1. Introduction
Lining performance has a key role in the sustainability of ferronickel production from nickeliferous laterites by rotary kiln-electric furnace (RKEF) smelting, since the iron-rich slags commonly produced are very aggressive to basic magnesia refractories, thus posing significant operational and safety risks. Due to the low nickel content in the Fe-Ni alloy produced in electric arc furnaces, it is further enriched in OBM (Oxygen Bottom Maxhütte) converters where iron as well as other impurities existing in the EAF alloy are preferentially oxidized by oxygen introduced and removed in slag, leading to a substantial increase of the final nickel content. The slag generated in this stage has very high iron content constituting a very aggressive environment for magnesia refractories used for OBM lining. Therefore, extensive refractories corrosion takes place that progressively decreases the depth of lining and causes unpredictable shutdowns, thus limiting equipment availability and posing significant threats to the operational security of the installations.
Generally, the corrosion of the refractories takes place in stages. When fluids, like slags, come into contact with refractories, their pores are filled and chemical wearing starts as a result of the differences in the chemical potential of elements between the refractory and the liquid. In a magnesia refractories–ferrous slag system, the grains of periclase progressively react with liquid iron, which substitutes for magnesium to form magnesiowustite. At the same time, magnesium is dissolved into the slag. A magnesiowustite layer is progressively developed in the boundary of the pores while diffusion of iron ions in the bulk mass of the refractory continues. Thus, an iron penetration zone is formed in refractories, which significantly affects the chemical and mechanical stability of the lining, as the refractories’ properties are substantially altered.
In almost all the studies of refractory–ferronickel slag corrosion systems available in the literature [
1,
2], electric arc furnace slag has been used. This slag is characterized by high concentrations of Si and Ca oxides, in contrast to the slag of Fe-Ni converters of this study, where slag consists almost exclusively of iron oxides, with Si and Ca oxides content being below 6%. This creates different corrosion conditions, due to the more aggressive nature of FeO that has not been studied in detail to date, with the exception of a study performed by the authors of this study [
3] at lower temperatures up to 1650 °C.
In this study, the mechanism of iron infiltration in magnesia refractories at very high temperatures was examined and the rate of intermediate zone development was determined at different temperature levels and contact time.
2. Materials and Methods
OBM converter slag samples and crucibles made of high purity commercial magnesia refractories currently used for OBM lining were provided by LARCO SA (Larymna, Greece). Chemical analysis of both materials was performed following fusion and determination of elements in the solution derived by Atomic Absorption Spectrometry (Flame-AAS) and ICP-OES. Mineralogical analyses of magnesia refractories and slag were conducted by X-ray Diffraction (XRD, Bruker D8 Focus, Billerica, MA, USA) and Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM/EDS, Jeol6380LV, Akishima, Japan).
Experimental tests were performed using crucibles filled with 100 g of OBM converter slag, heated at 4.5 °C/min up to 1600–1700 °C and remained at this temperature for 1 to 8 h (
Table 1). The temperature levels examined have been selected based on the actual conditions prevailing in OBM converters during their different phases of operation. Following overnight cooling, refractories were cut across the slag-refractory surface and thin polished sections were prepared for further examination with SEM-EDS.
Three types of SEM/EDS analyses/measurements were performed on thin polished sections from all the experimental conditions investigated. First, the depth of the intermediate zone, otherwise, the infiltration or penetration zone, was measured. Then, microanalyses were carried out for the determination of elements content, mainly iron and magnesium, at different locations. Finally, mapping of the main elements (Fe, Mg, Si, Ca), either linear across an affected area or surface mapping covering unaffected refractory, the intermediate zone, or slag areas was performed.
In addition, the software FactSage 7.0 (CRCT, Montreal, Canada and GTT-Technologies, Aachen, Germany) was used to thermodynamically determine the different phases that are formed at equilibrium when liquid slag is in contact with magnesia refractories at different slag to magnesia refractories mass ratios in the mixture, thus providing information to conclude about the refractories corrosion mechanism. The parameter Alpha in FactSage has been used for this purpose, with Alpha being equal to zero and 1, when the chemical composition of the mixture is the same as the one of the refractory and slag, respectively.
