Mercury Intake Estimation in Adult Individuals from Trieste, Italy: Hair Mercury Assessment and Validation of a Newly Developed Food Frequency Questionnaire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Location
2.2. FFQ Development and Administration
2.3. Hg Intake Estimation from the FFQ
2.4. Hair Sampling and THg Measurement
2.5. Hg Intake Estimation from Hair THg
2.6. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Seafood Consumption
3.3. Exposure Assessment
3.3.1. FFQ
3.3.2. Hair Analysis
3.4. FFQ Validation
4. Discussion
4.1. FFQ Reliability
4.2. Hg Exposure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szefer, P. Safety assessment of seafood with respect to chemical pollutants in European Seas. Oceanol. Hydrobiol. Stud. 2013, 42, 110–118. [Google Scholar] [CrossRef]
- Cossa, D.; Knoery, J.; Bănaru, D.; Harmelin-Vivien, M.; Sonke, J.E.; Hedgecock, I.M.; Bravo, A.G.; Rosati, G.; Canu, D.; Horvat, M.; et al. Mediterranean Mercury Assessment 2022: An Updated Budget, Health Consequences, and Research Perspectives. Environ. Sci. Technol. 2022, 56, 3840–3862. [Google Scholar] [CrossRef] [PubMed]
- Storelli, A.; Barone, G.; Garofalo, R.; Busco, A.; Storelli, M.M. Determination of Mercury, Methylmercury and Selenium Concentrations in Elasmobranch Meat: Fish Consumption Safety. Int. J. Environ. Res. Public Health 2022, 19, 788. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef] [Green Version]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human Health and Ocean Pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef]
- Rice, D.C.; Schoeny, R.; Mahaffey, K. Methods and Rationale for Derivation of a Reference Dose for Methylmercury by the U.S. EPA. Risk Anal. 2003, 23, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Rice, D.C. The US EPA reference dose for methylmercury: Sources of uncertainty. Environ. Res. 2004, 95, 406–413. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Statement on the Benefits of Fish/Seafood Consumption Compared to the Risks of Methylmercury in Fish/Seafood. EFSA J. 2015, 13, 3982. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, G.; Abete, M.C.; Binato, G.; Chiaravalle, E.; Cossu, M.; Dellatte, E.; Miniero, R.; Orletti, R.; Piras, P.; Roncarati, A.; et al. Mercury occurrence in Italian seafood from the Mediterranean Sea and possible intake scenarios of the Italian coastal population. Regul. Toxicol. Pharmacol. 2013, 65, 269–277. [Google Scholar] [CrossRef]
- WHO Guidance for Identifying Populations at Risk from Mercury Exposure 2008. Available online: https://Wedocs.Unep.Org/Bitstream/Handle/20.500.11822/11786/IdentifyingPopnatRiskExposuretoMercury_2008Web.Pdf?Sequence=1&isAllowed=y (accessed on 18 March 2022).
- Berglund, M.; Lind, B.; Björnberg, K.A.; Palm, B.; Einarsson, Ö.; Vahter, M. Inter-individual variations of human mercury exposure biomarkers: A cross-sectional assessment. Environ. Health 2005, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Weihe, P.; Grandjean, P.; Debes, F.; White, R. Health implications for Faroe Islanders of heavy metals and PCBs from pilot whales. Sci. Total Environ. 1996, 186, 141–148. [Google Scholar] [CrossRef]
- Elhamri, H.; Idrissi, L.; Coquery, M.; Azemard, S.; El Abidi, A.; Benlemlih, M.; Saghi, M.; Cubadda, F. Hair mercury levels in relation to fish consumption in a community of the Moroccan Mediterranean coast. Food Addit. Contam. 2007, 24, 1236–1246. [Google Scholar] [CrossRef] [Green Version]
- Díez, S.; Montuori, P.; Pagano, A.; Sarnacchiaro, P.; Bayona, J.M.; Triassi, M. Hair mercury levels in an urban population from southern Italy: Fish consumption as a determinant of exposure. Environ. Int. 2008, 34, 162–167. [Google Scholar] [CrossRef]
