1. Introduction
The global lithium-ion battery (LIB) market is expected to continue to grow, owing to the growing interest of consumers for electric vehicles at a compound annual growth rate (CAGR) of 15% from 2020 to 2026 [
1,
2]. The use of LIBs has led to an increase in the amount of spent batteries. However, the estimated recycling rates reported have still been very low [
2,
3]. In Europe, for example, a total of 65,500 tons of the LIB was consumed between 2013 and 2014, but only about 1900 tons were recycled in the same period [
2]. Currently, only the metals Co, Ni and Mn, with high economic values which are contained in the cathode active materials, are the main targets for most emerging recycling processes by pyro-hydrometallurgical techniques, and Li is mainly recovered as part of the cathode active materials. The metals, Cu, Al and Fe, are mostly recovered through mechanical and physical processing operations such as magnetic separation. Graphite is presently not of major interest for the battery recycling industry and is mostly lost in the recycling process [
2,
3]. From a circular economy perspective, recycling graphite is worth considering. Meanwhile, the separation of graphite could promote the efficiency of subsequent metal recovery. Graphite recovery from a mixture of shredded anode and cathodes via selective flotation after thermal or chemical treatment has been studied or patented [
4,
5,
6,
7,
8]. In the black mass (BM) material, both graphite and Li-containing metal oxides display very good floatability because their surfaces are coated with organic binders such as polyvinylidene fluoride (PVDF). Thus, direct flotation of the BM material showed poor selectivity between graphite and Ni, Co, and Mn. Thermal treatment by pyrolysis or roasting has proved to be an effective approach for the removal of organic binder’s coatings prior to flotation.
In this study, based on the BM material of a recycling process, the separation efficiencies of graphite from the cathode active materials containing Co, Ni, and Mn, and the metals Cu and Al via flotation was evaluated. The effects of roasting as pre-treatment at a temperature of 350–450 °C on the flotation efficiency of graphite were investigated.
2. Materials and Methods
The black mass (BM) material of spent Li-ion batteries used in this study was sourced from a recycling facility, where the NMC (Ni-Mn-Co) batteries were treated first by shredding and then the shredded material was sieved. The BM material was the undersize (<~2 mm) fraction of the sieving operation.
A sample of BM weighing about 50 kg was split into subsamples of 500 g each for chemical and mineralogical analyses and beneficiation test work.
A subsample of 500 g was sieved into the fractions of +1.0 mm, −1.0 + 0.5 mm, −0.5 + 0.25 mm, −0.25 + 0.15 mm, −0.15 + 0.106 mm, −0.106 + 0.075 mm, −0.075 + 0.045 mm and −0.045 mm and separately analysed by XRF for chemical compositions including Si, Fe, Mn, P, Cu, Ni, Co and Al, etc. The contents of carbon in the fractions were determined by Leco carbon analysis.
The compositions of compound phases, including graphite, the cathode active materials and metals Cu and Al, and textural characterization, were measured by the mineral liberation analysis (MLA) using an FEI QUANTA 650 FEG-SEM MLA instrument with two BRUKER XFLASF EDS-detectors. The mineralogical analysis was conducted for two fractions of −0.25 + 0.106 mm and −0.106 mm of the BM sample.
The 500 g subsamples were classified by sieving into two fractions of +0.25 mm and −0.25 mm as the feeds for flotation. Flotation trials on a laboratory flotation machine with a 1.5 L cell were carried out. Kerosene was used as the collector for graphite and Dowfroth 250 was added as the frother as needed. Roasting, as pre-treatment prior to flotation, was conducted with a laboratory muffle furnace at a temperature of 350 to 450 °C for a duration of 30 min.
