1. Introduction
Many efforts and researches have been conducted on the valorization of bauxite residue (BR), with the most successful—concerning industrial application—being in clinker production, in road construction and in iron/steel production. However, this does not exceed 3% of its annual production, which is around 150 million tons [
1]. With many years of research and hundreds of publications and equal patents, this number is insignificant and an immediate solution should be found. The process that will deal with the BR challenge should engage the main obstacles of its smooth utilization—quality diversity, complex mineralogy and logistics [
1,
2]—and counter them. Moreover, with technology evolving rapidly a novel process should follow the trends and directions in industry, and be, of course, as eco-friendly as possible. To this direction, lower temperature and less polluting agents should be applied.
The presented combinatory processes attempt to deal with the BR storage capacity while being greener and employing the latest trends in industry, specifically utilizing the capabilities of H
2 gas [
3,
4]. Moreover, recovery of both Al and Fe combined through one process, is more cost-effective [
5].
The concept of combining BR with NaOH under roasting was introduced by Borra et al. [
6], and proved that a low temperature process—more than 400 °C but less than 900 °C—is enough to create water soluble Na-aluminates and subsequently recover a significant fraction of the Al using water leaching. Our study elaborates on this concept: in combination with H
2 gas, which is also effective at lower temperature (<900 °C), both Al and Fe recovery is targeted.
2. Materials and Methods
For the experiments Greek BR was provided by Mytilineos S.A., Aluminium of Greece, after it was filter-pressed and dried. It was mixed with a NaOH solution—NaOH pellets of Sigma Aldrich—of 8.75 M in a 100 mL volume stainless steel crucible. The crucible was placed in a box furnace that was initially purged with N2 gas to eliminate the air. Immediately when the target temperature was reached, the gas was switched to pure H2 with a flow of around 30 L/h. The duration of the thermal treatment was two hours at 600 °C.
Subsequently, the thermal product was milled with a mortar and pestle. For the first two processes, it was introduced to water for water leaching at 60 °C for one hour. As a result, an Al and Na ion rich liquid (leachate or pregnant solution) and a Fe rich solid residue arose. The latter was in one case imposed to dry magnetic separation with a NdFeB magnet 2 × 2 × 1 inches, and in another case to melting at 1550 °C for 30 min.
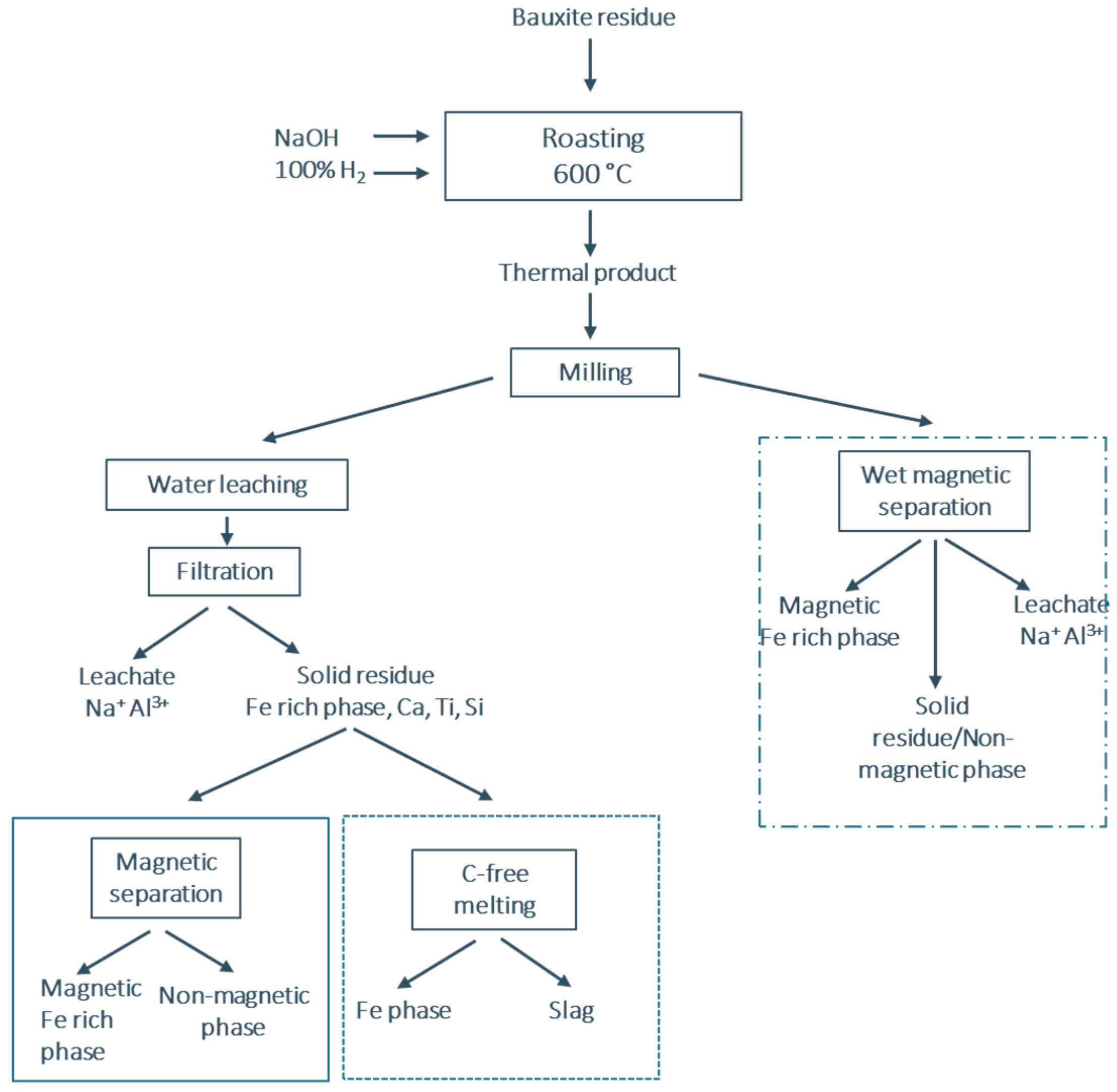