3. Results and Discussion
3.1. Chemical and Mineralogical Analysis
The chemical analysis of the refractory and slag samples is given in
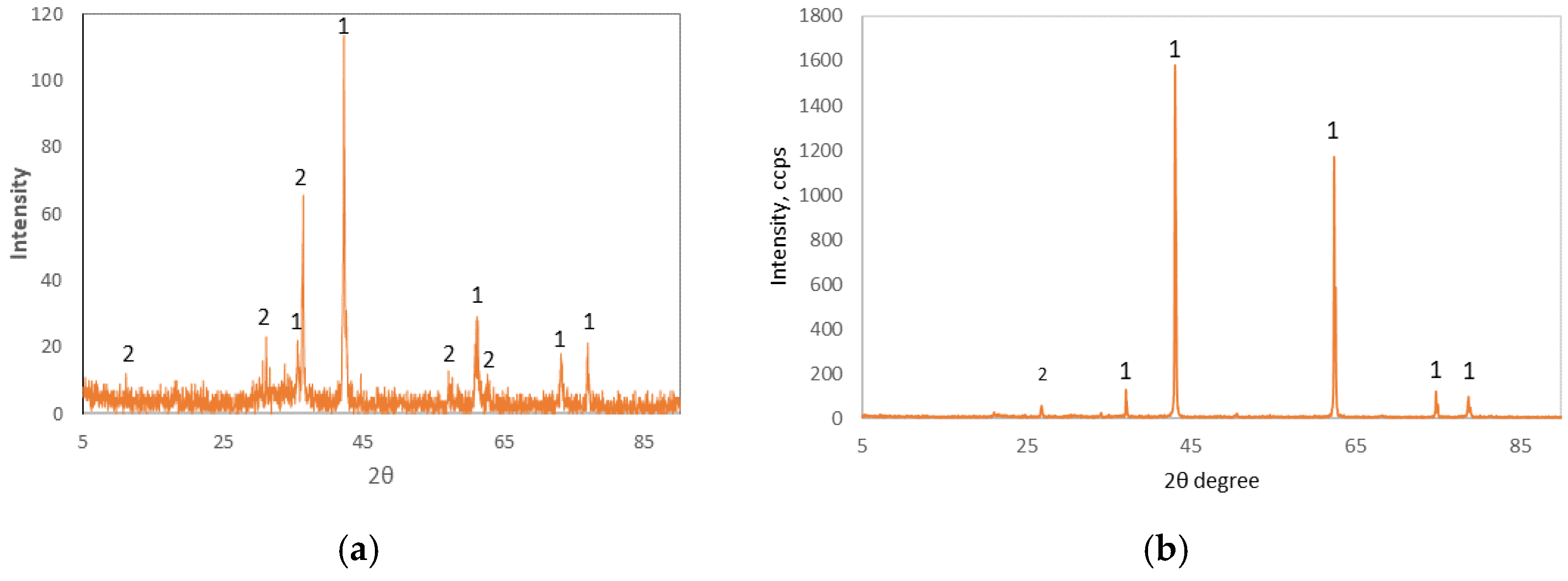
Table 2. XRD diagrams of the samples examined are shown in
Figure 1.
The OBM slag sample examined consists mainly of iron oxides with a total content of 82% wt. and a small amount of Si, Ca, Mg, Al and Cr oxides. A significant amount of ferrous oxides, mainly wustite, is contained in the OBM slag, whereas magnetite is coordinated with other oxides to create several phases of the spinel group and chromite (
Figure 1a). As for the refractory, the main mineral identified is periclase (MgO) with a small amount of quartz (SiO
2) (
Figure 1b).
