover | 3569 | 6742 |
| Leachate mechanicals | 10,708 | 14,277 |
| Landfill gas mechanicals | 5354 | 6782 |
| Wells/probes | 237,955 | 356,933 |
| Environmental monitoring | 5354 | 6841 |
| Total | 25,382 | 34,900 |
| Demolition Costs for Industrial Plants | |||||
|---|---|---|---|---|---|
| Industrial | |||||
| PHASE I | PHASE II | ||||
| Removal of redundant services | Fixed: tot € per site | 35.40 | 112.10 | 112.10 | 188.80 |
| Site clearance | Variable: tot € per m2 | 17.70 | 53.10 | 53.10 | 88.50 |
| Demolitions | Variable: tot € per m2 | 37.76 | 56.05 | 56.05 | 74.34 |
| Site investigation | Fixed: tot € per site | 47.20 | 177.00 | 177.00 | 306.80 |
| Fees | Fixed: tot € per site | 224.20 | 507.40 | 507.40 | 790.60 |
References
- Toth, G.; Szigeti, C. The historical ecological footprint: From over-population to over-consumption. Ecol. Indic. 2016, 60, 283–291. [Google Scholar] [CrossRef]
- Kassas, M. Desertification: A general review. J. Arid Environ. 1995, 30, 115–128. [Google Scholar] [CrossRef]
- Nelson, G.C.; Rosegrant, M.W.; Koo, J.; Robertson, R.D.; Sulser, T.; Zhu, T.; Ringler, C.; Msangi, S.; Palazzo, A.; Batka, M.; et al. Climate Change: Impact on Agriculture and Costs of Adaptation. 2009. Available online: https://books.google.it/books?hl=it&lr=&id=1Vpe0JvYTJYC&oi=fnd&pg=PR7&dq=climate+change+negative+impact&ots=Xns86aTvka&sig=mkwZExO60CZOzLvp5ivlMioWDSc#v=onepage&q=climate%20change%20negative%20impact&f=false (accessed on 2 February 2021).
- Tallis, H.; Kareiva, P. Essay Ecosystem Services. Curr. Biol. 2007, 15, R746–R748. Available online: https://www.perc.org/wp-content/uploads/2016/10/EcosystemServices_RDSimpson.pdf (accessed on 3 February 2021). [CrossRef] [PubMed]
- Rafoss, T. Sustainable Biomass Production from Oceans and the Potential for Circular Bioeconomy. In The Bioeconomy Approach; Taylor & Francis Group: Oxfordshire, UK, 2020; pp. 24–44. [Google Scholar] [CrossRef]
- Tičina, V. Marine Aquaculture Impacts on Marine Biota in Oligotrophic Environments of the Mediterranean Sea—A Review. Front. Mar. Sci. 2020, 7, 517704. [Google Scholar] [CrossRef]
- Maulu, S. Climate Change Effects on Aquaculture Production: Sustainability Implications, Mitigation, and Adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Ben Chekroun, K.; Sánchez, E.; Baghour, M. The role of algae in bioremediation of organic pollutants. Int. Res. J. Public Environ. Health 2014, 1, 19–32. [Google Scholar]
- Zeraatkar, A.K. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Thirukumaran, R. Resource recovery from fish waste: Prospects and the usage of intensified extraction technologies. Chemosphere 2022, 299, 134361. [Google Scholar] [CrossRef] [PubMed]
- Poff, N.L.; Day, J.W. Aquatic Ecosystems & Global Climate Change—Potential Impacts on Inland Freshwater and Coastal Wetland Ecosystems in the United States Characterizing Contemporary Drivers of the Occurrence of Coldwater Fishes in the Western United States View Project. 2002. Available online: https://www.researchgate.net/publication/248528187 (accessed on 4 February 2021).
- Dórea, J.G. Persistent, bioaccumulative and toxic substances in fish: Human health considerations. Sci. Total Environ. 2008, 400, 93–114. [Google Scholar] [CrossRef]
- Suja, F.; Pramanik, B.K.; Zain, S.M. Contamination, bioaccumulation and toxic effects of perfluorinated chemicals (PFCs) in the water environment: A review paper. Water Sci. Technol. 2009, 60, 1533–1554. [Google Scholar] [CrossRef]
- Check, L.; Marteel-Parrish, A. The fate and behavior of persistent, bioaccumulative, and toxic (PBT) chemicals: Examining lead (Pb) as a PBT metal. Rev. Environ. Health 2013, 28, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.M.; Sutton, P.A.; Lewis, C.A.; Lewis, A.C.; Scarlett, A.; Chau, W.; Widdows, J.; Rowland, S.J. Unresolved complex mixtures of aromatic hydrocarbons: Thousands of overlooked persistent, bioaccumulative, and toxic contaminants in mussels. Environ. Sci. Technol. 2007, 41, 457–464. [Google Scholar] [CrossRef]
- Murray, T.M. PBT (Persistent, Bioaccumulative, and Toxic) Chemicals. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 762–764. [Google Scholar] [CrossRef]
- Werner, W. Fertilizers, 6. Environmental Aspects. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Zaldívar, J.-M.; Cardoso, A.C.; Viaroli, P.; Newton, A.; de Wit, R.; Ibañez, C.; Reizopoulou, S.; Somma, F.; Razinkovas, A.; Basset, A.; et al. Eutrophication in transitional waters: An overview. Transit. Waters Monogr. 2008, 1, 1–78. [Google Scholar]
- Joniver, C.F.H.; Photiades, A.; Moore, P.J.; Winters, A.L.; Woolmer, A.; Adams, J.M.M. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal Res. 2021, 58, 102407. [Google Scholar] [CrossRef]
- Chislock, M.F. Eutrofizzazione. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Al-Yamani, F.Y.; Polikarpov, I.; Saburova, M. Marine life mortalities and Harmful Algal Blooms in the Northern Arabian Gulf. Aquat. Ecosyst. Health Manag. 2020, 23, 196–209. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, T.; Zhou, J. Historical occurrence of algal blooms in the northern Beibu Gulf of China and implications for future trends. Front. Microbiol. 2019, 10, 451. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of marine macroalgae into high-tech bioproducts: A review. Environ. Chem. Lett. 2021, 19, 969–1000. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Horner, R.A.; Caron, D.A.; Garcia-Mendoza, E.; Hickey, B.M.; Hunter, M.; Huppert, D.D.; Kudela, R.M.; Langlois, G.W.; Largier, J.L.; et al. Harmful algal blooms along the North American west coast region: History, trends, causes, and impacts. Harmful Algae 2012, 19, 133–159. [Google Scholar] [CrossRef]
- Glibert, P.M.; Allen, J.I.; Artioli, Y.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: Projections based on model analysis. Glob. Change Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef]
- Moore, S.K.; Mantua, N.J.; Salathé, E.P. Past trends and future scenarios for environmental conditions favoring the accumulation of paralytic shellfish toxins in Puget Sound shellfish. Harmful Algae 2011, 10, 521–529. [Google Scholar] [CrossRef]
- Townhill, B.L.; Tinker, J.; Jones, M.; Pitois, S.; Creach, V.; Simpson, S.D.; Dye, S.; Bear, E.; Pinnegar, J.K. Harmful algal blooms and climate change: Exploring future distribution changes. ICES J. Mar. Sci. 2018, 75, 1882–1893. [Google Scholar] [CrossRef]
- Facca, C. Ecological status assessment of transitional waters. Water 2020, 12, 3159. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development | Department of Economic and Social Affairs. 2022. Available online: https://sdgs.un.org/2030agenda (accessed on 14 May 2021).