- Endo, T.; Haraguchi, K. High mercury levels in hair samples from residents of Taiji, a Japanese whaling town. Mar. Pollut. Bull. 2010, 60, 743–747. [Google Scholar] [CrossRef]
- Tian, W.; Egeland, G.M.; Sobol, I.; Chan, H.M. Mercury hair concentrations and dietary exposure among Inuit preschool children in Nunavut, Canada. Environ. Int. 2011, 37, 42–48. [Google Scholar] [CrossRef]
- Fang, T.; Aronson, K.J.; Campbell, L.M. Freshwater Fish—Consumption Relations with Total Hair Mercury and Selenium Among Women in Eastern China. Arch. Environ. Contam. Toxicol. 2012, 62, 323–332. [Google Scholar] [CrossRef]
- Višnjevec, A.M.; Kocman, D.; Horvat, M. Human mercury exposure and effects in Europe. Environ. Toxicol. Chem. 2014, 33, 1259–1270. [Google Scholar] [CrossRef]
- Giangrosso, G.; Cammilleri, G.; Macaluso, A.; Vella, A.; D’orazio, N.; Graci, S.; Dico, G.M.L.; Galvano, F.; Giangrosso, M.; Ferrantelli, V. Hair Mercury Levels Detection in Fishermen from Sicily (Italy) by ICP-MS Method after Microwave-Assisted Digestion. Bioinorg. Chem. Appl. 2016, 2016, 5408014. [Google Scholar] [CrossRef] [Green Version]
- Okati, N.; Esmaili-Sari, A. Determination of Mercury Daily Intake and Hair-to-Blood Mercury Concentration Ratio in People Resident of the Coast of the Persian Gulf, Iran. Arch. Environ. Contam. Toxicol. 2017, 74, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Horvat, M.; Evers, D.C.; Zastenskaya, I.; Weihe, P.; Tempowski, J. A State-of-the-Science Review of Mercury Biomarkers in Human Populations Worldwide between 2000 and 2018. Environ. Health Perspect. 2018, 126, 106001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbone, F.; Rosolen, V.; Mariuz, M.; Parpinel, M.; Casetta, A.; Sammartano, F.; Ronfani, L.; Brumatti, L.V.; Bin, M.; Castriotta, L.; et al. Prenatal mercury exposure and child neurodevelopment outcomes at 18 months: Results from the Mediterranean PHIME cohort. Int. J. Hyg. Environ. Health 2019, 222, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-W.; Kim, B.-G.; Hong, Y.-S. The Relationship between Mercury Exposure Indices and Dietary Intake of Fish and Shellfish in Women of Childbearing Age. Int. J. Environ. Res. Public Health 2020, 17, 4907. [Google Scholar] [CrossRef] [PubMed]
- Sekovanić, A.; Piasek, M.; Orct, T.; Grgec, A.S.; Sarić, M.M.; Stasenko, S.; Jurasović, J. Mercury Exposure Assessment in Mother—Infant Pairs from Continental and Coastal Croatia. Biomolecules 2020, 10, 821. [Google Scholar] [CrossRef]
- Droghini, E.; Annibaldi, A.; Prezioso, E.; Tramontana, M.; Frapiccini, E.; De Marco, R.; Illuminati, S.; Truzzi, C.; Spagnoli, F. Mercury Content in Central and Southern Adriatic Sea Sediments in Relation to Seafloor Geochemistry and Sedimentology. Molecules 2019, 24, 4467. [Google Scholar] [CrossRef] [Green Version]
- EEA. Mercury in Europe’s Environment, a Priority for European and Global Action; European Environment Agency: Copenhagen, Denmark, 2018. [Google Scholar]
- Copat, C.; Conti, G.O.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy Metals in Fish from the Mediterranean Sea: Potential Impact on Diet. In The Mediterranean Diet An Evidence-Based Approach; Elsevier: Amsterdam, The Netherlands, 2015; pp. 547–562. ISBN 978-0-12-407849-9. [Google Scholar]
- Gallo, P.; De Carlo, E.; Marigliano, L.; Maglio, P.; Amato, A.; Improta, A.; Caruso, C.; De Roma, A. Food safety assessment of heavy metals in uncommon and abyssal fish and cephalopod from the Tyrrhenian Sea. J. Consum. Prot. Food Saf. 2018, 13, 399–402. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Manera, M.; Abete, M.C.; Gavinelli, S.; Amorena, M. Contamination of different portions of raw and boiled specimens of Norway lobster by mercury and selenium. Environ. Sci. Pollut. Res. 2013, 20, 8255–8262. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Manera, M.; Zaccaroni, A.; Olivieri, V.; Amorena, M. Heavy metal (As, Cd, Hg, Pb, Cu, Zn, Se) concentrations in muscle and bone of four commercial fish caught in the central Adriatic Sea, Italy. Environ. Monit. Assess. 2013, 186, 2205–2213. [Google Scholar] [CrossRef]