The third process included, after the thermal treatment, a wet magnetic separation to combine the dissolution of the water-soluble aluminates with the recovery of Fe on the magnet. This resulted immediately in three products: the Al, Na ion-rich liquid (leachate or pregnant solution), the Fe-rich phase (on the magnet) and the non-magnetic phase which was recovered during the filtration of the liquid. The processes are presented in
Figure 1. For the chemical analysis and the characterization of the individual products in bulk and micro-scale WDXRF and ICP–OES along with powder XRD and SEM-EDS were applied. In particular, a Bruker S8 Tiger equipment was used for the WDXRF, whilst the mineralogical composition (XRD) was determined using a Bruker D2 Focus with Cu-Ka radiation, 10° to 50° diffraction angle and 0.02 step size. For the characterization on a micro-scale, the products were firstly embedded in epoxy resin, grinded with SiC paper up to 4000 grit size and polished with oil-based solution from 1 to 3 μm. Then a Philips XL30 FEG scanning electron microscope was utilized, and for the energy-dispersive X-ray spectroscopy (EDX) analysis an Ametek, EDAX, Apollo SDD 10 mm
2 detector was used. A Varian 720 ES axial ICP-OES instrument was used to provide analysis of the liquid products, after they were proper diluted. Moreover, the use of duplicates, blanks and standards provided accuracy and precision. Lastly, for particle size distribution measurements with laser particle analysis a Beckman Coulter LS was used.