3. Results
3.1. Particles Size and Elemental Distributions
3.1.1. Particle Size Distribution
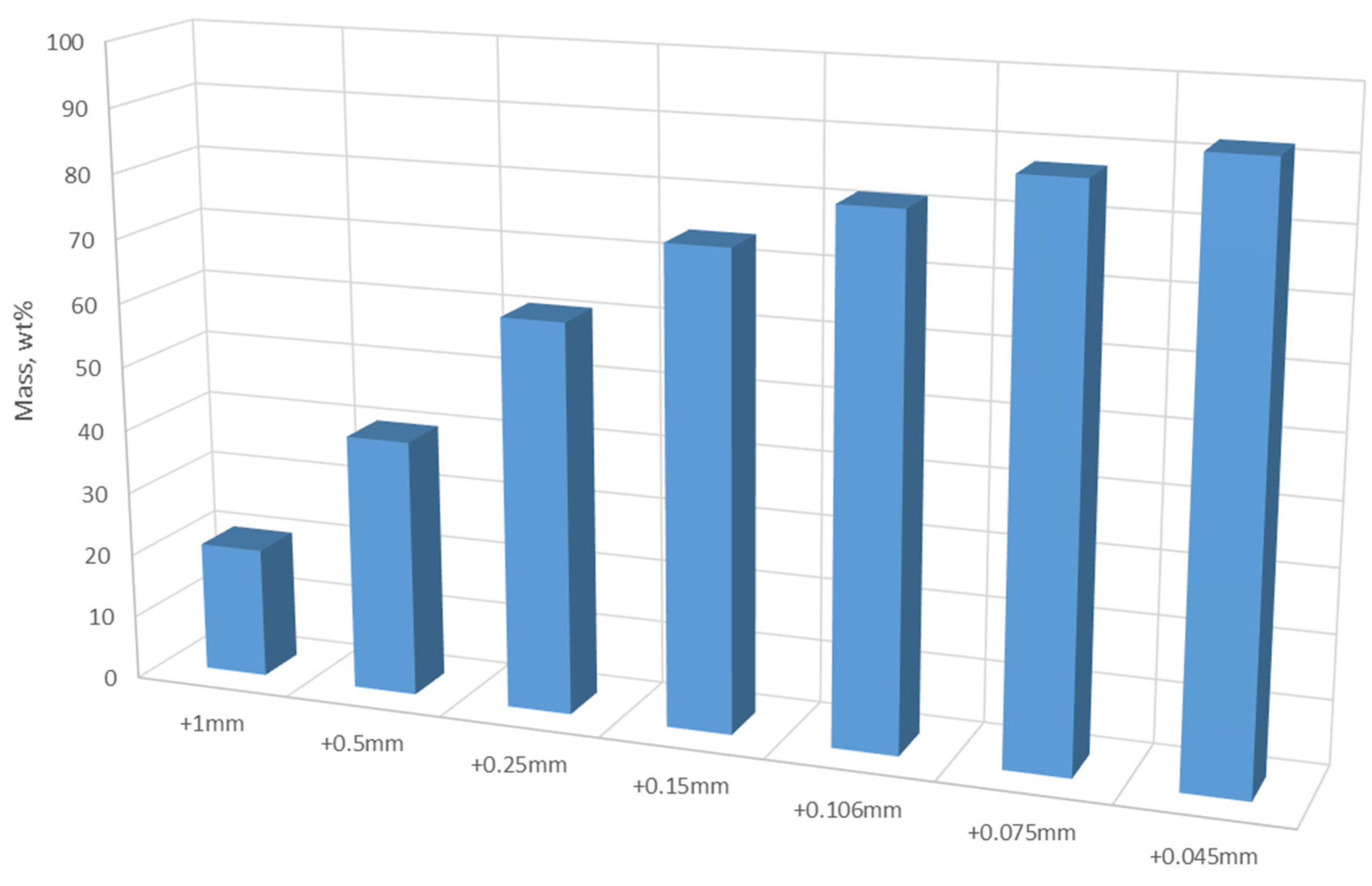
The particle size distribution of the BM material was obtained by sieving.
Figure 1 showed that about 60% mass in the material was in +0.25 mm fraction and the masses in the +0.75 mm and +0.045 mm were over 87% and 93%, respectively. In other words, fine size particles below 0.075 mm and 0.045mm were less than 13% and 8% in weight, respectively.