- Saeid, A.; Chojnacka, K. Algae Biomass as a Raw Material for Production of Algal Extracts; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Gupta, S.K.; Malik, A.; Bux, F. Algal Biofuels: Recent Advances and Future Prospects; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Bikaki, N. Technoeconomic Considerations for Biomass Fractionation in a Biorefinery Context. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 587–610. [Google Scholar] [CrossRef]
- Mominur Rahman, M.; Shajib Rahman, M.; Chandra Deb, B. Competitive Cost Advantage: An Application of Environmental Accounting and Management Approach with reference to Bangladesh. Cost Manag. 2021, 49, 47–59. [Google Scholar]
- Renaud, S.M.; Luong-Van, J.T. Seasonal variation in the chemical composition of tropical Australian marine macroalgae. J. Appl. Phycol. 2006, 18, 381–387. [Google Scholar] [CrossRef]
- Sutkowy, M.; Lenarczyk, J.; Kłosowski, G. Effect of culture medium on the growth of microscopic algae (Chlorophyceae) biomass showing biosorption potential: A case study Pseudopediastrum boryanum. Psychol. Res. 2018, 67, 112–119. [Google Scholar] [CrossRef]
- Pilatti, F.K.; Ramlov, F.; Schmidt, E.C.; Kreusch, M.; Pereira, D.T.; Costa, C.; de Oliveira, E.R.; Bauer, C.M.; Rocha, M.; Bouzon, Z.L.; et al. In vitro exposure of Ulva lactuca Linnaeus (Chlorophyta) to gasoline—Biochemical and morphological alterations. Chemosphere 2016, 156, 428–437. [Google Scholar] [CrossRef]
- Henriques, B.; Teixeira, A.; Figueira, P.; Reis, A.T.; Almeida, J.; Vale, C.; Pereira, E. Simultaneous removal of trace elements from contaminated waters by living Ulva lactuca. Sci. Total Environ. 2019, 652, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Mirza, N.; Shaheen, S. Phytoremediation using algae and macrophytes: I. In Phytoremediation: Management of Environmental Contaminants; Springer International Publishing: Cham, Switzerland, 2015; Volume 2, pp. 265–289. [Google Scholar] [CrossRef]
- Chekroun, B.; Ben Chekroun, K.; Baghour, M. The role of algae in phytoremediation of heavy metals: A review. J. Mater. Environ. Sci. 2013, 4, 873–880. [Google Scholar]
- Nanda, S.; Rana, R.; Sarangi, P.K.; Dalai, A.K.; Kozinski, J.A. A broad introduction to first-, second-, and third-generation biofuels. In Recent Advancements in Biofuels and Bioenergy Utilization; Springer: Singapore, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Linares-Pastén, J.A.; Andersson, M.; Karlsson, E.N. Thermostable Glycoside Hydrolases in Biorefinery Technologies. Curr. Biotechnol. 2014, 3, 26–44. Available online: https://www.borregaard.com (accessed on 28 November 2020). [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Stevens, C.V.; Clark, J.H.; Deswarte, F. Introduction to Chemicals from Biomass Wiley Series in Renewable Resources Series Editor Titles in the Series Renewables-Based Technology-Sustainability Assessment Handbook of Natural Colorants Surfactants from Renewable Resources Industrial Application of Natural Fibres-Structure, Properties and Technical Applications. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ahmad Ansari, F.; Nasr, M.; Guldhe, A.; Gupta, S.K.; Rawat, I.; Bux, F. Techno-economic feasibility of algal aquaculture via fish and biodiesel production pathways: A commercial-scale application. Sci. Total Environ. 2020, 704, 135259. [Google Scholar] [CrossRef] [PubMed]
- Ingle, K.; Vitkin, E.; Robin, A.; Yakhini, Z.; Mishori, D.; Golberg, A. Macroalgae Biorefinery from Kappaphycus alvarezii: Conversion Modeling and Performance Prediction for India and Philippines as Examples. Bioenergy Res. 2018, 11, 22–32. [Google Scholar] [CrossRef]
- Greene, J.M.; Gulden, J.; Wood, G.; Huesemann, M.; Quinn, J.C. Techno-economic analysis and global warming potential of a novel offshore macroalgae biorefinery. Algal Res. 2020, 51, 102032. [Google Scholar] [CrossRef]
- Zhang, X.; Thomsen, M. Techno-economic and environmental assessment of novel biorefinery designs for sequential extraction of high-value biomolecules from brown macroalgae Laminaria digitata, Fucus vesiculosus, and Saccharina latissima. Algal Res. 2021, 60, 102499. [Google Scholar] [CrossRef]
- Dickson, R.; Brigljevic, B.; Lim, H.; Liu, J. Maximizing the sustainability of a macroalgae biorefinery: A superstructure optimization of a volatile fatty acid platform. Green Chem. 2020, 22, 4174–4186. [Google Scholar] [CrossRef]
- Konda, N.V.S.N.M.; Singh, S.; Simmons, B.A.; Klein-Marcuschamer, D. An Investigation on the Economic Feasibility of Macroalgae as a Potential Feedstock for Biorefineries. Bioenergy Res. 2015, 8, 1046–1056. [Google Scholar] [CrossRef]
- Paoletti. Site Description (Sacca di Goro). 2008. Available online: https://www3.diism.unisi.it/~paoletti/teaching/ModGestSistAmb/download/Descrizione_SaccaDiGoro.pdf (accessed on 12 December 2020).
- National Order of Biologists. Sacca di Goro, la ‘Fabbrica’ delle Vongole: Un Paradiso da Preservare—Ordine Nazionale dei Biologi. 2018. Available online: https://iris.unife.it/bitstream/11392/2514210/2/tesi_digital_nofirme_AndreaBaldi.pdf (accessed on 15 December 2020).
- Nasa. Italy. 2022. Available online: https://visibleearth.nasa.gov/images/65788/italy (accessed on 17 December 2020).
- Parco del Delta del Po. Available online: http://www.parcodeltapo.it/it/index.php (accessed on 17 December 2020).
- Angonese, A.G. 2007. Available online: https://www3.diism.unisi.it/~paoletti/teaching/ModGestSistAmb/download/Monitoraggio_SaccaDiGoro.pdf (accessed on 17 December 2020).
- Lenzi, M.; Birardi, F. Confronto della Flora Marina Presente in Alcuni Ambienti di Transizione Italiani; Related Papers Flora and Veget a ion of the Italian Transitional Water Sysems the Lagoon of Orbetello; Academia: Florence, Italy, 2006. [Google Scholar]
- Andrea, P. L’alga Invade Ancora la Sacca di Goro La Nuova Ferrara. 2015. Available online: https://www.lanuovaferrara.it/ferrara/cronaca/2015/09/11/news/l-alga-invade-ancora-la-sacca-di-goro-1.12074080 (accessed on 20 December 2020).
- Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Nagendra Gandhi, N.; Renganathan, S. Production of algal biodiesel from marine macroalgae Enteromorpha compressa by two step process: Optimization and kinetic study. Bioresour. Technol. 2013, 128, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, B.; Brahmachari, K.; Ghosh, A.; Zodape, S.T. Effect of Seaweed Saps Derived from Two Marine Algae Kappaphycus and Gracilaria on Growth and Yield Improvement of Blackgram. Indian J. Geo-Mar. Sci. 2016, 45, 789–794. [Google Scholar]
- Yildiz, G.; Vatan, Ö.; Çelikler, S.; Dere, Ş. Determination of the phenolic compounds and antioxidative capacity in red algae Gracilaria bursa-pastoris. Int. J. Food Prop. 2011, 14, 496–502. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef]
- Hani Norziah, M.; Yen Ching, C. Nutritional Composition of Edible Seaweed Gracilaria Changgi. 1998. Available online: https://www.elsevier.com/locate/foodchem (accessed on 20 May 2021).
- Boardman, A.; Greenberg, D.; Vining, A.; Weimer, D. Cost-Benefit Analysis: Concepts and Practice. 1998. Available online: https://www.jstor.org/stable/30024403?casa_token=xVty-uS9nP4AAAAA%3A2eJdicVfv3Tm2BFHuM61UL2cA57tjkqaBuxGcgaF8cvSgsXI-HL7r4r9o0nHyyymw4Et3OypE9alZDxjuVrkNXn3ojmJlEDrMSOoou07LoIQxTJWpPw#metadata_info_tab_contents (accessed on 20 May 2021).
- Pearce, D.; Atkinson, G.; Mourato, S. Cost-Benefit Analysis and the Environment: Recent Developments; OECD: Paris, France, 2006; pp. 15–27. [Google Scholar]
- Hanley, N.; Barbier, E. Pricing Nature: Cost-Benefit Analysis and Environmental Policy; Edward Elgar: Cheltenham, UK, 2009. [Google Scholar]
- Prest, A.R.; Turvey, R. Cost-Benefit Analysis: A Survey. In Surveys of Economic Theory; Springer: Cham, Switzerland, 1966; pp. 155–207. [Google Scholar] [CrossRef]
- Perman. Natural Resource and Environmental Economics, 4th ed. 2003. Available online: https://www.pearson.com/uk/educators/higher-education-educators/program/Perman-Natural-Resource-and-Environmental-Economics-4th-Edition/PGM848461.html (accessed on 20 May 2021).
- Cariola, M.; Rolfo, S. Evolution in the rationales of foresight in Europe. Futures 2004, 36, 1063–1075. [Google Scholar] [CrossRef]
- Martin, B.R.; Johnston, R. Technology foresight for wiring up the national innovation system: Experiences in Britain, Australia, and New Zealand. Technol. Forecast. Soc. Change 1999, 60, 37–54. [Google Scholar] [CrossRef]
- Martin, B.R. Foresight in Science and Technology. Technol. Anal. Strateg. Manag. 1995, 7, 139–168. [Google Scholar] [CrossRef]
- Popper, R. Foresight Methodology. In Handbook of Technology Foresight: Concepts and Practice; Georghiou, L., Cassingena, H.J., Keenan, M., Miles, I., Popper, R., Eds.; Edward Elgar: Cheltenham, UK, 2008; pp. 44–88, 428. [Google Scholar]
- Georghiou, L. The UK technology foresight programme. Futures 1996, 28, 359–377. [Google Scholar] [CrossRef]
- Rialland, A.; Wold, K.E. Future Studies, Foresight and Scenarios as Basis for Better Strategic Decisions; Norwegian University of Science and Technology: Trondheim, Norway, 2009. [Google Scholar]
- Jain, A.; Ewurum, N. SWOT Analysis in Thirukkural: Comparative Analysis with Humphrey SWOT Matrix Related papers Foreign Direct Investment in Nigeria’s Commercial Real Estate Market: A SWOT Analysis Swot Analysis of Food-Equipment Importance through Processing and Use Of Refrigered Flour. IOSR J. 2015, 17, 17–20. [Google Scholar]
- Bison, G.O. Organizzazione Produttori Vongola di Goro|Edizioni Pubblicità Italia Srl. 2012. Available online: https://pubblicitaitalia.com/it/pesce/prodotti/il-pesce/2012/2/11664 (accessed on 30 May 2021).
- ANSA. Siccità: Delta Po; a Pesca di Alghe Contro Asfissia Vongole—Emilia-Romagna—ANSA.it. 2022. Available online: https://www.ansa.it/emiliaromagna/notizie/2022/07/17/siccita-delta-po-a-pesca-di-alghe-contro-asfissia-vongole_426128c0-c09d-4993-8e3e-c64c47490e1a.html (accessed on 10 June 2021).
- Mario, B. Ferrara, un Mare di Alghe Invade la Laguna: A Rischio gli Allevamenti di Vongole—Cronaca. 2022. Available online: https://www.ilrestodelcarlino.it/ferrara/cronaca/alghe-vongole-1.7818006 (accessed on 15 June 2021).
- Duffy, D.P. Landfill Economics: Getting Down to Business—Part 2|MSW Management. 2016. Available online: https://www.mswmanagement.com/landfills/article/13022732/landfill-economics-getting-down-to-business-part-2 (accessed on 1 July 2021).
- Nardelli, A.E.; Chiozzini, V.G.; Braga, E.S.; Chow, F. Integrated multi-trophic farming system between the green seaweed Ulva lactuca, mussel, and fish: A production and bioremediation solution. J. Appl. Phycol. 2019, 31, 847–856. [Google Scholar] [CrossRef]
- Coker, C. Composting Business Management: Capital Cost of Composting Facility Construction|BioCycle. 2020. Available online: https://www.biocycle.net/composting-business-management-capital-cost-composting-facility-construction/ (accessed on 3 July 2021).
- Troell, M.; Rönnbäck, P.; Halling, C.; Kautsky, N.; Buschmann, A. Ecological engineering in aquaculture: Use of seaweeds for removing nutrients from intensive mariculture. In Proceedings of the Sixteenth International Seaweed Symposium, Cebu City, Philippines, 12–17 April 1998; pp. 603–611. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, L.; Cheung, W.W.L.; Sumaila, U.R. Global estimates of suitable areas for marine algae farming. Environ. Res. Lett. 2023, 18, 064028. [Google Scholar] [CrossRef]
- Nazemi, F.; Karimi, K.; Denayer, J.F.M.; Shafiei, M. Techno-economic aspects of different process approaches based on brown macroalgae feedstock: A step toward commercialization of seaweed-based biorefineries. Algal Res. 2021, 58, 102366. [Google Scholar] [CrossRef]
- Davis, R.; Kinchin, C.; Markham, J.; Tan, E.; Laurens, L.; Sexton, D.; Knorr, D.; Schoen, P.; Lukas, J. Process Design and Economics for the Conversion of Algal Biomass to Biofuels: Algal Biomass Fractionation to Lipid- and Carbohydrate-Derived Fuel Products. 2013. Available online: https://www.nrel.gov/publications (accessed on 5 July 2021).
- Weitzman, M.L. Gamma discounting. Am. Econ. Rev. 2001, 91, 260–271. [Google Scholar] [CrossRef]
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van de Bund, W. Human pressures and ecological status of European rivers. Sci. Rep. 2017, 7, 205. [Google Scholar]
- Chaudhary, M.; Mishra, S.; Kumar, A. Estimation of water pollution and probability of health risk due to imbalanced nutrients in River Ganga, India. Int. J. River Basin Manag. 2017, 15, 53–60. [Google Scholar] [CrossRef]
- Rastogi, T.; Mahmoud, W.M.M.; Kümmerer, K. Human and veterinary drugs in the environment. In Encyclopedia of the Anthropocene; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1–5, pp. 263–268. [Google Scholar]
- Messina. Nel Ferrarese un Impianto a Biogas Alimentato ad Alghe—Corriere.it. 2013. Available online: https://www.corriere.it/ambiente/13_agosto_14/biogas-alghe-ferrara-goro_4581adb4-0420-11e3-b7de-a2b03b792de4.shtml (accessed on 2 September 2021).