3. Results
3.1. Bauxite Residue
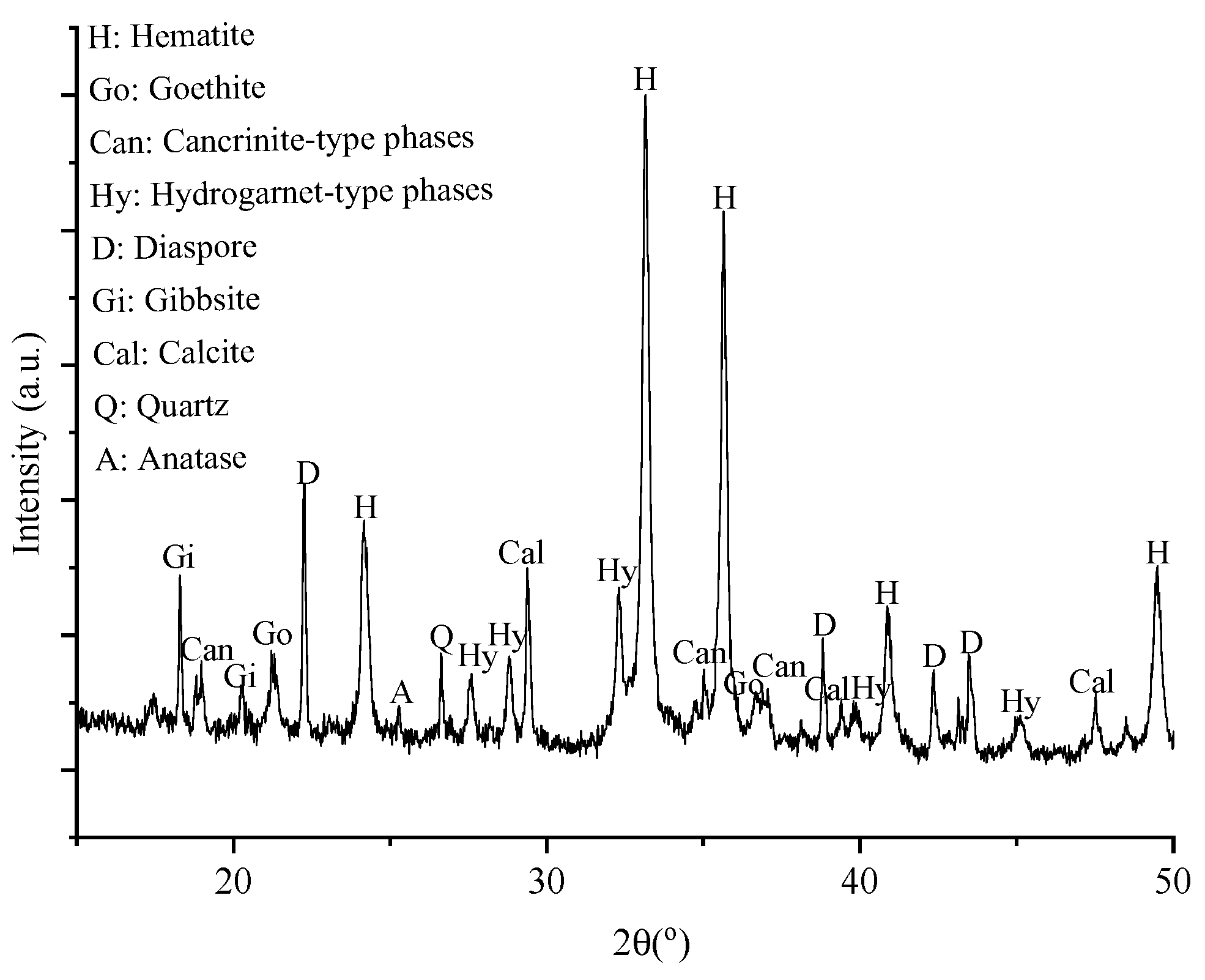
The chemical and mineralogical composition of the applied BR are shown in
Table 1 and
Figure 2. It consists mainly of iron oxide in the form of hematite and Al-bearing phases, for instance cancrinite and hydrogarnet or Al-hydroxides, like diaspore.
3.2. Thermal Treatment (Common for all the Processes)
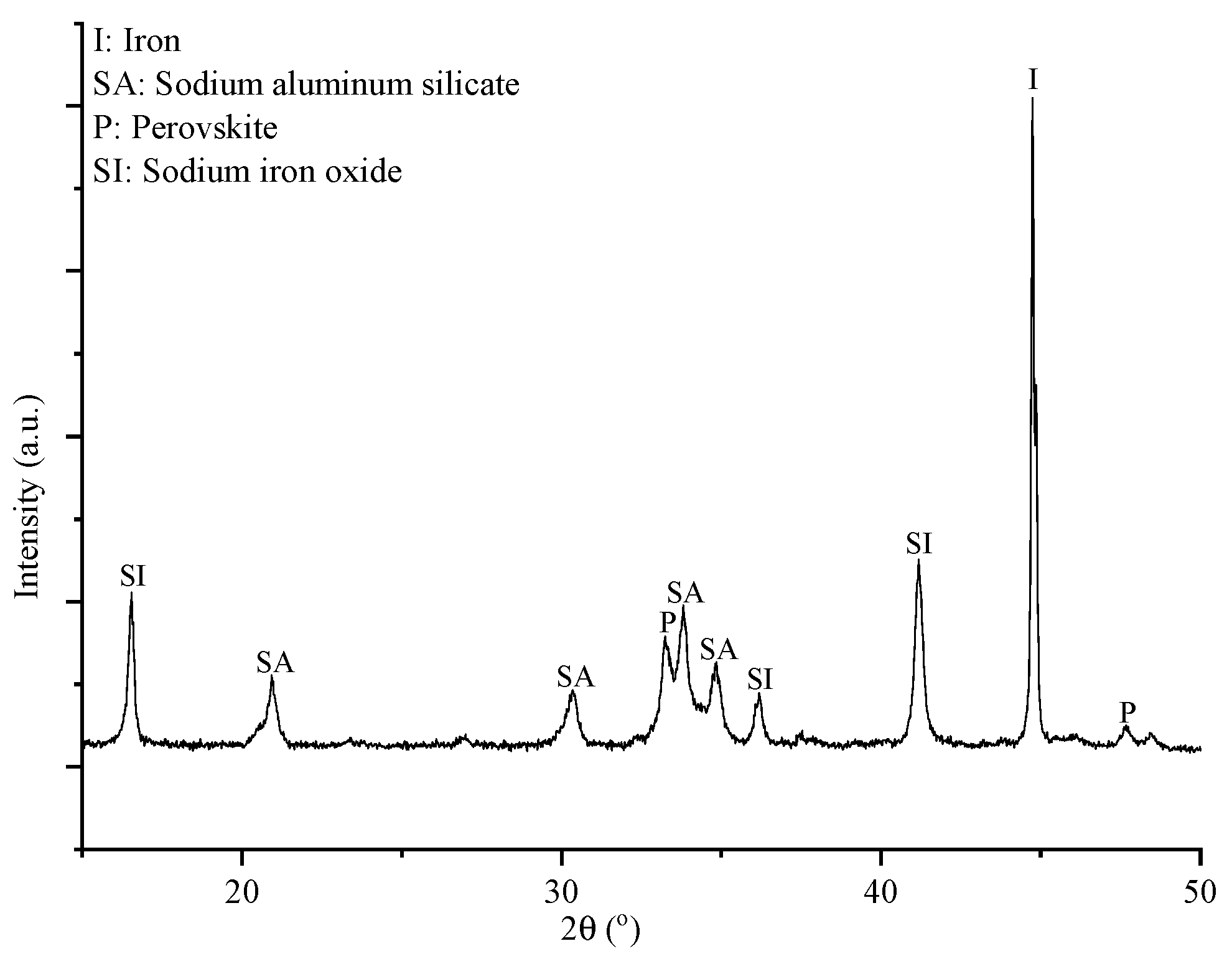
The major resulting phases of the product after roasting BR and NaOH at 600 °C, under H
2 gas, which are illustrated in
Figure 3, are: metallic Fe, perovskite (CaTiO
3) and sodium aluminum silicate (Na
1.95Al
1.95Si
0.05O
4). Moreover, in
Table 2 the wt.% composition of this product is presented, and it is useful for comparison with the Al, Na-depleted solid residue.
3.3. Magnetic Separation or Melting of the Resulting Solid Residue (Two Separate Processes)
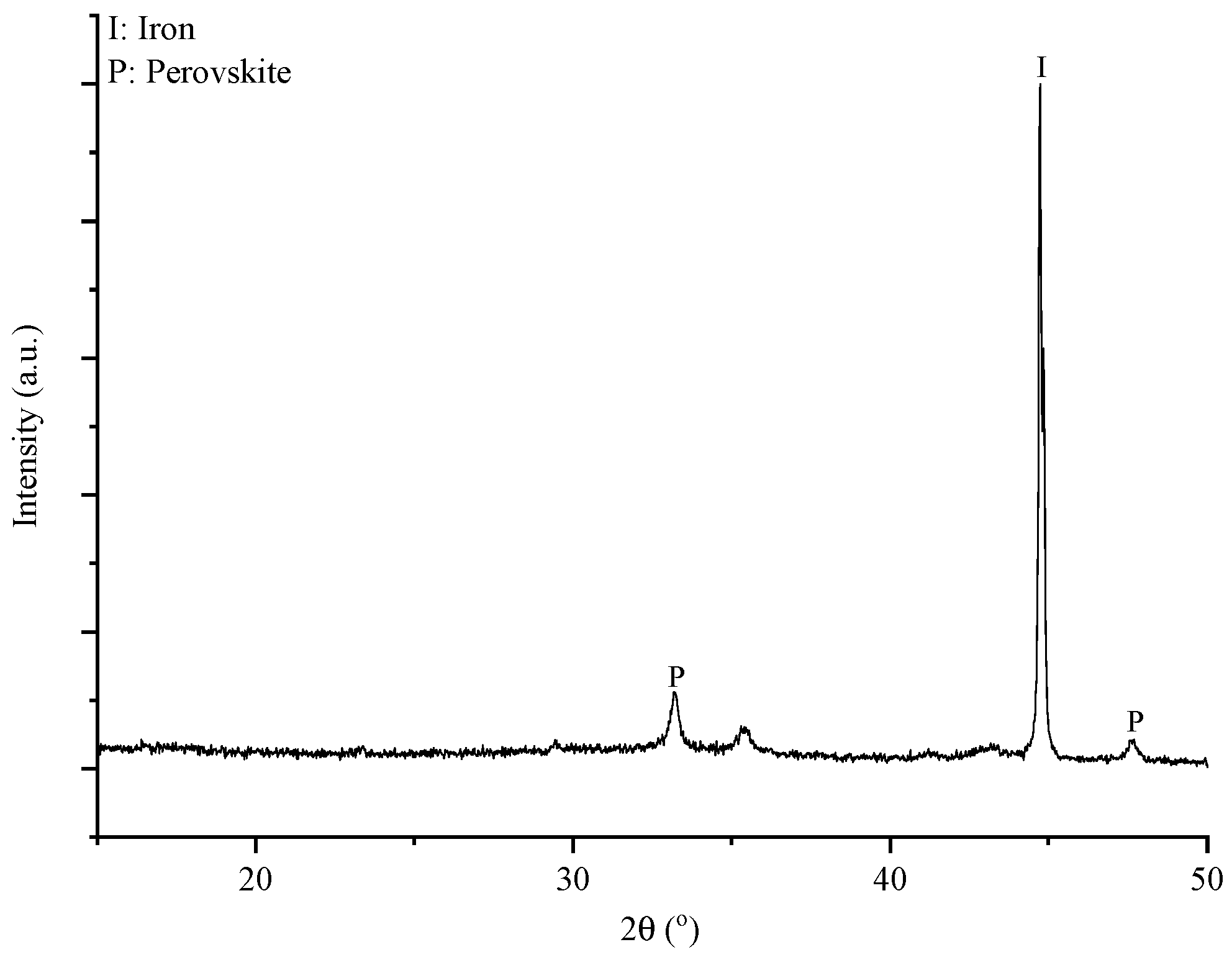
As it is indicated in
Figure 1 the first two processes are differentiated in the treatment of the resulting solid residue. The mineralogical and chemical composition of the latter are shown in
Figure 4 and
Table 3, respectively. The Al recovery, calculated based on the initial BR composition and ICP-OES measurements, was 78% in one case and 84% in another.