- Perugini, M.; Zezza, D.; Tulini, S.M.R.; Abete, M.C.; Monaco, G.; Conte, A.; Olivieri, V.; Amorena, M. Effect of cooking on total mercury content in Norway lobster and European hake and public health impact. Mar. Pollut. Bull. 2016, 109, 521–525. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G. Toxic Metals (Hg, Pb, and Cd) in Commercially Important Demersal Fish from Mediterranean Sea: Contamination Levels and Dietary Exposure Assessment: Toxic Metals in Fish. J. Food Sci. 2013, 78, T362–T366. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Caproni, R.; Orban, E. Total mercury levels in crustacean species from Italian fishery. Food Addit. Contam. Part B 2018, 11, 175–182. [Google Scholar] [CrossRef]
- De Giovanni, A.; Giuliani, C.; Marini, M.; Luiselli, D. Methylmercury and Polycyclic Aromatic Hydrocarbons in Mediterranean Seafood: A Molecular Anthropological Perspective. Appl. Sci. 2021, 11, 11179. [Google Scholar] [CrossRef]
- Hond, E.D.; Govarts, E.; Willems, H.; Smolders, R.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Seiwert, M.; Fiddicke, U.; Castaño, A.; et al. First Steps toward Harmonized Human Biomonitoring in Europe: Demonstration Project to Perform Human Biomonitoring on a European Scale. Environ. Health Perspect. 2015, 123, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Junqué, E.; Garí, M.; Llull, R.M.; Grimalt, J.O. Drivers of the accumulation of mercury and organochlorine pollutants in Mediterranean lean fish and dietary significance. Sci. Total. Environ. 2018, 634, 170–180. [Google Scholar] [CrossRef]
- Birgisdottir, B.; Kiely, M.; Martinez, J.; Thorsdottir, I. Validity of a food frequency questionnaire to assess intake of seafood in adults in three European countries. Food Control 2008, 19, 648–653. [Google Scholar] [CrossRef]
- Miklavčič, A.; Casetta, A.; Tratnik, J.S.; Mazej, D.; Krsnik, M.; Mariuz, M.; Sofianou, K.; Špirić, Z.; Barbone, F.; Horvat, M. Mercury, arsenic and selenium exposure levels in relation to fish consumption in the Mediterranean area. Environ. Res. 2013, 120, 7–17. [Google Scholar] [CrossRef]
- Woods, R.K.; Stoney, R.M.; Ireland, P.D.; Bailey, M.J.; Raven, J.M.; Thien, F.C.; Walters, E.H.; Abramson, M.J. A valid food frequency questionnaire for measuring dietary fish intake. Asia Pac. J. Clin. Nutr. 2002, 11, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, B.L.; Brown, J.; Williams, P.G.; Meyer, B.J. Dietary validation of a new Australian food-frequency questionnaire that estimates long-chain-n-3 polyunsaturated fatty acids. Br. J. Nutr. 2008, 99, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Filippini, T.; Malavolti, M.; Cilloni, S.; Wise, L.A.; Violi, F.; Malagoli, C.; Vescovi, L.; Vinceti, M. Intake of arsenic and mercury from fish and seafood in a Northern Italy community. Food Chem. Toxicol. 2018, 116, 20–26. [Google Scholar] [CrossRef]
- FAO. Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings; FAO: Roma, Italy, 2018; ISBN 978-92-5-130635-2. [Google Scholar]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a food frequency questionnaire for use in Italian adults living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, E.; Kyrkou, C.; Fotiou, M.; Tsakoumaki, F.; Dimitropoulou, A.; Polychroniadou, E.; Menexes, G.; Athanasiadis, A.P.; Biliaderis, C.G.; Michaelidou, A.