3.2. Microscopic Examination of Refractories-Slag Interface
3.2.1. SEM Images-Depth of Infiltration Zone
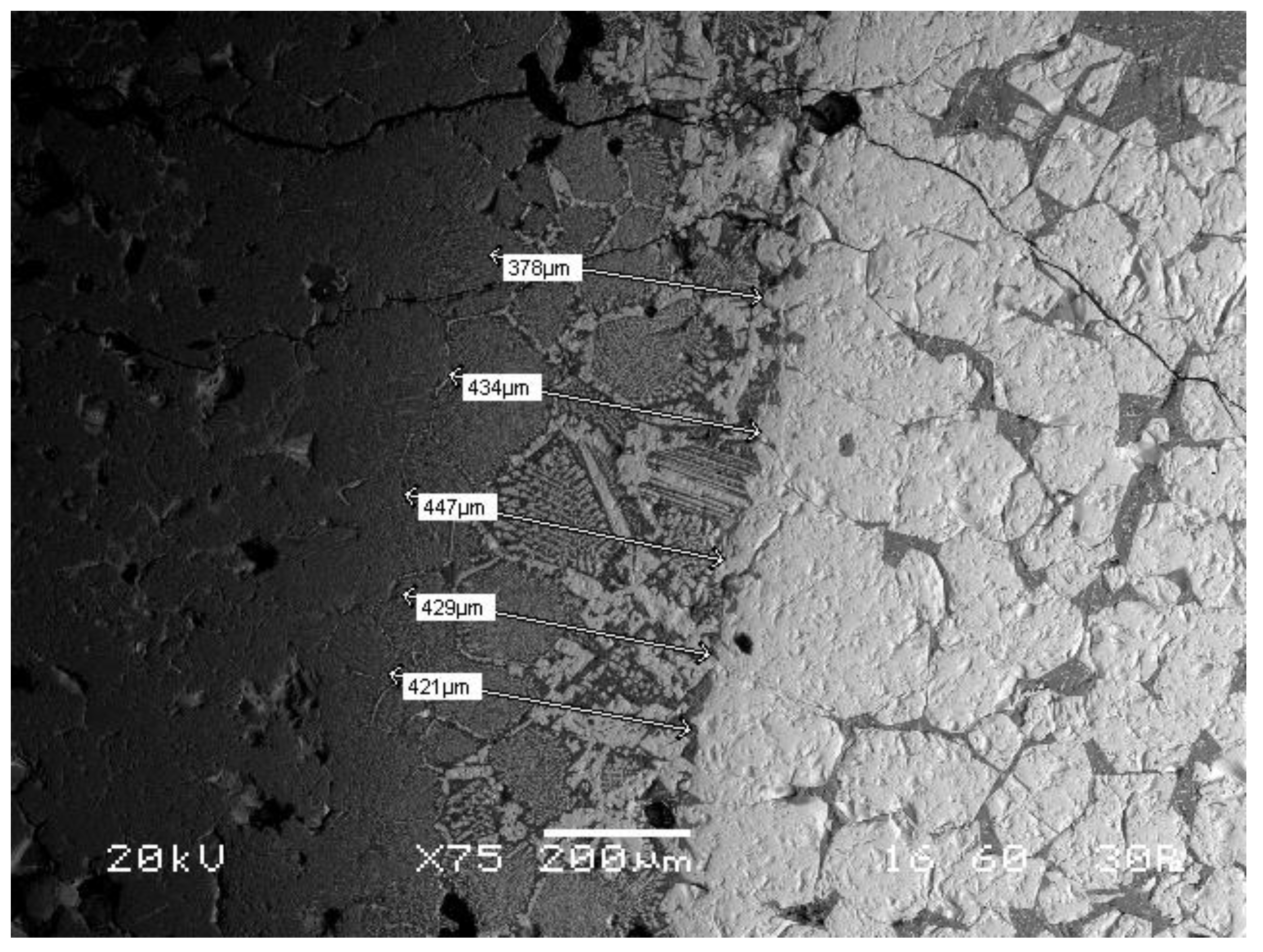
A typical SEM image of a slag-refractory interface is shown in
Figure 2. Magnesia refractory (dark gray color, left side), slag (white gray color, right side) and the intermediate zone of slag affected refractories are clearly seen in SEM image. The white grey color formations are attributed to iron, mainly in the form of iron oxides, whereas dark grey complexes in slag are calcium silicates. As seen in
Figure 2, iron has penetrated into the refractories mass, resulting in the formation of several different patterns, with cellular shapes presenting high iron content in the periphery and several different patterns in their interior part.