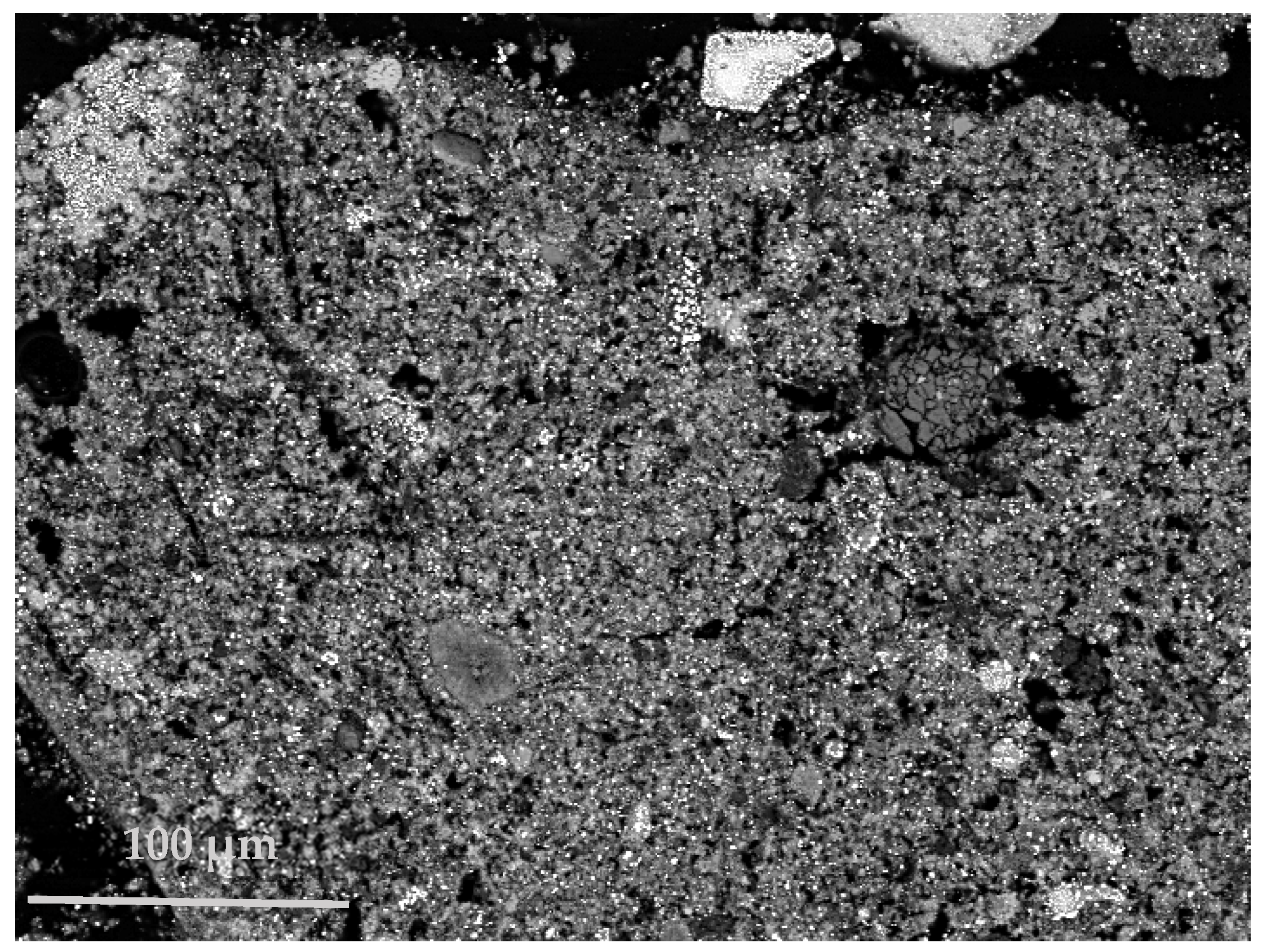
To finalize the first process, the iron-rich solid residue was imposed to a dry magnetic separation with the NdFeB magnet, after it was milled with a mortar and pestle. The particle size analysis revealed a median particle size (D50) equal to 4.9 μm. Specifically, the iron particles have an extended size range and are spread in the matrix of the solid residue, as can be observed in the SEM—BSE image, shown in
Figure 5—iron particles correspond to the brightest areas. This is challenging for the magnetic separation and an approach would be the target of a specific particle size of the iron phase, an identical and effective size translates into an efficient separation. Nevertheless, a solid mixture attracted by the magnet was obtained, which in the best case was consistent of 38.5 wt.% Fe.