-M. Development and Validation of a Mediterranean Oriented Culture-Specific Semi-Quantitative Food Frequency Questionnaire. Nutrients 2016, 8, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoun, C.; Daher, R.B.; El Osta, N.; Papazian, T.; Khabbaz, L.R. Reproducibility and relative validity of a food frequency questionnaire to assess dietary intake of adults living in a Mediterranean country. PLoS ONE 2019, 14, e0218541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garneau, V.; Rudkowska, I.; Paradis, A.-M.; Godin, G.; Julien, P.; Pérusse, L.; Vohl, M.-C. Omega-3 fatty acids status in human subjects estimated using a food frequency questionnaire and plasma phospholipids levels. Nutr. J. 2012, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Weaver, A.M.; Salazar, C.; Samet, J.M.; Diaz-Sanchez, D.; Tong, H. Validation of a Dietary Questionnaire to Screen Omega-3 Fatty Acids Levels in Healthy Adults. Nutrients 2019, 11, 1470. [Google Scholar] [CrossRef] [Green Version]
- Cinnirella, S.; Bruno, D.E.; Pirrone, N.; Horvat, M.; Živković, I.; Evers, D.C.; Johnson, S.; Sunderland, E.M. Mercury concentrations in biota in the Mediterranean Sea, a compilation of 40 years of surveys. Sci. Data 2019, 6, 205. [Google Scholar] [CrossRef] [Green Version]
- Bajt, O.; Ramšak, A.; Milun, V.; Andral, B.; Romanelli, G.; Scarpato, A.; Mitrić, M.; Kupusović, T.; Kljajić, Z.; Angelidis, M.; et al. Assessing chemical contamination in the coastal waters of the Adriatic Sea using active mussel biomonitoring with Mytilus galloprovincialis. Mar. Pollut. Bull. 2019, 141, 283–298. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Storelli, A.; D’Addabbo, R.; Palermo, C.; Marcotrigiano, G.O. Survey of total mercury and methylmercury levels in edible fish from the Adriatic Sea. Food Addit. Contam. 2003, 20, 1114–1119. [Google Scholar] [CrossRef]
- Jureša, D.; Blanuša, M. Mercury, arsenic, lead and cadmium in fish and shellfish from the Adriatic Sea. Food Addit. Contam. 2003, 20, 241–246. [Google Scholar] [CrossRef]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Caló, M.; Naccari, F. Heavy Metals in Liver and Muscle of Bluefin Tuna (Thunnus thynnus) Caught in the Straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Relationship between Total Mercury Concentration and Fish Size in Two Pelagic Fish Species: Implications for Consumer Health. J. Food Prot. 2006, 69, 1402–1405. [Google Scholar] [CrossRef]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Manera, M.; Zaccaroni, A.; Olivieri, V.; Amorena, M. Levels of Total Mercury in Marine Organisms from Adriatic Sea, Italy. Bull. Environ. Contam. Toxicol. 2009, 83, 244–248. [Google Scholar] [CrossRef]
- Conti, G.O.; Copat, C.; Ledda, C.; Fiore, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Evaluation of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Mullus barbatus from Sicily Channel and Risk-Based Consumption Limits. Bull. Environ. Contam. Toxicol. 2012, 88, 946–950. [Google Scholar] [CrossRef]
- Praticò, V.; Normanno, G.; Dambrosio, A.; Anaclerio, D.; Serpe, L.; Gallo, P.; Federico, G.; Borruto, S. Heavy Metals Contamination in Fish Caught in the Strait of Messina. Ind. Aliment. 2013, 52, 12–16. [Google Scholar]
- Miniero, R.; Beccaloni, E.; Carere, M.; Ubaldi, A.; Mancini, L.; Marchegiani, S.; Cicero, M.; Scenati, R.; Lucchetti, D.; Ziemacki, G.; et al. Mercury (Hg) and methyl mercury (MeHg) concentrations in fish from the coastal lagoon of Orbetello, central Italy. Mar. Pollut. Bull. 2013, 76, 365–369. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G.; Perrone, V.G.; Storelli, A. Risk characterization for polycyclic aromatic hydrocarbons and toxic metals associated with fish consumption. J. Food Compos. Anal. 2013, 31, 115–119. [Google Scholar] [CrossRef]
- Copat, C.; Vinceti, M.; D’Agati, M.G.; Arena, G.; Mauceri, V.; Grasso, A.; Fallico, R.; Sciacca, S.; Ferrante, M. Mercury and selenium intake by seafood from the Ionian Sea: A risk evaluation. Ecotoxicol. Environ. Saf. 2014, 100, 87–92. [Google Scholar] [CrossRef]