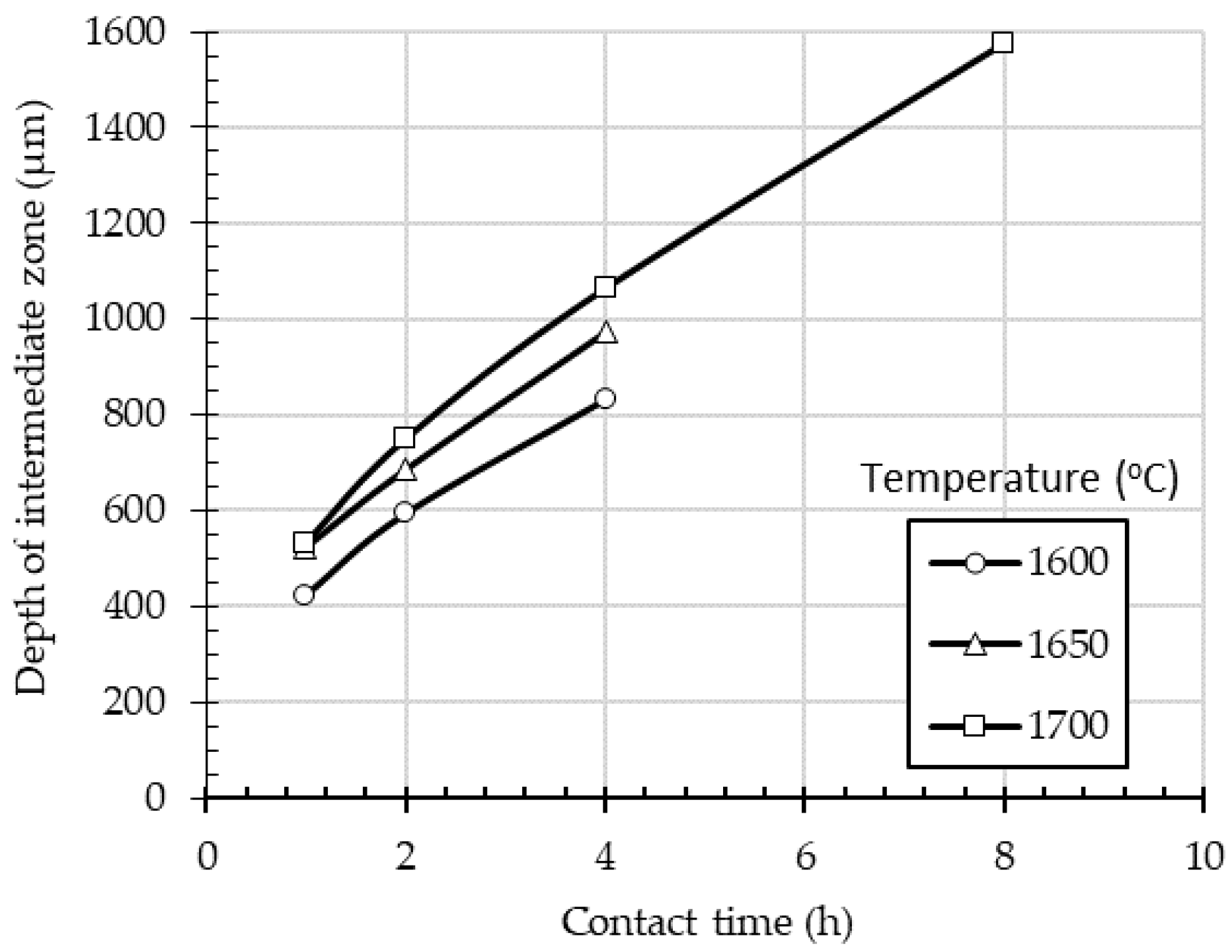
The depth of infiltration zone was measured using SEM images, and the average values for each set of experimental conditions (temperature, contact time) were plotted against time (
Figure 3). As seen in
Figure 3, the increase of the retention time or temperature results in the increase of the intermediate zone, with maximum value of 1.57 mm obtained at 1700 °C and 8 h contact time.