For the second process, instead of a dry magnetic separation, the solid residue was melted at 1550 °C for half an hour, under Ar gas and pO
2 around 10
−17 atm. Then it was allowed to cool down in the furnace. This C-free melting process imitates the EAF (electric arc furnace) process, which is utilized in steel production and consumes scrap or sponge iron or both. In this study sponge iron was produced using H
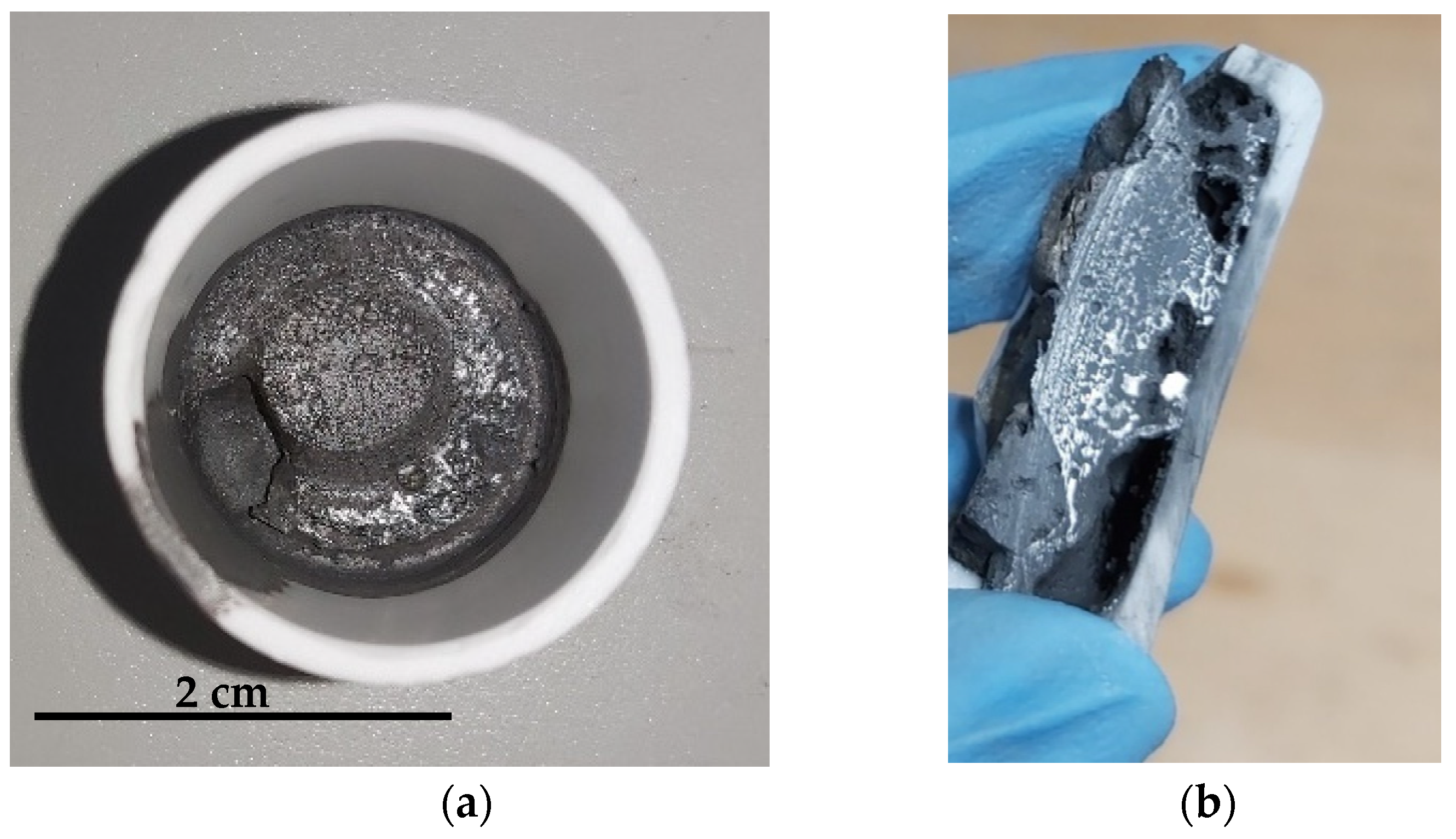
2 gas, with the difference that in-between Al (and some of the Na) were obtained. The final product after melting and cooling down can be found in
Figure 6. Since the Fe phase was not separated immediately, as normally occurs during melting, the process should be optimized, probably higher temperature will give better results.