- Conte, F.; Copat, C.; Longo, S.; Conti, G.O.; Grasso, A.; Arena, G.; Brundo, M.V.; Ferrante, M. First data on trace elements in Haliotis tuberculata (Linnaeus, 1758) from southern Italy: Safety issues. Food Chem. Toxicol. 2015, 81, 143–150. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Garofalo, R.; Busco, V.P.; Quaglia, N.C.; Centrone, G.; Storelli, M.M. Assessment of mercury and cadmium via seafood consumption in Italy: Estimated dietary intake (EWI) and target hazard quotient (THQ). Food Addit. Contam. Part A 2015, 32, 1277–1286. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Caproni, R.; Fusari, A.; Orban, E. Total mercury levels in commercial fish species from Italian fishery and aquaculture. Food Addit. Contam. Part B 2017, 10, 118–127. [Google Scholar] [CrossRef]
- Signa, G.; Mazzola, A.; Tramati, C.D.; Vizzini, S. Diet and habitat use influence Hg and Cd transfer to fish and consequent biomagnification in a highly contaminated area: Augusta Bay (Mediterranean Sea). Environ. Pollut. 2017, 230, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, M.; Franzellitti, S.; Kiwan, A.; Valbonesi, P.; Dinelli, E.; Pignotti, E.; Birke, M.; Fabbri, E. A comprehensive evaluation of the environmental quality of a coastal lagoon (Ravenna, Italy): Integrating chemical and physiological analyses in mussels as a biomonitoring strategy. Sci. Total. Environ. 2017, 598, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Bajc, Z.; Kirbiš, A. Trace Element Concentrations in Mussels (Mytilus galloprovincialis) from the Gulf of Trieste, Slovenia. J. Food Prot. 2019, 82, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Ariano, A.; Marrone, R.; Andreini, R.; Smaldone, G.; Velotto, S.; Montagnaro, S.; Anastasio, A.; Severino, L. Metal Concentration in Muscle and Digestive Gland of Common Octopus (Octopus vulgaris) from Two Coastal Site in Southern Tyrrhenian Sea (Italy). Molecules 2019, 24, 2401. [Google Scholar] [CrossRef] [Green Version]
- Arienzo, M.; Toscanesi, M.; Trifuoggi, M.; Ferrara, L.; Stanislao, C.; Donadio, C.; Grazia, V.; Gionata, D.V.; Carella, F. Contaminants bioaccumulation and pathological assessment in Mytilus galloprovincialis in coastal waters facing the brownfield site of Bagnoli, Italy. Mar. Pollut. Bull. 2019, 140, 341–352. [Google Scholar] [CrossRef]
- Ancora, S.; Mariotti, G.; Ponchia, R.; Fossi, M.C.; Leonzio, C.; Bianchi, N. Trace elements levels in muscle and liver of a rarely investigated large pelagic fish: The Mediterranean spearfish Tetrapturus belone (Rafinesque, 1810). Mar. Pollut. Bull. 2020, 151, 110878. [Google Scholar] [CrossRef]
- La Torre, G.L.; Cicero, N.; Bartolomeo, G.; Rando, R.; Vadalà, R.; Santini, A.; Durazzo, A.; Lucarini, M.; Dugo, G.; Salvo, A. Assessment and Monitoring of Fish Quality from a Coastal Ecosystem under High Anthropic Pressure: A Case Study in Southern Italy. Int. J. Environ. Res. Public Health 2020, 17, 3285. [Google Scholar] [CrossRef]
- Grgec, A.S.; Kljaković-Gašpić, Z.; Orct, T.; Tičina, V.; Sekovanić, A.; Jurasović, J.; Piasek, M. Mercury and selenium in fish from the eastern part of the Adriatic Sea: A risk-benefit assessment in vulnerable population groups. Chemosphere 2020, 261, 127742. [Google Scholar] [CrossRef]
- Ramon, D.; Morick, D.; Croot, P.; Berzak, R.; Scheinin, A.; Tchernov, D.; Davidovich, N.; Britzi, M. A survey of arsenic, mercury, cadmium, and lead residues in seafood (fish, crustaceans, and cephalopods) from the south-eastern Mediterranean Sea. J. Food Sci. 2021, 86, 1153–1161. [Google Scholar] [CrossRef]
- EEA Commission Regulation (EC) No 1881/2006 of 19 December 2006. Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 30 June 2023).