- Valentini. A Ferrara il Primo Impianto a Biogas Alimentato con Alghe—ItaliaOggi.it. 2013. Available online: https://www.italiaoggi.it/archivio/a-ferrara-il-primo-impianto-a-biogas-alimentato-con-alghe-1838628 (accessed on 2 September 2021).
- Forti. La Centrale a Biogas di Goro fa Paura, si Profila una Marcia Indietro—Ferraraitalia.it—Quotidiano Glocal Indipendente. 2014. Available online: https://www.ferraraitalia.it/probabile-marcia-indietro-sulla-costruzione-della-centrale-biogas-di-goro-evidente-la-pericolosita-per-salute-e-ambiente-7977.html (accessed on 3 February 2022).
- HERAmbiente. Termovalorizzatore di Ferrara—HERAmbiente. 2022. Available online: https://ha.gruppohera.it/impianti/termovalorizzatori/ferrara (accessed on 1 April 2022).
- Dall’Oca. Biogas, a Goro Vincono i Cittadini. Stop Delle Istituzioni, la Centrale non si Farà—Il Fatto Quotidiano. 2014. Available online: https://www.ilfattoquotidiano.it/2014/04/12/biogas-a-goro-vincono-i-cittadini-dietrofront-delle-istituzioni-la-centrale-non-si-fara/948877/ (accessed on 1 March 2022).
- Margulies, P. Building Communities of Virtue: Political Theory, Land Use Policy, and the ‘Not in my Backyard’. Syracuse L. Rev. 1992, 43, 945. [Google Scholar]
- Wexler, M.N. A Sociological Framing of the Nimby (not-in-my-backyard) Syndrome. Int. Rev. Mod. Sociol. 1996, 26, 91–110. Available online: https://about.jstor.org/terms (accessed on 18 March 2022).
- Rasmussen, T.H. Not in My Backyard: The Politics of Siting Prisons, Landfills, and Incinerators. State Local Gov. Rev. 1992, 24, 128–134. Available online: https://www.jstor.org/stable/4355046 (accessed on 15 March 2022).