3.4. Wet Magnetic Separation (Final Process)
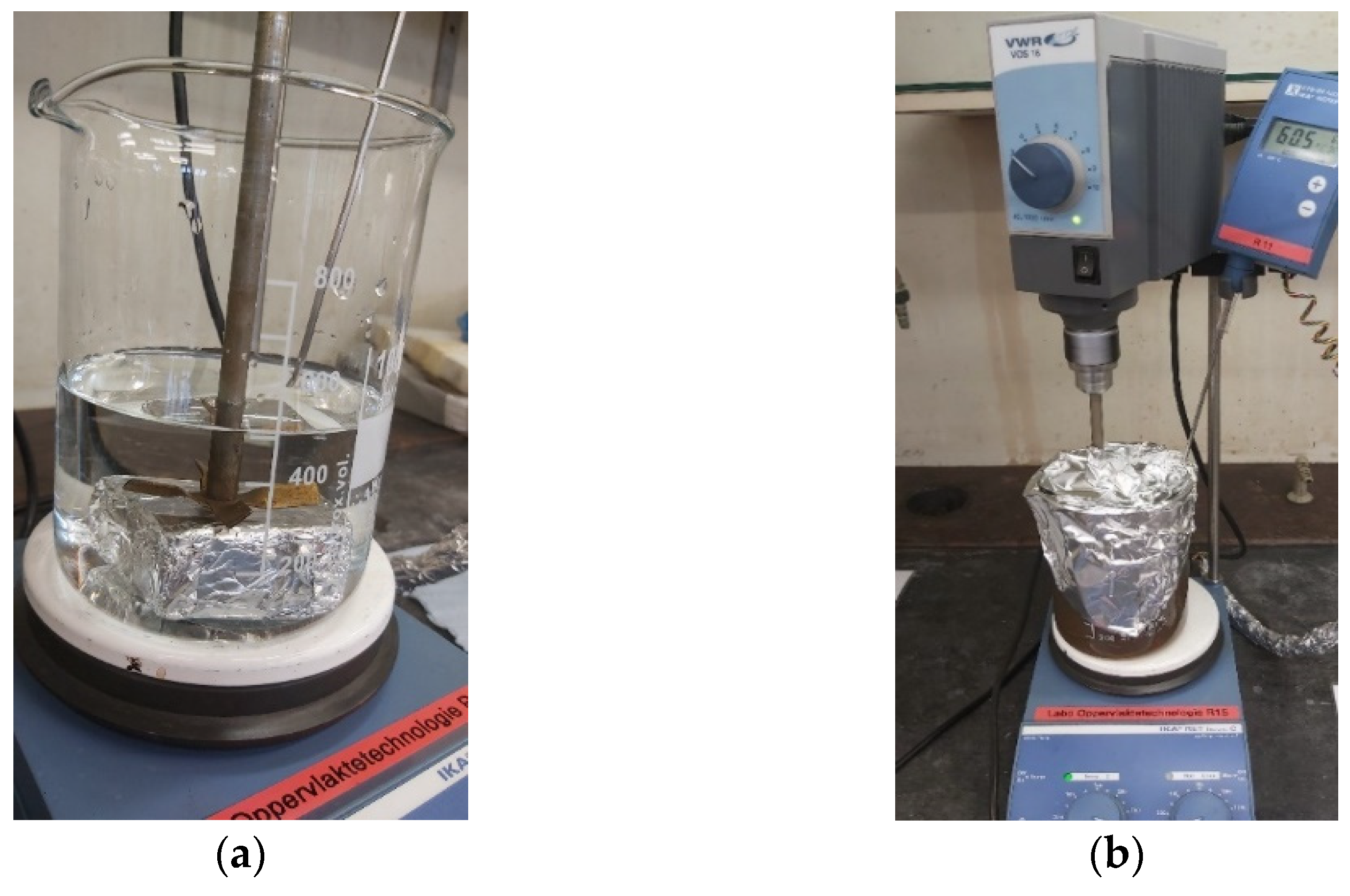
The final process led immediately to an ion-rich leachate and two solids, one magnetic and one non-magnetic fraction. The water leaching conditions were identical to the previous processes—water at 60 °C for one hour—the difference was the magnet placed inside the beaker during the water leaching. The set-up is illustrated in
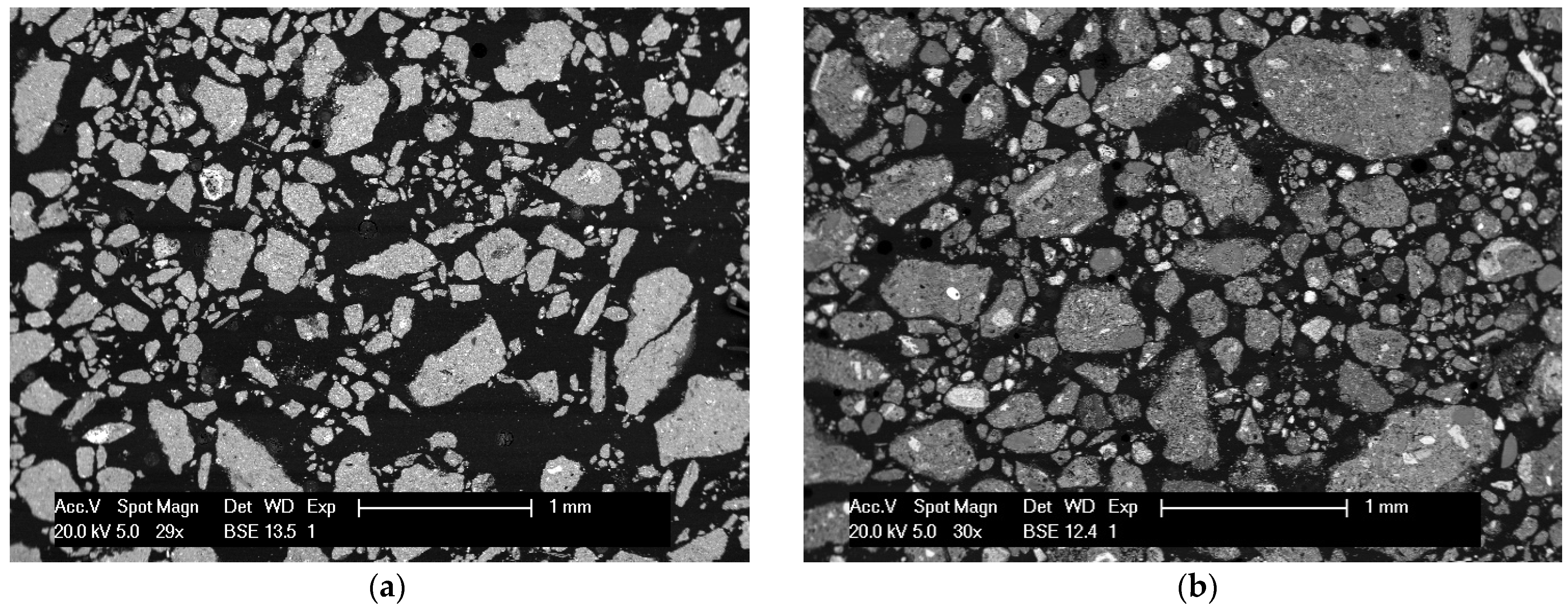
Figure 7. The recovery for Al reached 91%, which is higher than in the previous plain water leaching. Two SEM-BSE images of the solid attracted by the magnet and the solid obtained during the filtration of the leachate are given in
Figure 8. It seems that the non-magnetic solid sample still contains some Fe particles (bright areas), based on PXRD and EDS measurements, however they are much smaller in size compared with the ones in the solid attracted by the magnet. A plausible explanation is that bigger Fe particles, or particles consistent of many Fe microparticles, can be attracted easier from the magnet, because the average magnetic susceptibility is higher due to a higher metallic Fe fraction, while the nanoparticles trapped in the non-magnetic matrix result in an overall lower magnetic susceptibility. However, there is still a considerable amount of other phases interfering with the larger Fe particles, as it can be seen from
Figure 8b. The Fe content in the magnetic fraction was 31.85 wt.%.
4. Conclusions and Remarks
Three processes for Al and Fe recovery from bauxite residue were described above, after addition of NaOH and thermal treatment under H2 gas at 600 °C. The first process, that included water leaching and dry magnetic separation lead to an iron phase fraction of 38.5 wt.% Fe content. The second process included melting of the solid residue after the water leaching, instead of magnetic separation, and adjustment of the experimental parameters is necessary to achieve the maximum Fe recovery. The third process, simultaneous water leaching and magnetic separation (wet magnetic separation) resulted in a solid of 31.85 wt.% Fe content. The metallic Fe content in each case can be improved, and it was proven that larger particles, for instance more than 40 μm, can be separated more effectively using magnetic separation. Concerning the Al recovery in all cases was high enough, especially during the wet magnetic separation that reached 91% and further investigation is necessary to examine the reason behind this difference. Once the ideal Fe particle size for the maximum liberation is achieved, the wet magnetic separation can be a highly efficient and practical approach to the bauxite residue issue, since during one step both Fe and Al can be recovered, with a low energy consumption and with a relatively ‘green’ process.