- Vicent, A.; Cervera, M.; Morales-Rubio, A. Fast and accurate methodology for direct mercury determination in hair and nails. Sample Prep. 2016, 2, 91–98. [Google Scholar] [CrossRef]
- Legrand, M.; Passos, C.J.S.; Mergler, D.; Chan, H.M. Biomonitoring of Mercury Exposure with Single Human Hair Strand. Environ. Sci. Technol. 2005, 39, 4594–4598. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Kalra, A. Decoding the Bland–Altman plot: Basic review. J. Pract. Cardiovasc. Sci. 2017, 3, 36. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Frey, M.E.; Petersen, H.C.; Gerke, O. Nonparametric Limits of Agreement for Small to Moderate Sample Sizes: A Simulation Study. Stats 2020, 3, 343–355. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- US EPA. Water Quality Criterion for the Protection of Human Health: Methylmercury Chapter 4: Risk Assessment for Methylmercury. Office of Science and Technology, Office of Water. 2001. Available online: https://clu-in.org/Download/Contaminantfocus/Mercury/Water-Quality-Criterion-Methyl-Mercury.Pdf (accessed on 6 April 2022).
- Joint FAO/WHO Expert Committee on Food Additives. Meeting (67th: 2006: Geneva, S.; International Programme on Chemical Safety. Evaluation of Certain Food Additives and Contaminants. Prepared by the Sixty-Seventh Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA). 2007. Available online: https://apps.who.int/iris/handle/10665/43592 (accessed on 30 June 2023).