3.1.2. Distributions of Elemental Contents in Size Fractions
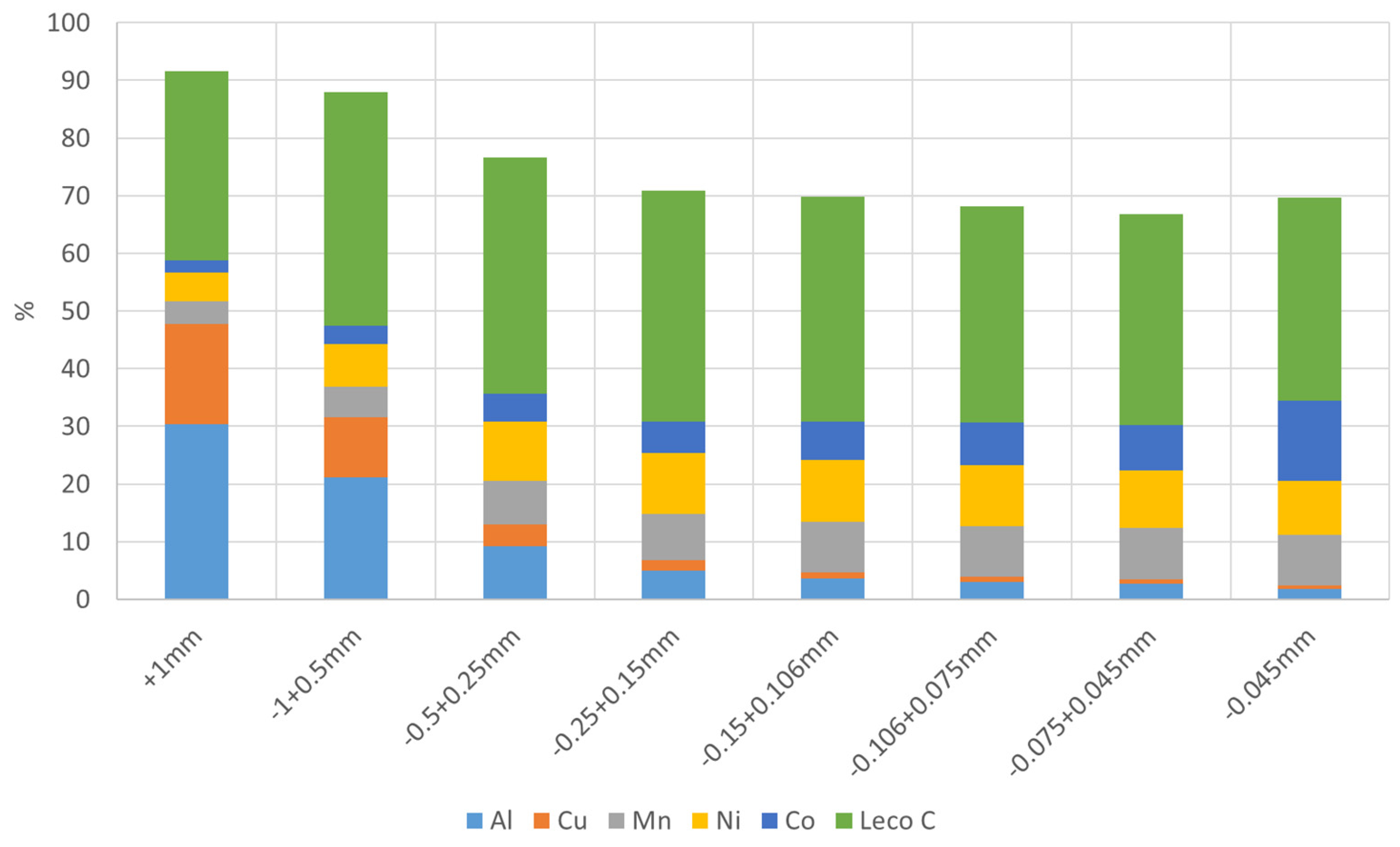
The BM material was classified according to size by sieving and all the fractions were analysed by XRF and Leco C. The analysis results showed that Al, Cu, Ni, Co, Mn and C are the main elements in the sample with significant contents. The contents of the elements Al, Cu, Ni, Co, Mn and C in all the fractions are presented in
Figure 2 (Li was also one of main elements measured by ICP-MS with an overall content of around 3.1%). It is seen that the contents of Cu (3.8–17.5%) and Al (9.2–30.3%) are obviously higher in the coarser fractions (+0.25 mm) and the contents of Ni, Co and Mn appear higher in the finer fractions (−0.25 mm). However, C with the contents of 32.8–41.0% distributes quite evenly in all the factions.
3.2. Mineralogical Analysis
Graphite and the cathode active materials (Li)-Co-oxide (LiCoO
2,), (Li)-Mn-Ni-Co-oxide (LiNixMnyCozO
2), and (Li)-Ni-oxide (LiNi
0.8Co
0.2O
2) were identified by MLA as major phases in the studied samples. The theoretical chemical formulas and densities for the metal-oxides were adopted from the internet (
www.americanelements.com (accessed on 22 October 2021)). The metal oxides are abbreviated as (Li)-compounds because Li cannot be detected by the EDS analyses due to its small atomic number (Z=3)). The contents of graphite, the Li-containing metal oxides and other phases with significant amounts determined in the two fractions are shown in
Table 1. It is seen that the compositions of the material are very complex. However, graphite and the four Li-containing metal oxides take up 92–94% of the amount.