- Xu, M.; Lin, B. Exploring the “not in my backyard” effect in the construction of waste incineration power plants—Based on a survey in metropolises of China. Environ. Impact Assess. Rev. 2020, 82, 106377. [Google Scholar] [CrossRef]
- ANSA. Stop alla Vendita di Auto a Benzina e Diesel dal 2035—Attualità—ANSA.it. 2022. Available online: https://www.ansa.it/canale_motori/notizie/attualita/2022/06/08/stop-alla-vendita-di-auto-benzina-diesel-gpl-dal-2035-via-libera-dal-parlamento-europeo_32037239-8d4a-4a3c-98e9-0c933fb7e168.html (accessed on 20 March 2022).
- Mu, D.; Seager, T.P.; Rao, P.S.C.; Park, J.; Zhao, F. A resilience perspective on biofuel production. Integr. Environ. Assess. Manag. 2011, 7, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Show, P.L.; Lau, B.F.; Chang, J.S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Cruz-Suárez, L.E.; León, A.; Peña-Rodríguez, A.; Rodríguez-Peña, G.; Moll, B.; Ricque-Marie, D. Shrimp/Ulva co-culture: A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture 2010, 301, 64–68. [Google Scholar] [CrossRef]
- Kanno, K.; Fujita, Y.; Honda, S.; Takahashi, S.; Kato, S. Urethane foam of sulfated polysaccharide ulvan derived from green-tide-forming chlorophyta: Synthesis and application in the removal of heavy metal ions from aqueous solutions. Polym. J. 2014, 46, 813–818. [Google Scholar] [CrossRef]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Koçer, A.T.; Özçimen, D. Investigation of the biogas production potential from algal wastes. Waste Manag. Res. 2018, 36, 1100–1105. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef]