- Schulz, C.; Angerer, J.; Ewers, U.; Kolossa-Gehring, M. The German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health 2007, 210, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Barbone, F.; Valent, F.; Pisa, F.; Daris, F.; Fajon, V.; Gibicar, D.; Logar, M.; Horvat, M. Prenatal low-level methyl mercury exposure and child development in an Italian coastal area. Neurotoxicology 2020, 81, 376–381. [Google Scholar] [CrossRef] [PubMed]

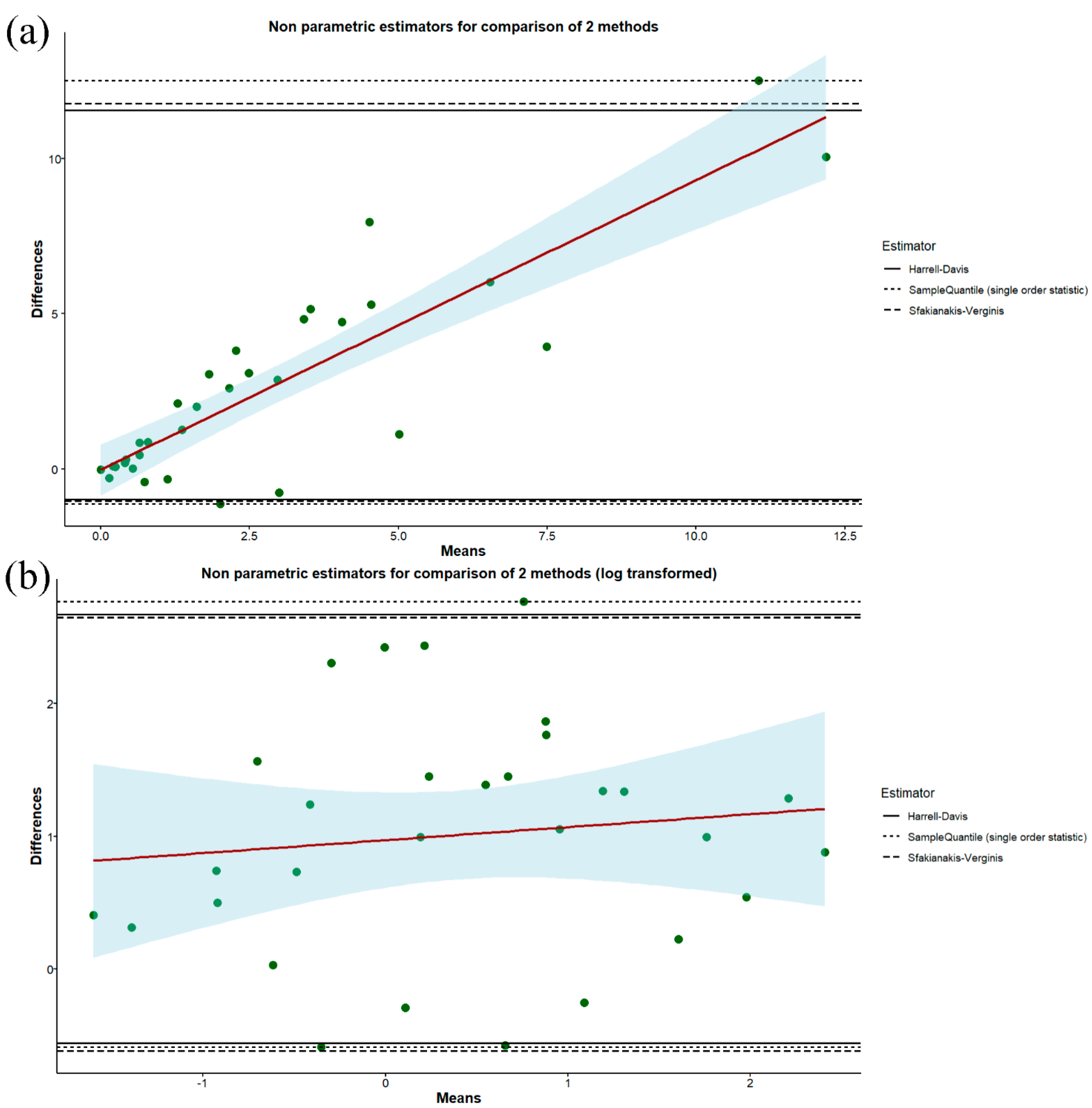
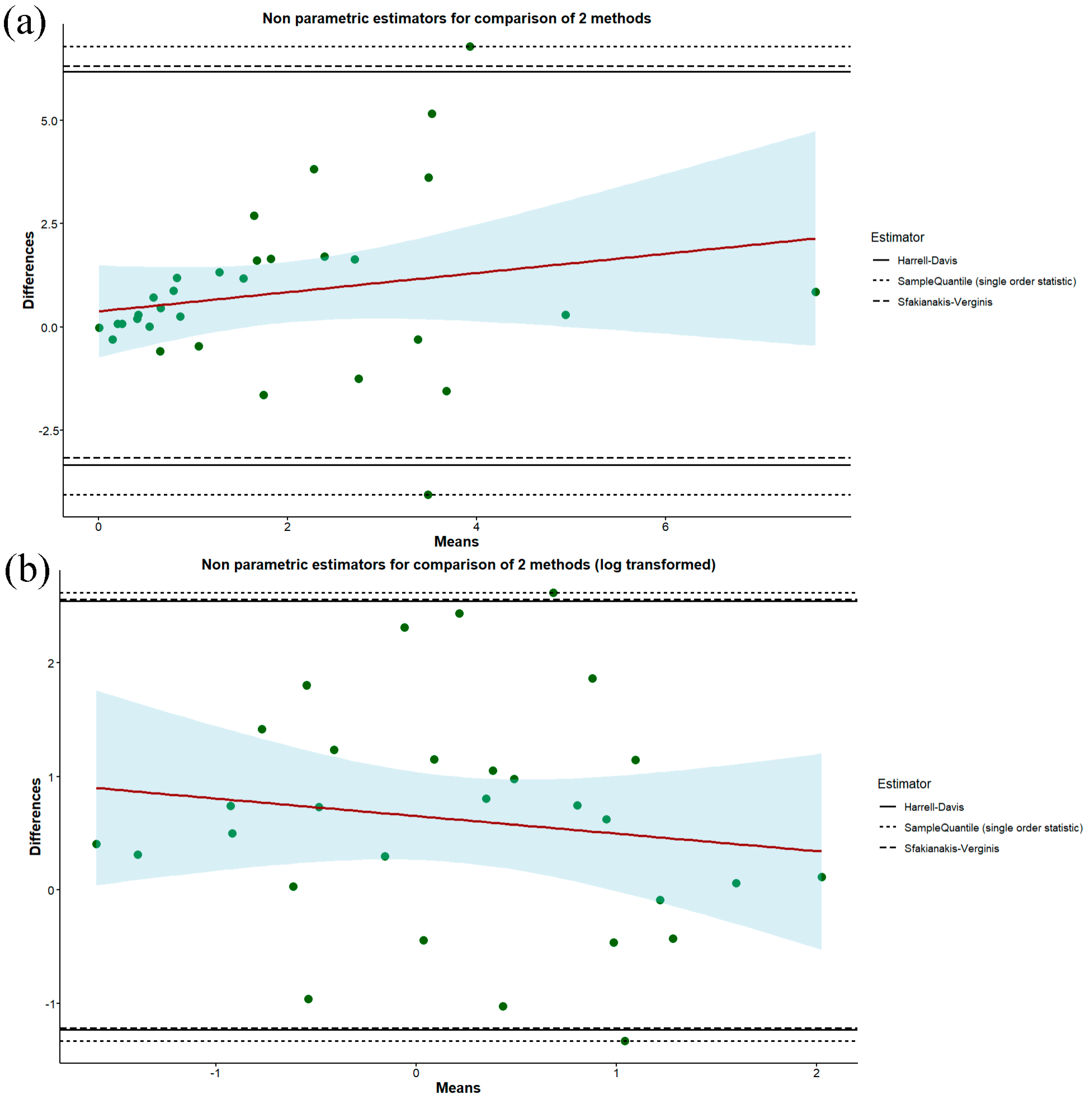
| Variables | Male Participants (n = 13) | Female Participants (n = 19) | Overall (n = 32) |
|---|---|---|---|
| Mean age ± SD (median, range) | 35 ± 10.90 (32, 21–55) | 48 ± 11.63 (51, 20–65) | 42.81 ± 12.82 (45.50, 20–65) |
| Current smokers | 7 | 2 | 9 |
| Dental amalgam fillings | 1 | 5 | 6 |
| Mean number of seafood servings (times a week) | 1.32 | 1.25 | 1.28 |
| Mean Hg w.i. 1 from n.f. 2 FFQ (μg kg−1 b.w.) ± SD (median, range) | 4.03 ± 3.13 (3.46, 0.52–9.55) | 4.11 ± 5.28 (1.99, 0.00–17.64) | 4.08 ± 4.47 (2.62, 0.00–17.21) |
| Mean Hg w.i. 1 from f. 3 FFQ (μg kg−1 b.w.) ± SD (median, range) | 1.98 ± 1.08 (2.12, 0.36–3.53) | 2.57 ± 2.63 (1.23, 0.00–8.01) | 2.33 ± 2.13 (1.70, 0.00–8.00) |
| Mean hair THg (mg kg−1) ± SD (median, range) | 2.91 ± 2.16 (2.07, 0.72–7.43) | 1.42 ± 0.48 (0.48, 0.02–9.63) | 2.02 ± 2.42 (1.08, 0.01–9.63) |
| Mean Hg w.i. 1 from hair THg (μg kg−1 b.w.) ± SD (median, range) | 2.16 ± 1.61 (1.54, 0.53–5.52) | 1.05 ± 1.82 (0.36, 0.01–7.15) | 1.50 ± 1.80 (0.80, 0.01–7.15) |
| ρ (p-Value) | Cohen’s Weighted Kappa | |
|---|---|---|
| Hg w.i. 1 from n.f. 2 FFQ—Hg w.i. 1 from hair THg | 0.76 (5.54 × 10−7) | 0.69 |
