A Novel One-Step Green Method to Synthesis of Palladium Nanoparticles †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PdNPs
2.3. Characterization
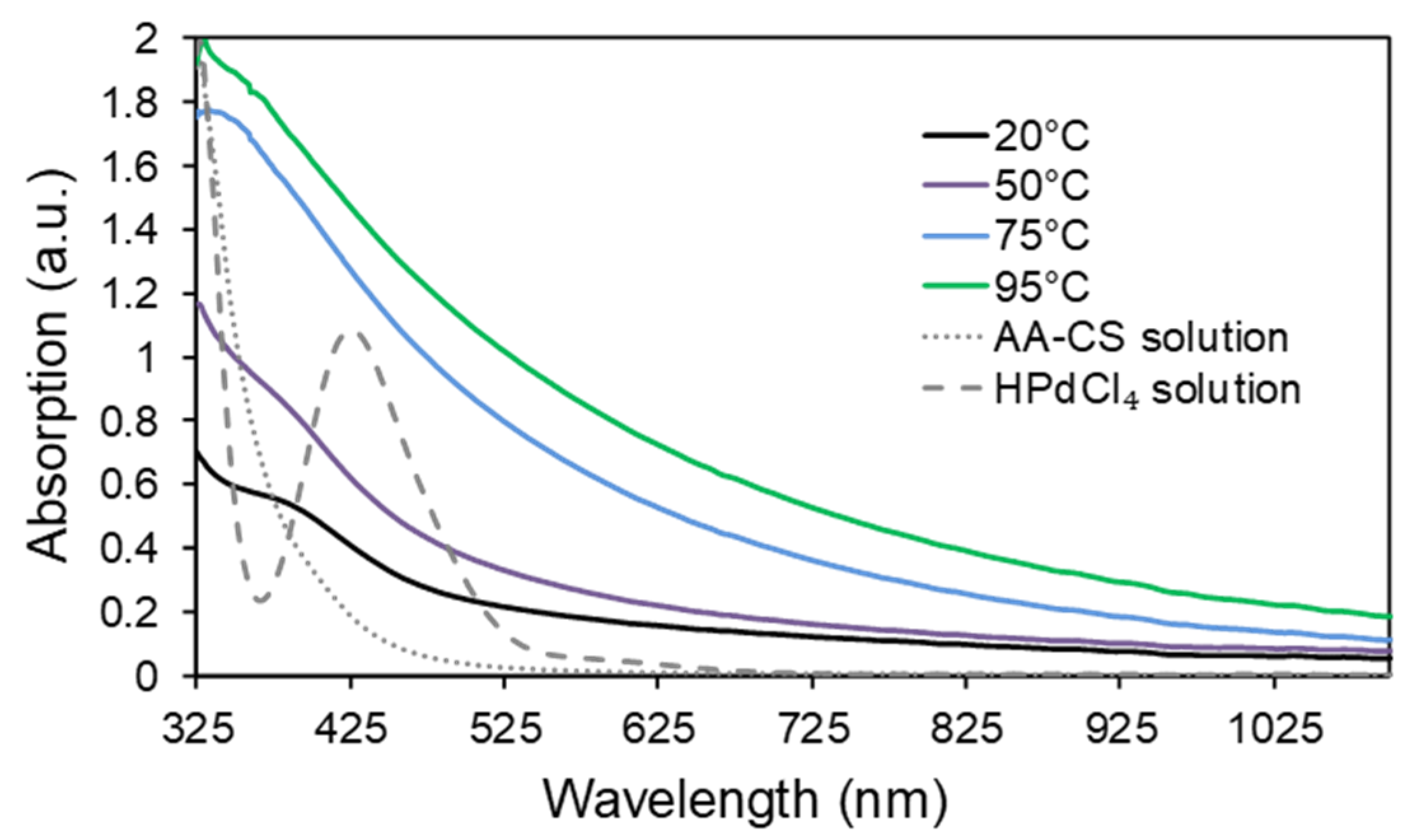
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed]
- Long, N.V.; Nguyen, C.; Hirata, H.; Ohtaki, M.; Hayakawa, T.; Nogami, M. Chemical synthesis and characterization of palladium nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jin, M.; Lee, K.J.; Cheon, J.Y.; Joo, S.H.; Kim, J.M.; Moon, H.R. In situ-generated metal oxide catalyst during CO oxidation reaction transformed from redox-active metal-organic framework-supported palladium nanoparticles. Nanoscale Res. Lett. 2012, 7, 461–461. [Google Scholar] [CrossRef] [PubMed]
- Long, N.V.; Thi, C.M.; Yong, Y.; Nogami, M.; Ohtaki, M. Platinum and palladium nano-structured catalysts for polymer electrolyte fuel cells and direct methanol fuel cells. J. Nanosci. Nanotechnol. 2013, 13, 4799–4824. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Couvreur, P. Palladium: A future key player in the nanomedical field? Chem. Sci. 2015, 6, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Fraser, C.; Rubio-Ruiz, B.; Myers, S.; Crispin, R.; Dawson, J.; Brunton, V.; Patton, E.; Carragher, N.; Unciti-Broceta, A. N-Alkynyl Derivatives of 5-Fluorouracil: Susceptibility to Palladium-Mediated Dealkylation and Toxigenicity in Cancer Cell Culture. Front. Chem. 2014, 2, 56. [Google Scholar] [CrossRef]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Lovanh, N.; Seo, S.-K.; Jayanthi, P.; Park, Y.-J.; Cho, M.; Oh, B.-T. Synthesis and antimicrobial activity of palladium nanoparticles from Prunus × yedoensis leaf extract. Mater. Lett. 2016, 185, 335–338. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Hoang, G.; Nguyen, V.T.; Nguyen, T.P.; Kim, H.H.; Mondal, S.; Manivasagan, P.; Moorthy, M.S.; Lee, K.D.; Junghwan, O. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohydr. Polym. 2019, 205, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D.; et al. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, F.; Gunn, T.M.; Zhao, D.; Roberts, C.B. Precise Seed-Mediated Growth and Size-Controlled Synthesis of Palladium Nanoparticles Using a Green Chemistry Approach. Langmuir 2009, 25, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, M.; Xu, J.; Yang, P.; Du, Y. Size-Controlled Synthesis of Palladium Nanoparticles. J. Dispers. Sci. Technol. 2008, 29, 891–894. [Google Scholar] [CrossRef]