- el Harchi, M.; Fakihi Kachkach, F.Z.; el Mtili, N. Optimization of thermal acid hydrolysis for bioethanol production from Ulva rigida with yeast Pachysolen tannophilus. S. Afr. J. Bot. 2018, 115, 161–169. [Google Scholar] [CrossRef]
- Suganya, T.; Renganathan, S. Optimization and kinetic studies on algal oil extraction from marine macroalgae Ulva lactuca. Bioresour. Technol. 2012, 107, 319–326. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, J.; Sim, S.J.; Lee, J.H. Production of hydrogen from marine macro-algae biomass using anaerobic sewage sludge microflora. Biotechnol. Bioprocess. Eng. 2009, 14, 307–315. [Google Scholar] [CrossRef]
- Mhatre, A.; Gore, S.; Mhatre, A.; Trivedi, N.; Sharma, M.; Pandit, R.; Anil, A.; Lali, A. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renew. Energy 2019, 132, 742–751. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Bourret, E. Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae, Rhodophyta). Bioresour. Technol. 2003, 90, 329–333. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Marhaeni, B.; Oktaviani, D.F.; Jeong, G.T.; Hong, Y.K. Comparison of bioethanol production from cultivated versus wild Gracilaria verrucosa and Gracilaria gigas. J. Appl. Phycol. 2018, 30, 143–147. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef]
- Guimarães, M.; Viana, A.G.; Duarte, M.E.R.; Ascêncio, S.D.; Plastino, E.M.; Noseda, M.D. Low-molecular-mass carbohydrates and soluble polysaccharides of green and red morphs of Gracilaria domingensis (Gracilariales, Rhodophyta). Bot. Mar. 2007, 50, 314–317. [Google Scholar] [CrossRef]