| Hg w.i. 1 from f. 3 FFQ—Hg w.i. 1 from hair THg | 0.61 (1.90 × 10−4) | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giovanni, A.; Iannuzzi, V.; Gallello, G.; Giuliani, C.; Marini, M.; Cervera, M.L.; Luiselli, D. Mercury Intake Estimation in Adult Individuals from Trieste, Italy: Hair Mercury Assessment and Validation of a Newly Developed Food Frequency Questionnaire. Pollutants 2023, 3, 320-336. https://doi.org/10.3390/pollutants3030022
De Giovanni A, Iannuzzi V, Gallello G, Giuliani C, Marini M, Cervera ML, Luiselli D. Mercury Intake Estimation in Adult Individuals from Trieste, Italy: Hair Mercury Assessment and Validation of a Newly Developed Food Frequency Questionnaire. Pollutants. 2023; 3(3):320-336. https://doi.org/10.3390/pollutants3030022
Chicago/Turabian StyleDe Giovanni, Andrea, Vincenzo Iannuzzi, Gianni Gallello, Cristina Giuliani, Mauro Marini, M. Luisa Cervera, and Donata Luiselli. 2023. "Mercury Intake Estimation in Adult Individuals from Trieste, Italy: Hair Mercury Assessment and Validation of a Newly Developed Food Frequency Questionnaire" Pollutants 3, no. 3: 320-336. https://doi.org/10.3390/pollutants3030022
APA StyleDe Giovanni, A., Iannuzzi, V., Gallello, G., Giuliani, C., Marini, M., Cervera, M. L., & Luiselli, D. (2023). Mercury Intake Estimation in Adult Individuals from Trieste, Italy: Hair Mercury Assessment and Validation of a Newly Developed Food Frequency Questionnaire. Pollutants, 3(3), 320-336. https://doi.org/10.3390/pollutants3030022