- Coronado, E.; Ribera, A.; García-Martínez, J.; Linares, N.; Liz-Marzán, L.M. Synthesis, characterization and magnetism of monodispersed water soluble palladium nanoparticles. J. Mater. Chem. 2008, 18, 5682–5688. [Google Scholar] [CrossRef]
- Castellanos-Rubio, I.; Insausti, M.; Gil de Muro, I.; Rojo, T.; Lezama, L. Tuning the Size of Palladium Nanoparticles in Organic and Aqueous Solutions: Influence of Aminated and Thiolated Ligands. J. Nanosci. Nanotechnol. 2016, 16, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Jang, Y.; Shokouhimehr, M.; Lee, I.S.; Hyeon, T. Facile Aqueous-Phase Synthesis of Uniform Palladium Nanoparticles of Various Shapes and Sizes. Small 2007, 3, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Park, J.; Jang, Y.; Chung, Y.; Hwang, S.; Hyeon, T.; Kim, Y.W. Synthesis of Monodisperse Palladium Nanoparticles. Nano Lett. 2003, 3, 1289–1291. [Google Scholar] [CrossRef]
- Yang, Z.; Klabunde, K.J. Synthesis of nearly monodisperse palladium (Pd) nanoparticles by using oleylamine and trioctylphosphine mixed ligands. J. Organomet. Chem. 2009, 694, 1016–1021. [Google Scholar] [CrossRef]
- Teranishi, T.; Miyake, M. Size Control of Palladium Nanoparticles and Their Crystal Structures. Chem. Mater. 1998, 10, 594–600. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.T.V. A Novel One-Step Green Method to Synthesis of Palladium Nanoparticles. Mater. Proc. 2021, 4, 57. https://doi.org/10.3390/IOCN2020-07860
Phan TTV. A Novel One-Step Green Method to Synthesis of Palladium Nanoparticles. Materials Proceedings. 2021; 4(1):57. https://doi.org/10.3390/IOCN2020-07860
Chicago/Turabian StylePhan, Thi Tuong Vy. 2021. "A Novel One-Step Green Method to Synthesis of Palladium Nanoparticles" Materials Proceedings 4, no. 1: 57. https://doi.org/10.3390/IOCN2020-07860
APA StylePhan, T. T. V. (2021). A Novel One-Step Green Method to Synthesis of Palladium Nanoparticles. Materials Proceedings, 4(1), 57. https://doi.org/10.3390/IOCN2020-07860