- Habig, C.; Debusk, T.A.; Ryther, J.H. The Effect of Nitrogen Content on Methane Production by the Marine Algae Gracilaria tikvahiae and Ulva sp. Biomass 1984, 4, 239–251. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Q.; Liu, Z.; Wang, S.; Long, A.; Yang, Y. Potential bioremediation effects of seaweed Gracilaria lemaneiformis on heavy metals in coastal sediment from a typical mariculture zone. Chemosphere 2020, 245, 125636. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Arunkumar, K.; Carvalho, I.S. Antioxidant activity and lipid profile of three seaweeds of Faro, Portugal. Rev. Bras. Bot. 2016, 39, 9–17. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef]
- Homes & Communities Agency. Guidance on Dereliction, Demolition and Remediation Costs; Homes and Communities Agency: London, UK, 2015. [Google Scholar]
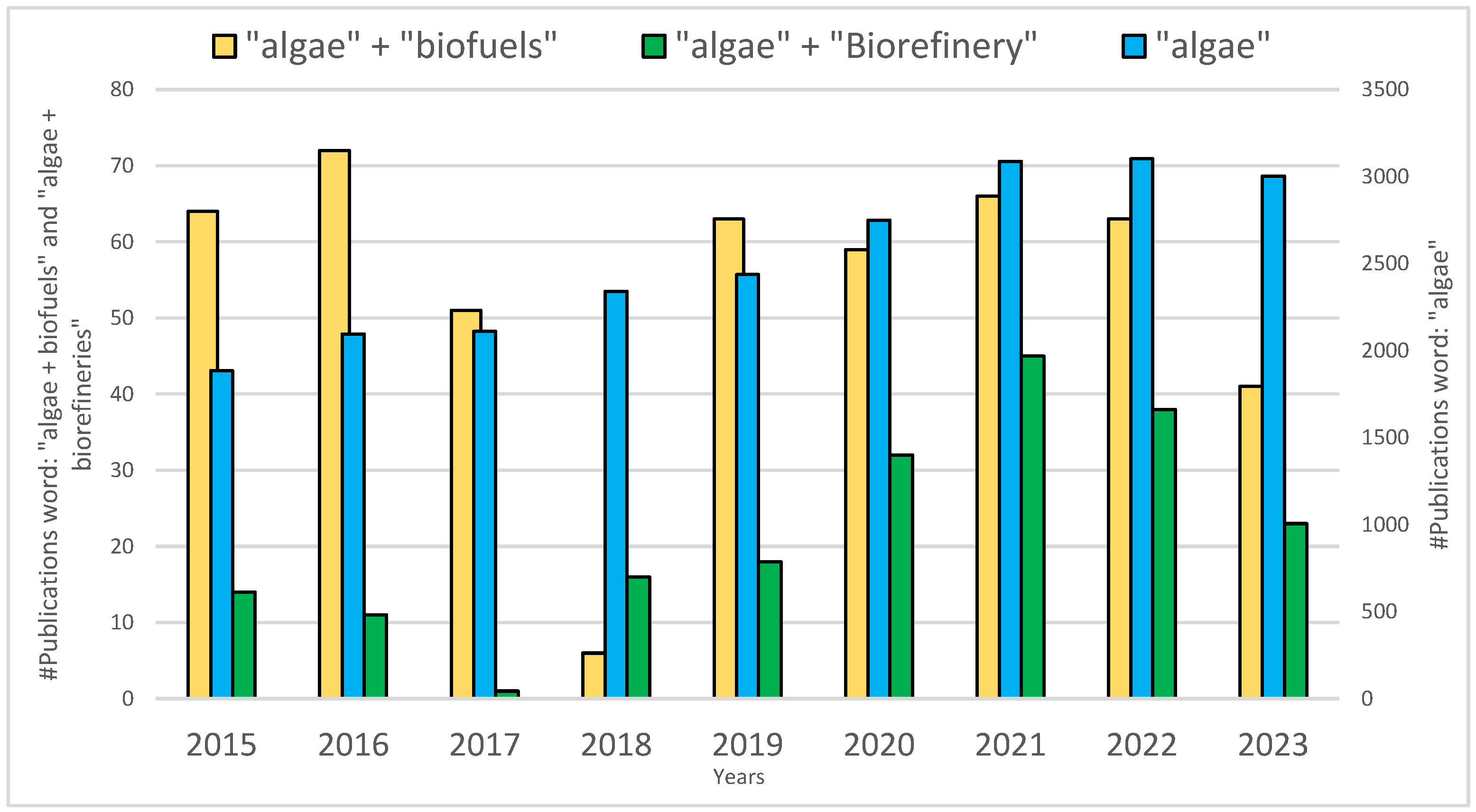
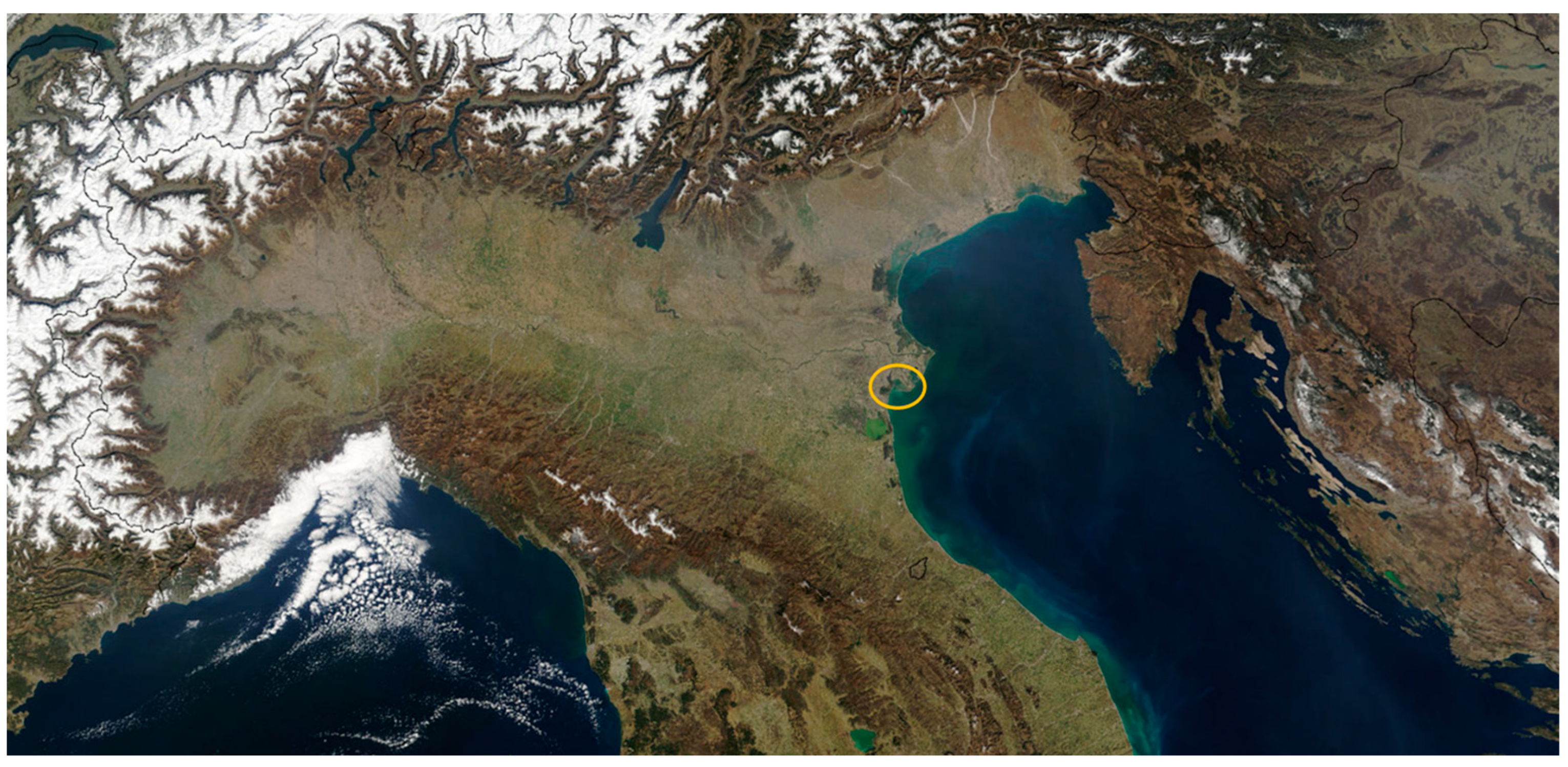
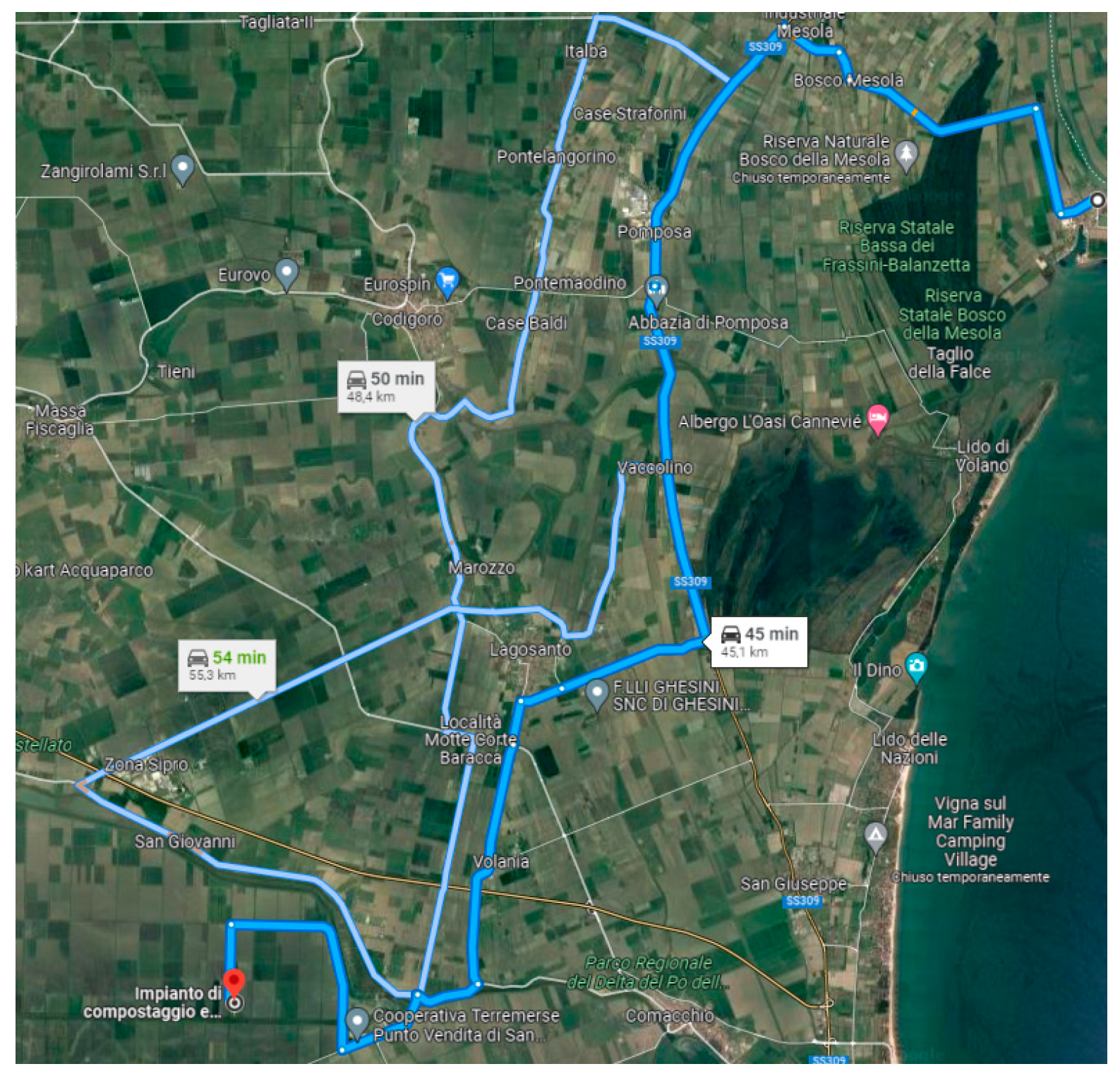
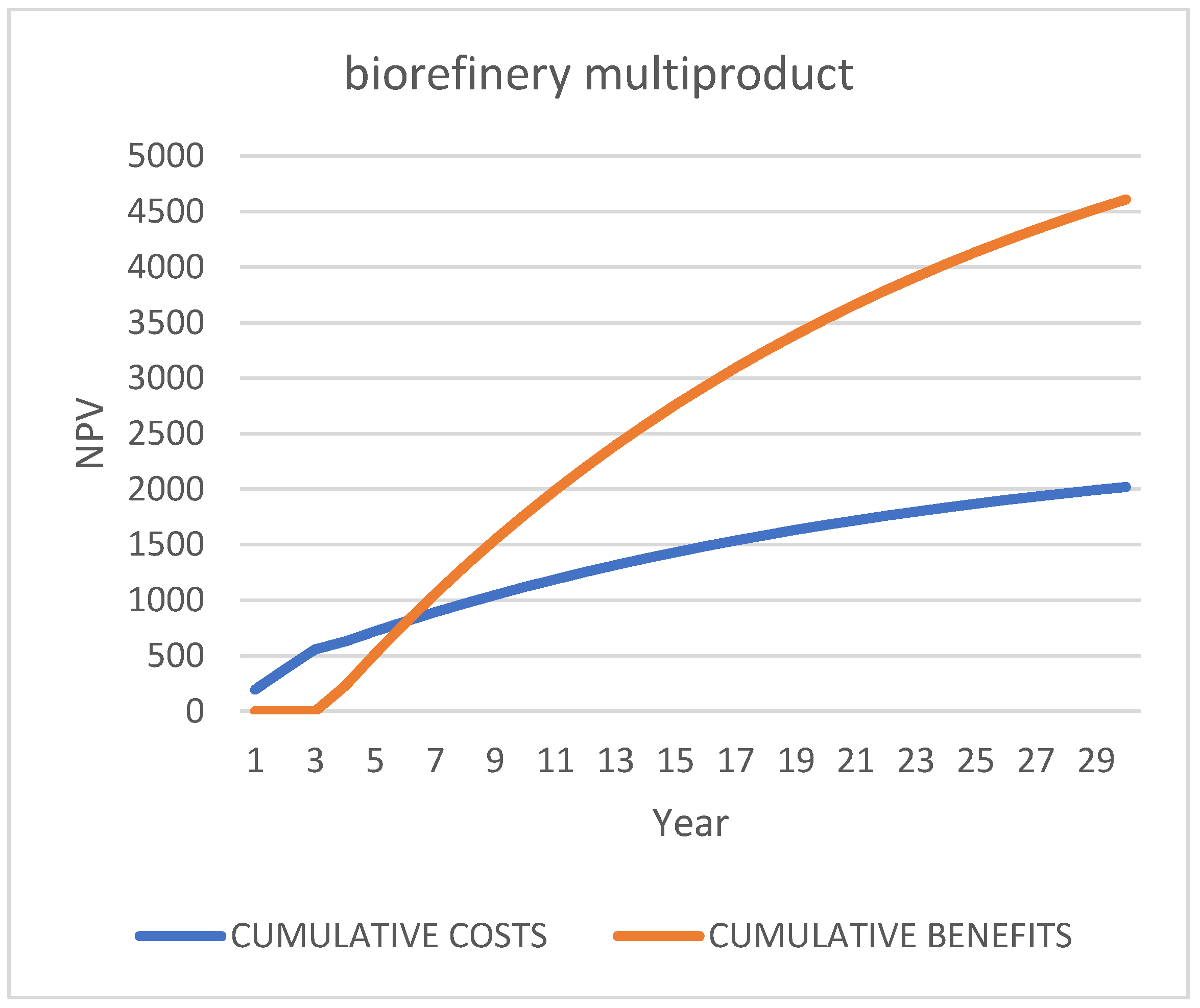


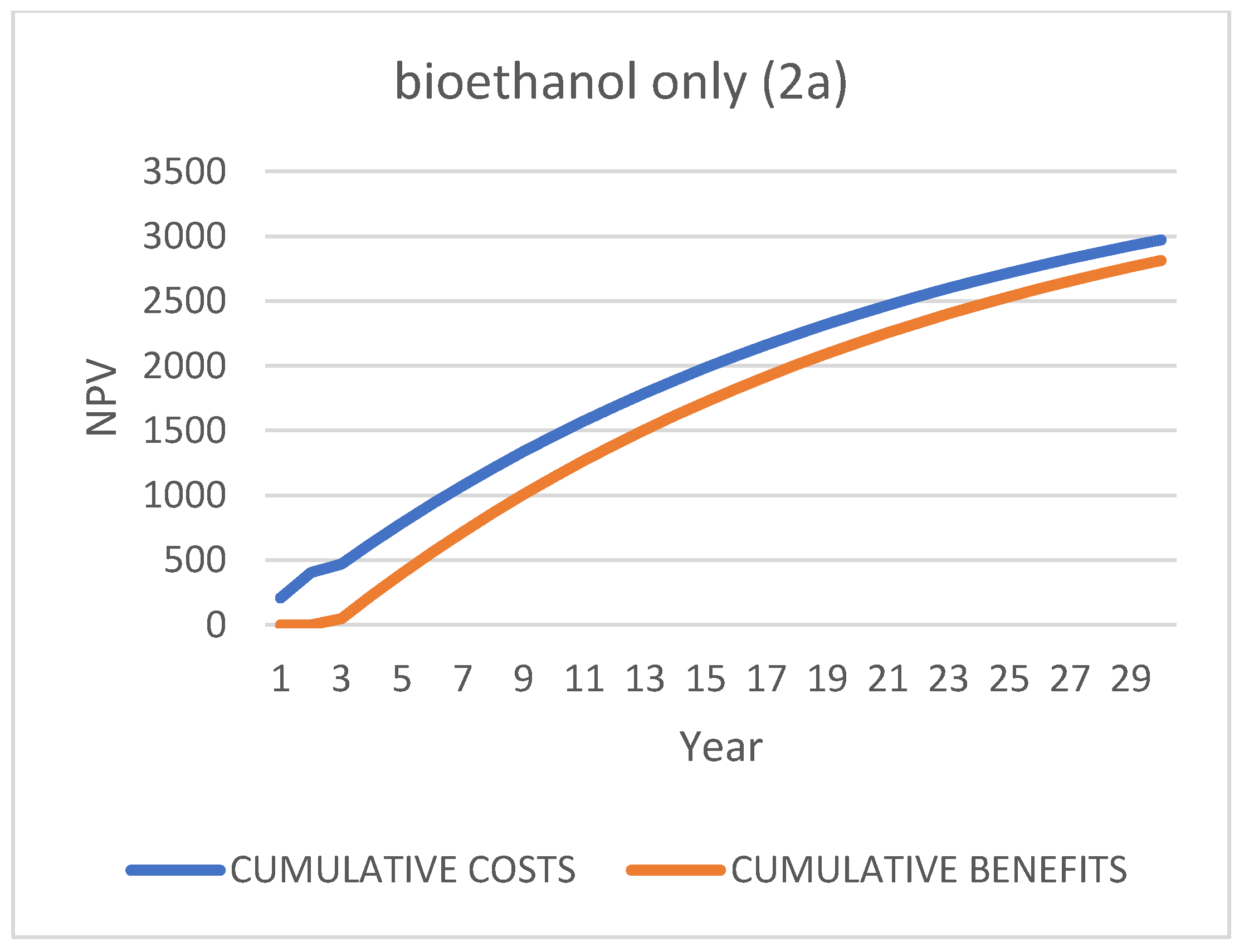
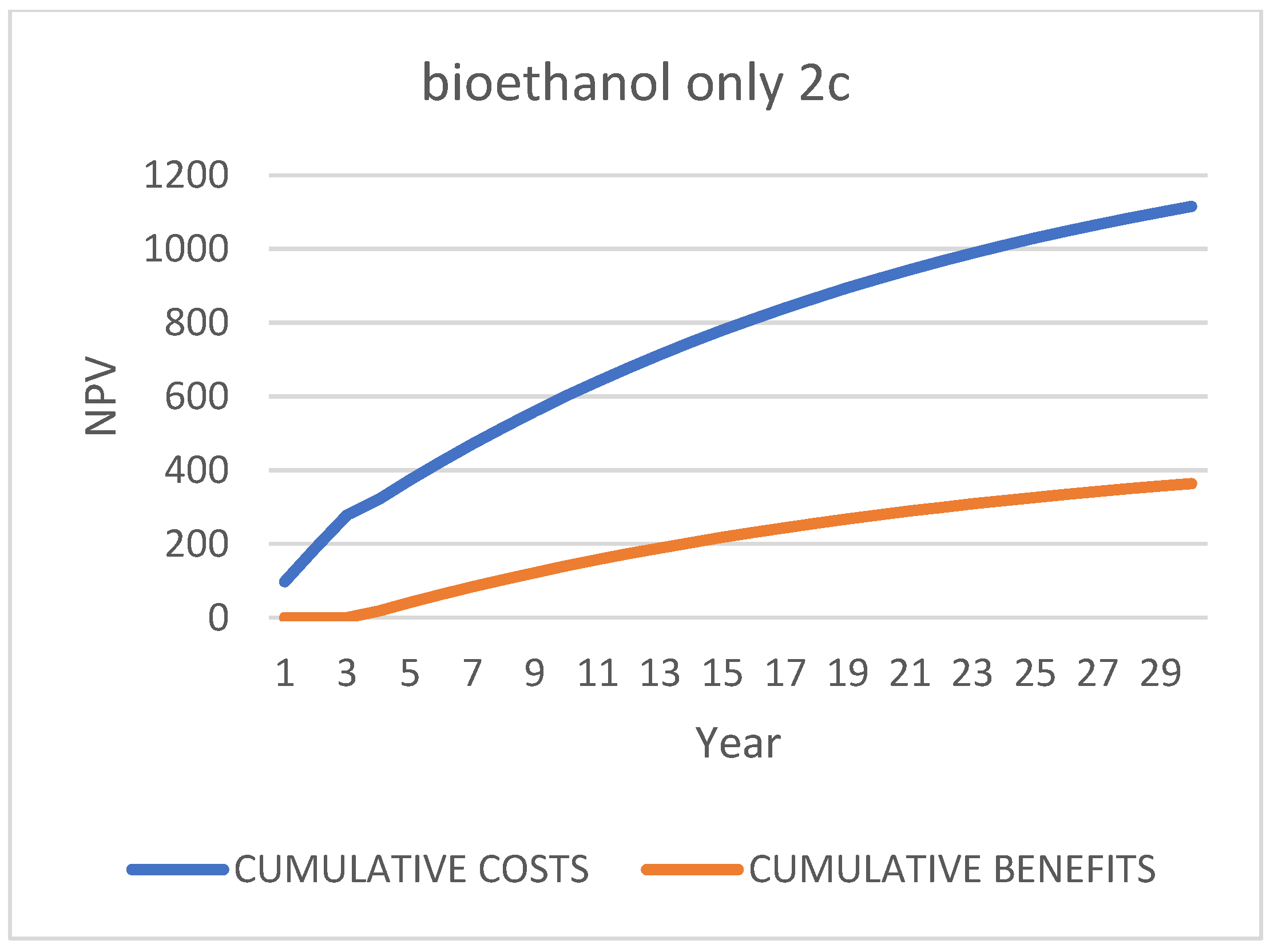

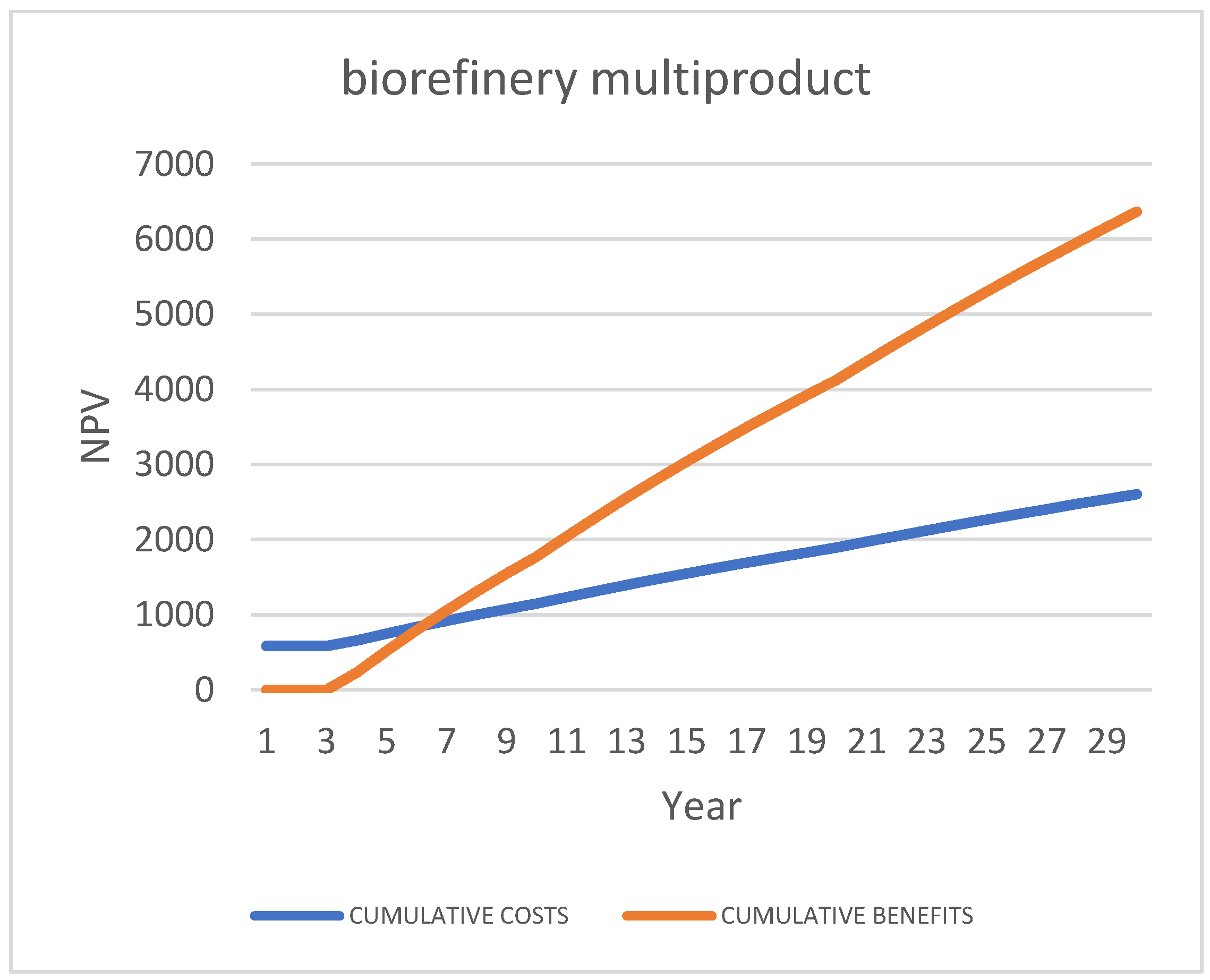
| Case 1: Do-Nothing (Status Quo): Bio-Stabilized for Landfill | ||||||
|---|---|---|---|---|---|---|
| Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| Biomass (tonn) | 912 | 95.44 | 19.74 | 71.94 | 0 | 120 |
| Maintenance cost | €32,150.00 | €14,280.00 | €19,600.00 | €15,600.00 | €0 | €36,750.00 |
| Sea-Transport Cost | €40,162.50 | €17,325.00 | €0 | €12,800.00 | €1800.00 | €10,800.00 |
| Land-Transport Cost | €14,350.00 | €1684.00 | €2800.00 | €1400.00 | €0 | €2100.00 |
| Disposal Cost (HERA) | €5000.00 | €5000.00 | €5000.00 | €1798.50 | €0 | €3003.50 |
| Total Expenditure Costs | €91,662.50 | €38,289.00 | €27,400.00 | €31,598.50 | €1800.00 | €52,653.50 |
| Discount Rate | NPV (Million Euros) | |
|---|---|---|