3.2.2. Mapping
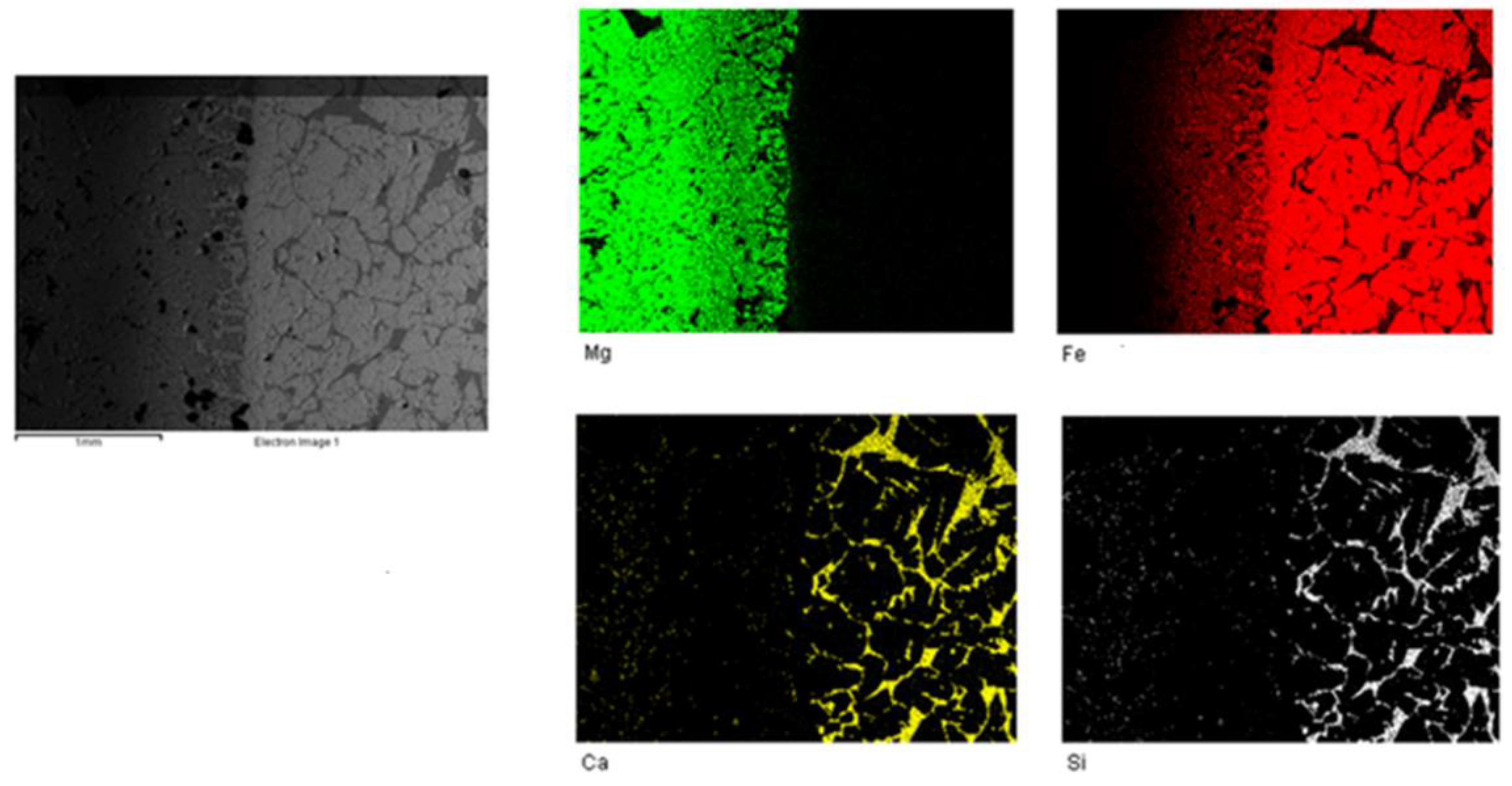
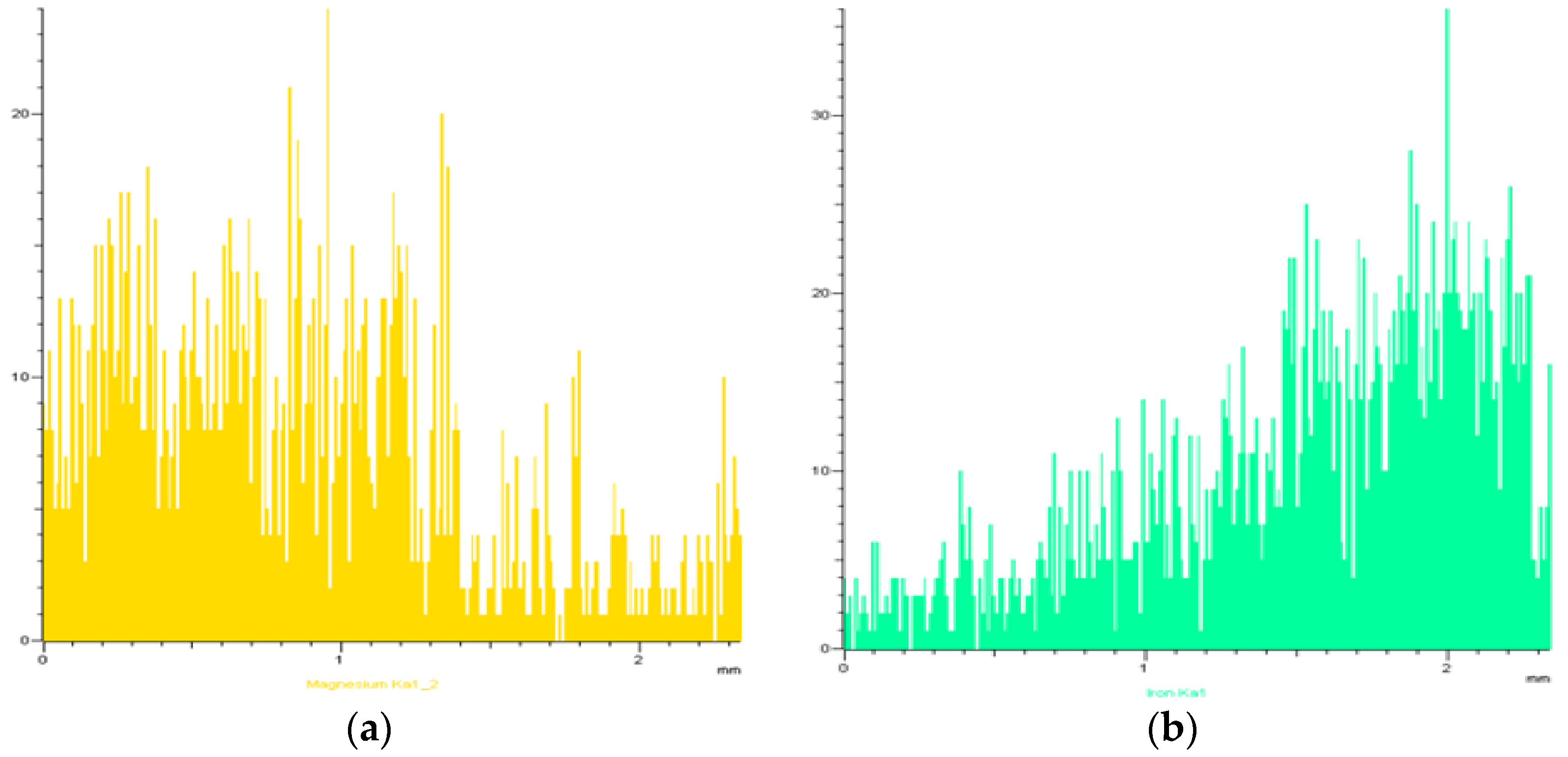
The surface distribution of the main elements investigated (Fe, Mg, Ca and Si) in an area covering unaffected refractory, slag and the intermediate as determined by SEM/EDS mapping on a section of sample derived from the experiment at 1650 °C for contact time 2 h is given in
Figure 4.
It is clear that the bulk mass of the refractory has a greater magnesium content up to the intermediate zone in which the intensity of the green color corresponding to Mg decreases and that of the red color corresponding to Fe increases. On the other hand, calcium and silicon create new phases in slag, with similar distributions of the two elements in the same particles.
It is seen that magnesium and iron act adjunctively, mainly in the intermediate zone, as it is also shown in SEM/EDS linear mapping of the slag–refractory interface after their contact at 1700 °C for 2 h (
Figure 5). As seen in
Figure 5, Fe content is very high and close to the slag–refractory interface, indicating substitution of magnesium by iron in the periclase phase, resulting in the formation of magnesiowustite, which is progressively decreased with a simultaneous increase of Mg content.