3.3. Classification by Sieving
The sample was classified by sieving with aperture sizes of 1.0 mm, 0.5 mm, 0.25 mm, 0.15 mm, 0.106 mm, 0.075 mm and 0.045 mm. The relationships between the recoveries of main elements Cu, Ni, Co, Al, Mn and C in the oversize fractions and the mass yields (wt.%) are shown in
Figure 3. It is indicated that the metals Cu and Al display obvious separation in the oversize fractions, that is, in the +1.0 mm, +0.5 mm and +0.25 mm fractions (related mass yields 20.4, 40.3 and 61.0 wt.%) the recoveries of Cu reach 51.9%, 82.0% and 93.4%, respectively, and those of Al reach 45.3%, 76.0% and 90.0%, respectively.
3.4. Flotation
The grades and recoveries of the main elements C, Cu, Ni, Co, Mn, and Al in flotation concentrates for the coarse fraction +0.25 mm for no roasting and being roasted at a temperature 350 °C prior to flotation are shown in
Figure 4. It is seen that no significant difference was found in flotation result between with and without roasting. Roasting only slightly hindered the flotation of graphite and Li metal oxides. Generally, for both cases with and without roasting graphite (indicated by C) and Li metal oxides (indicated by Ni, Co, Mn) were well floated with relatively high recoveries (85–95% for C, and 77–85% for Ni, Co and Mn). However, mainly Cu and Al foils were sunk in the tailings, e.g., only 15–18% of Cu was entrained into the concentrate.
The effects of roasting at different temperatures on the grades and recoveries of the main elements C, Cu, Ni, Co, Mn and Al in the flotation concentrates for the fine fraction −0.25 mm are shown in
Figure 5. The results indicate that roasting obviously improved the selectivity between the graphite and metallic elements Ni, Co, Mn, Cu and Al on flotation; that is, significantly lower recoveries of the metallic elements were obtained in the concentrates after roasting than no roasting treatment. Meanwhile, roasting temperature affects the flotation performances of both graphite and metals. At the lower temperatures of 350 °C and 400 °C, graphite still has almost the same high recoveries (>93%) as no roasting treatment, but when the roasting temperature increased to 450 °C, the flotation of both graphite and metallic matters were hindered. The recovery of graphite decreased to 50%, although there was still obvious difference between graphite and the metallic elements on recovery. Based on the results, the optimal temperature of roasting for graphite flotation is 400 °C.