| Biofuel only | 0.50% | 10,572.75 |
| 1.00% | 9707.21 | |
| 3.00% | 7012.87 | |
| 5% | 5192.06 | |
| 10% | 2676.16 | |
| Biofuel + bioproduct 2.a | 0.50% | 63.89 |
| 1.00% | 25.57 | |
| 3.00% | −91.87 | |
| 5% | −168.65 | |
| 10% | −266.46 | |
| Biofuel + bioproduct 2.b | 0.50% | 1118.20 |
| 1.00% | 977.99 | |
| 3.00% | 532.37 | |
| 5% | 223.15 | |
| 10% | −211.01 | |
| Multiproduct biorefinery 2.c | 0.50% | −1240.05 |
| 1.00% | −1164.32 | |
| 3.00% | −927.37 | |
| 5% | −765.58 | |
| 10% | −536.55 | |
| Multiproduct biorefinery 2.d | 0.50% | 5605.41 |
| 1.00% | 5114.74 | |
| 3.00% | 3589.83 | |
| 5% | 2562.79 | |
| 10% | 1154.43 | |
| Bioproducts only | 0.50% | 3103.44 |
| 1.00% | 2873.61 | |
| 3.00% | 2155.87 | |
| 5% | 1667.65 | |
| 10% | 983.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, A.; Pronti, A.; Mazzanti, M.; Pasti, L. Exploitation of Waste Algal Biomass in Northern Italy: A Cost–Benefit Analysis. Pollutants 2024, 4, 393-423. https://doi.org/10.3390/pollutants4030027
Baldi A, Pronti A, Mazzanti M, Pasti L. Exploitation of Waste Algal Biomass in Northern Italy: A Cost–Benefit Analysis. Pollutants. 2024; 4(3):393-423. https://doi.org/10.3390/pollutants4030027
Chicago/Turabian StyleBaldi, Andrea, Andrea Pronti, Massimiliano Mazzanti, and Luisa Pasti. 2024. "Exploitation of Waste Algal Biomass in Northern Italy: A Cost–Benefit Analysis" Pollutants 4, no. 3: 393-423. https://doi.org/10.3390/pollutants4030027
APA StyleBaldi, A., Pronti, A., Mazzanti, M., & Pasti, L. (2024). Exploitation of Waste Algal Biomass in Northern Italy: A Cost–Benefit Analysis. Pollutants, 4(3), 393-423. https://doi.org/10.3390/pollutants4030027