3.3. FactSage Thermodynamic Simulation
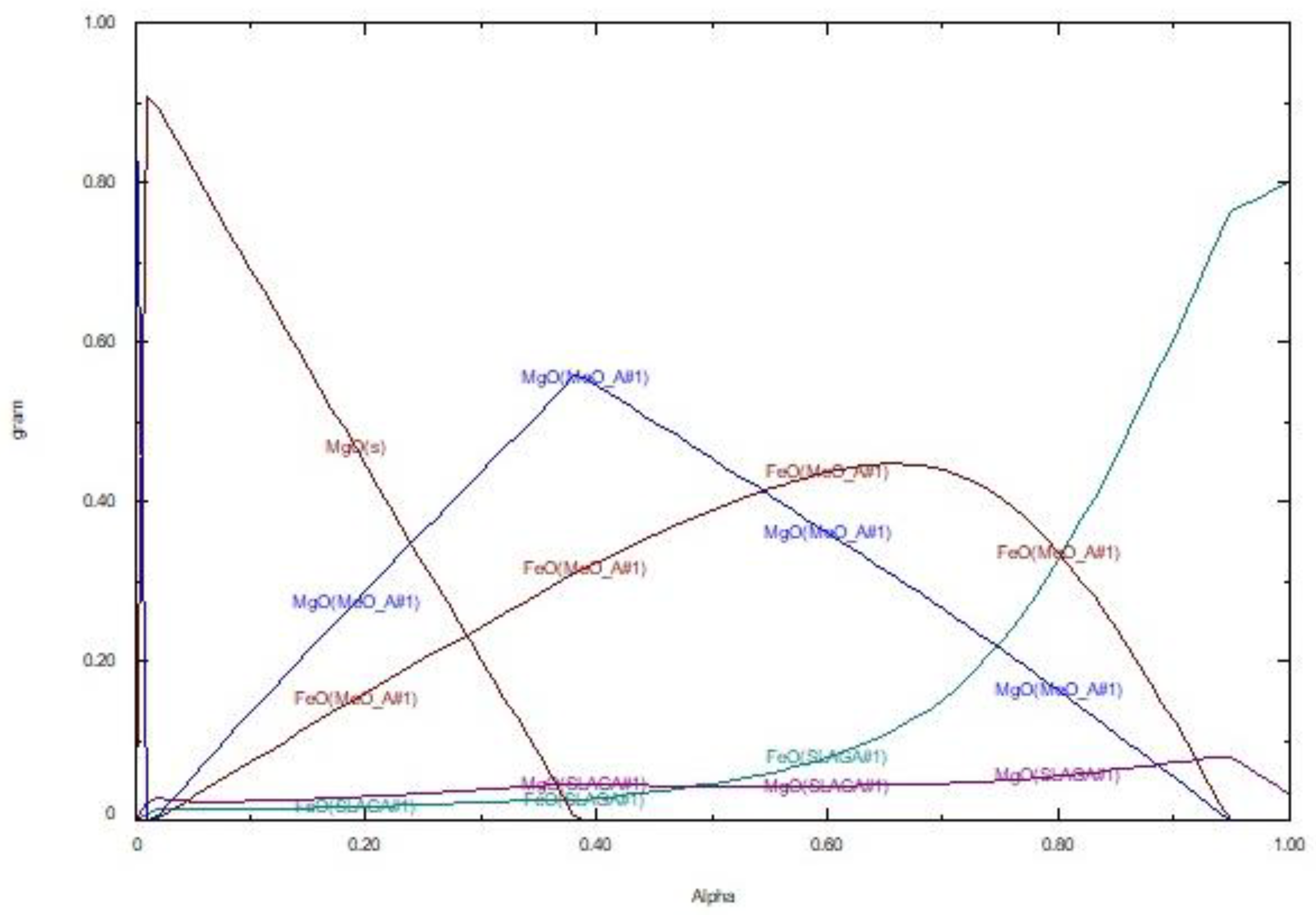
The behavior of the slag–refractory system in terms of the individual phases formed as well as their composition and properties strongly depend on the relative mass of the two components, i.e., slag and refractory, in the system, as well as the experimental conditions applied. The mass of the different phases formed as well as their compositions in the refractory–slag mixture at equilibrium at 1600 °C, as determined by FactSage, is given in
Figure 6. It is seen that only three phases appear in the system:
- (a)
Pure solid magnesium oxide (MgO(s)), which is the main phase of refractories (A = 0); its percentage decreases linearly with increasing percentage of slag in the mixture and becomes zero for a value of A = 0.36 at 1600 °C.