4. Discussion
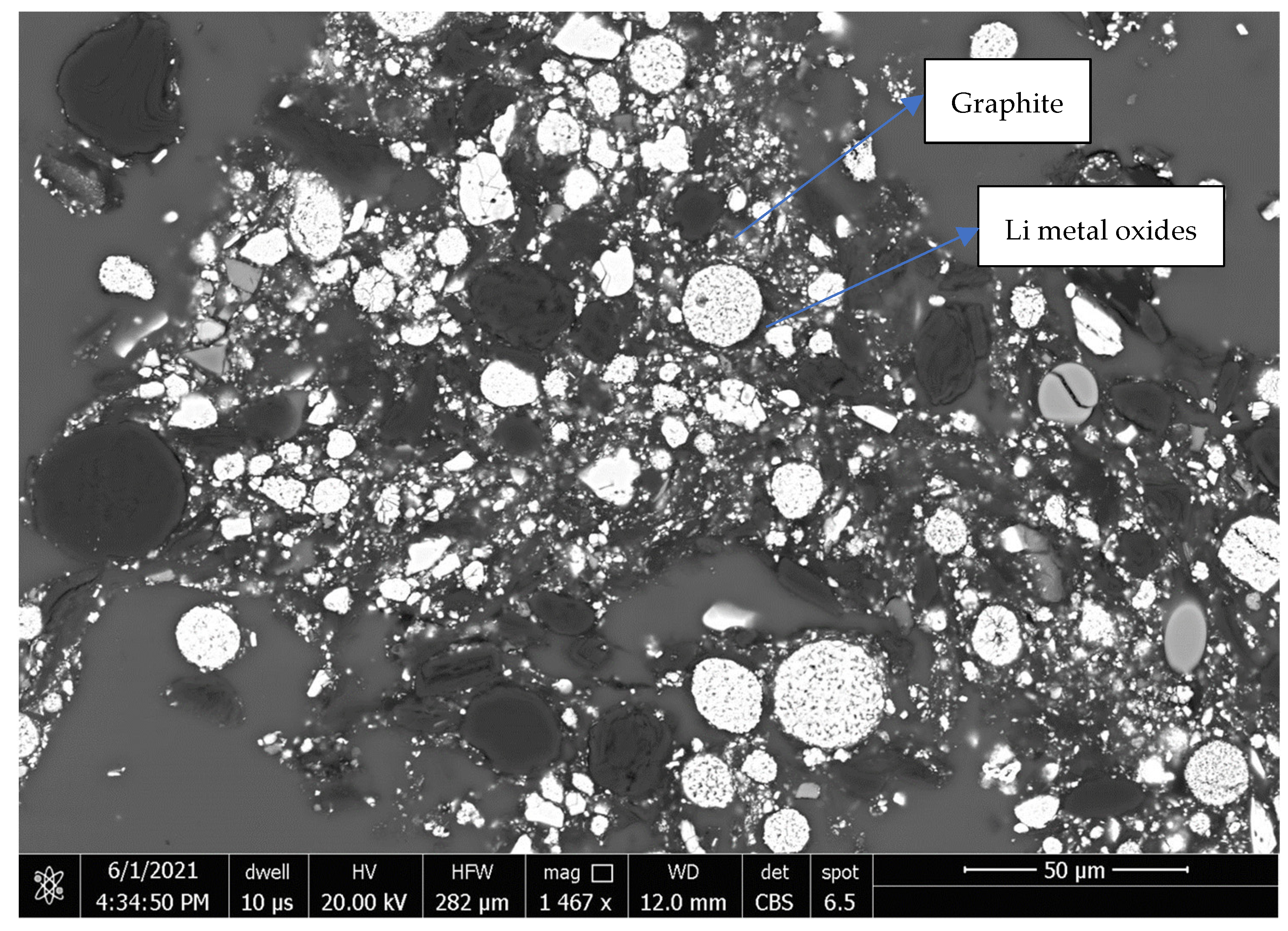
The study showed that the metals Cu and Al can be effectively separated by simple sieving and flotation operations at a coarse size (+0.25 mm). However, the separation of graphite from the cathode active metals Co, Ni and Mn was challenging. Further processing, including finely grinding, followed by roasting and flotation, could improve their separation. For the fine fraction (−0.25 mm) by flotation after roasting at a temperature of 350–450 °C for 30 min over 90% of graphite was recovered, but significant amounts (about 50%) of Co, Ni and Mn were floated into the graphite concentrate. This is mainly attributed to very fine particle sizes (~10 µm) of graphite and Li metal oxides and their very tight association, as shown in the back-scattered electron image (
Figure 6). Meanwhile, the coatings of the organic binders on the surfaces of graphite and the Li metal oxides might be very difficult to completely remove during roasting. Roasting at a higher temperature than 450 °C might cause significant surface oxidation of both graphite and metallic matters which hinders their flotation.
5. Conclusions
The main elements of recycled LIBs include Li, Al, Cu, Ni, Co, Mn and C with significant contents. The contents of Cu and Al were obviously higher in the coarser fractions (+0.25 mm) and Ni, Co and Mn appear in content higher in the finer fractions (−0.25 mm). However, C with content of 32.8–41.0% distributes quite evenly in all the sieve factions.
The BM has very complex compositions, but graphite and the cathode active materials (Li)-Co-oxide, (Li)-Mn-Ni-Co-oxide, and (Li)-Ni-oxide were identified to be the major components in the fraction of −0.25 mm, taking up 92–94% of the amount. Metals Cu and Al in the foils used as current collectors mostly exist in the coarse fractions (+0.25 mm).
Through sieving the BM material into +0.25 mm and −0.25 mm fractions, the metals Cu and Al were mostly (>90%) concentrated in the oversize fraction of +0.25 mm, from which Cu and Al were further separated by flotation from graphite and the cathode active materials.
For the undersize fraction of −0.25 mm, roasting at a temperature of 350–450 °C for 30 min as pre-treatment obviously improved the selectivity between graphite and the metals Ni, Co, Mn, Cu and Al by flotation, that is, significantly lower recoveries of the metals were obtained in the concentrates after roasting than no roasting treatment.