- (b)
The phase of magnesium–iron oxide, symbolized as MeO_A#1 (magnesiowustite, (Mg, Fe)O). The amount of this phase increases by increasing the slag percentage in the mixture, as more iron is provided that gradually substitutes magnesium forming (Mg, Fe)O. Thus, the amount of pure solid MgO decreases as the amount of (Mg, Fe)O increases. In
Figure 6, the amount of FeO and MgO as constituents of phase MeO_A#1 are given. Upon the increase of iron content in magnesiowustite, the melting point of magnesiowustite decreases; when the latter is equal or below 1600 °C, magnesiowustite melts and dissolves into the liquid phase formed.
- (c)
A small amount of liquid slag, which is symbolized as SLAGA # 1, is formed even at very low slag/refractory mass ratios. However, its quantity decreased exponentially when slag ratios in the mixture became very high.
4. Conclusions
Based on the high temperature slag–refractories interaction experiments performed, SEM/EDS observations and measurements, and FactSage 7.0 thermodynamic analyses of the slag–refractories system, the following conclusions have been derived:
Magnesia refractories are significantly corroded after their contact with iron rich slag under the conditions prevailing in OBM converters.
FeO in slag penetrates refractories, progressively substitutes for MgO thus forming magnesiowusite (Mg, Fe)O, and develops a zone of affected refractories, with very high iron content close to the refractories–slag interface.
Various cellular formations are mainly found close to the slag–refractory interface consisting of white grey magnesiowustite with high iron content in the periphery and black grey alternating with white grey magnesiowustite with low and high iron content, respectively, in their internal part.
The thickness of the iron penetration zone increases with contact time and temperature and reaches up to 1.57 mm at 1700 °C after 8 h of processing.
The thermodynamic analysis of the refractory–slag system at various mixing ratios performed by FactSage indicated the formation of pure periclase, magesiowustite and liquid slag phases. Their percentage after treatment at a certain temperature, as well as the composition of magnesiowustite and liquid slag, depend on the initial quantity of the slag in the mixture.
Based on the results of this work, it is proposed that the corrosion mechanism of refractories includes the following stages:
Penetration of FeO either through the pores or via the contact surface and substitution of Mg by Fe,
Further solid state diffusion of Fe and increase of its concentration in magnesiowustite that results in the decrease of the melting point of magnesiowustite,
Melting of the iron-enriched magnesiowustite when its melting point becomes lower than the operational temperature, and
Dissolution of magnesiowustite in the slag mass and consequently partial erosion of OBM lining.
Author Contributions
Conceptualization, K.B. and A.X.; methodology, A.X.; software, K.B. and K.K.; validation, A.X.; investigation, K.B. and A.K.; resources, K.B.; writing—original draft preparation, K.B.; writing—review and editing, K.B., K.K., A.K. and A.X.; visualization, K.B.; supervision, A.X.; project administration, A.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge LARCO SA for providing slag samples and magnesia refractories-based crucibles for conducting laboratory experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sagadin, C.; Luidold, S.; Wagner, C.; Spanring, A.; Kremmer, T. Phase Reactions Between Refractory and High-Acidic Synthetic CaO-Ferronickel Slag. JOM 2018, 70, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.; Wenzl, C.; Gregurek, D.; Kreuzer, D.; Luidold, S.; Schnideritsch, H. Thermodynamic and Experimental Investigations of High-Temperature Refractory Corrosion by Molten Slags. Metall. Mater. Trans. B 2017, 48, 119–131. [Google Scholar] [CrossRef]
- Betsis, K.; Kourtis, A.; Chatzitheodoridis, E.; Xenidis, A. Corrosion of high purity magnesia refractories from iron-rich slags of ferronickel enrichment in OBM converters. In Proceedings of the International Scientific Conference Summer Session Industry 4.0, Varna, Bulgaria, 22–25 June 2022. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).